Highlights
- ⁻
Many vector-borne diseases of public health importance originated in non-human primate hosts. Understanding transmission cycles requires vector surveillance at canopy-levels.
- ⁻
Globally, studies have observed substantial differences in vector species composition, diversity and biting preferences vertically.
- ⁻
Canopy-level vector surveillance has been conducted using human landing catches, and different types of mosquito traps to collect host seeking mosquitoes. However, few studies have been able to cross-validate different vector catching methods at canopy level.
- ⁻
Methods of accessing the canopy remains a key logistical barrier. Dependent on survey design, this may involve building fixed platforms or temporary deployment methods.
- ⁻
New technologies, such as drones, offer opportunities to access canopies and inaccessible areas but need further development for routine deployment.
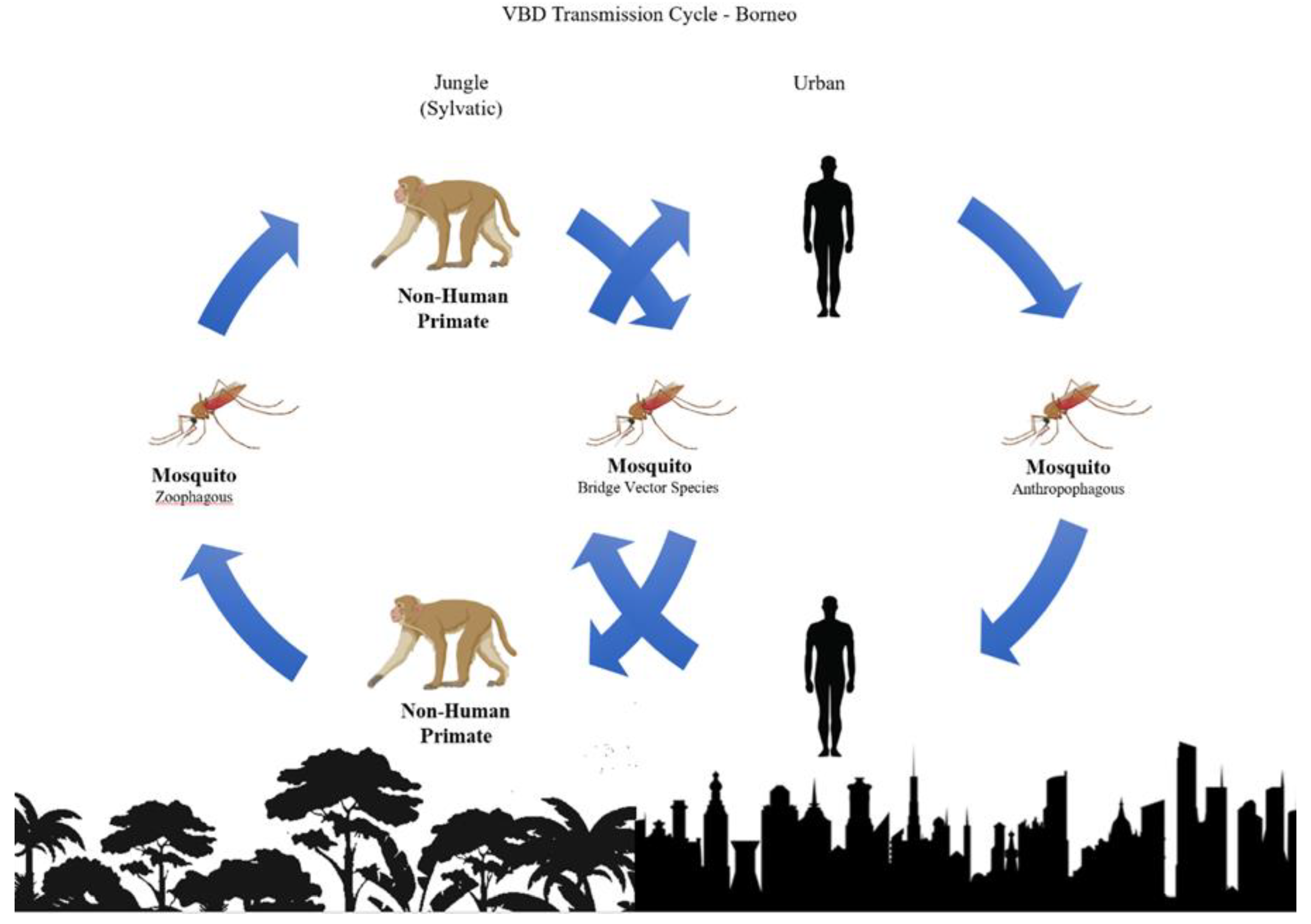
Surveillance for Vector-Borne Diseases with Non-Human Primate Hosts
Many vector-borne diseases (VBDs) originated in
non-human primates (NHPs, see
Glossary). Diseases, such as malaria, yellow fever and other arboviral diseases, often include
sylvatic disease cycles involving wildlife in natural forest habitats [
1]. In the natural habitats of NHPs,
zoophilic,
acrodendrophilic (canopy-dwelling)
mosquitoes transmit the pathogens between wildlife hosts [
2]. Human populations can become infected if they encroach into NHP habitats or when infected zoophilic
bridge vectors transmit pathogens from wildlife to humans, with VBD infections spreading rapidly if they can be transmitted by competent,
anthropophilic mosquito species [
3]. This results in spillover into human populations and potential transitions from a sylvatic disease cycle to an endemic human transmission cycle [
4] (See
Figure 1).
NHP reservoirs are a barrier to VBD elimination due to the risk of repeated spillover and pathogen maintenance in sylvatic transmission cycles. The emergence of dengue as a global health burden demonstrates the shift from a sylvatic cycle to a human-endemic cycle due to ecological disturbance and subsequent adaptation to nonzoonotic transmission [
5]. Characterised by infrequent but large epidemics from 1780 – 1940 [
6], the large-scale habitat degradation in the Pacific and South-East Asia theatres during World War II created optimal conditions for increased transmission in urban areas maintained by anthropophilic vector species with repeated spillover events mediated by bridge vectors [
7].
Applying traditional control measures for nonzoonotic VBD diseases to diseases with sylvatic cycles, has rarely succeeded given the complexity of transmission. Standard vector-borne disease control measures, such as insecticide treated nets, vaccine drives and rapid detection and treatment of cases, target nonzoonotic transmission and do not prevent spillover from wildlife. Wildlife reservoirs have become increasingly critical in areas where nonzoonotic disease transmission has been eliminated, such as zoonotic malaria outbreaks in otherwise malaria free areas of Malaysia and Brazil [
8]. Due to the complexity of sylvatic transmission cycles, there remain key research gaps on how to design and implement
zoonotic and vector-borne disease surveillance [
9].
For diseases with sylvatic cycles, surveillance systems are needed to monitor pathogen transmission between wildlife populations. Arboreal
zoophilic mosquitoes act as vectors for a number of sylvatic zoonotic pathogens, including
Plasmodium sp., West Nile virus, yellow fever virus (YFV) and Rift Valley fever virus [
10]. Data on the abundance, biting preferences, vectoral capacity and circulating pathogens in these vector species is essential to understand the epidemiology of these pathogens. Despite advances in genetic methods, which have led to increased use of
xenosurveillance to noninvasively detect circulating pathogens from vector bloodmeals [
11], data on pathogen epidemiology circulating between canopy dwelling wildlife remain limited [
12]. Targeted mosquito trapping has identified the vector species for sylvatic pathogens including Rift Valley fever, dengue and yellow fever and malaria [
6,
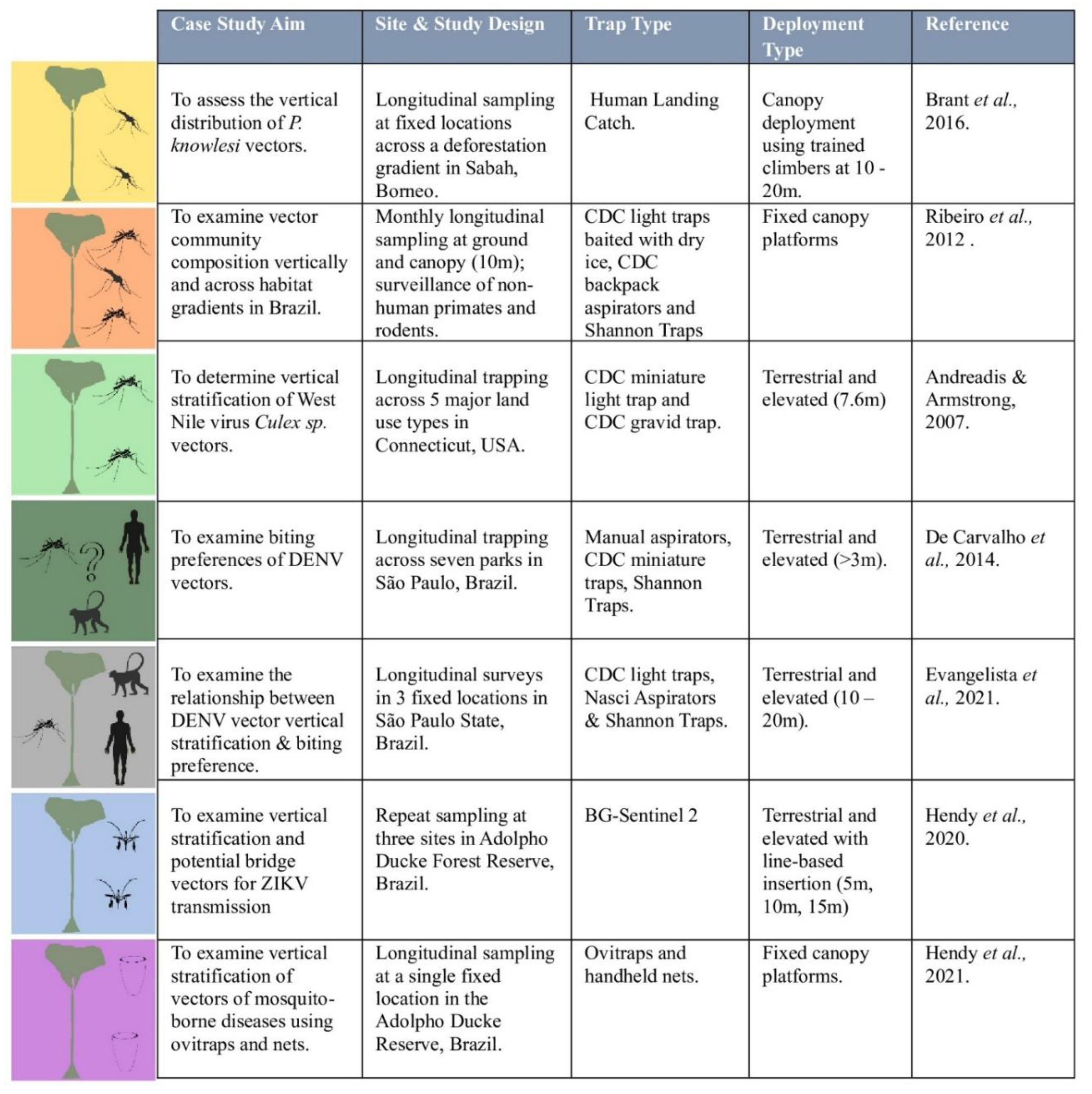
13]. Additionally, vector trapping data provide insights into spatial and temporal patterns of vector occurrence, vector community composition, blood feeding preferences, and niche specificity. Evaluation of mosquito trapping methods has primarily focused on design for terrestrial usage, creating a paucity of research on transmission cycles in the forest canopy.
Here, we review existing methods of entomological surveillance at canopy level, assess specific canopy-level trapping requirements and identify opportunities to develop canopy-level trapping tools for surveillance of sylvatic disease cycles. We examine case studies of canopy-level trapping tools used for monitoring zoonotic malaria in Malaysian Borneo and zoonotic malaria and arboviruses in Brazil. Placing canopy-level trapping in the broader framework of developing policy for effective surveillance and control strategies for zoonotic diseases, we discuss applications and future priorities.
Entomological Trapping Methods
Entomological trapping methods for research and operational use have been extensively developed to catch mosquitoes across all stages of their life cycle, with context-dependent efficacy for different methods [
14]. Host-seeking adult mosquitoes are commonly surveyed using traps that may incorporate live animals or human volunteers as attractants. For anthropophilic mosquito vectors, the gold standard remains
Human Landing Catch (HLC) [
15,
16,
17], which requires human volunteers to collect landing mosquitoes. Other traps utilise attractants such as carbon dioxide, odour, heat and light of different wavelengths to attract mosquitoes [
18,
19]. Alternatively, traps that target egg-laying behaviour include oviposition traps, which use small containers containing water to attract egg-laying mosquitoes and a substrate to collect eggs, and gravid traps, which similarly use water to attract gravid mosquitoes and methods such as sticky glue boards or oil to capture adult mosquitoes [
20,
21]. As these traps target blood fed mosquitoes, there is a higher probability of detecting infections.
Human Landing Catches in the Canopy
However, using these entomological methods at canopy levels creates new logistical challenges (see
Figure 2). First, there needs to be method of deployment to access the canopy. For HLCs or other human-baited traps, this may require using certified tree climbers or creating fixed platforms for people to access. Research by Hendy et al. [
22] evaluated the efficacy of volunteers using hand-nets to collect approaching mosquitoes from platforms in Manaus, Brazil, and observed a decreasing mosquito species richness with altitude, and the presence of flavivirus vectors at all elevations. However, developing these platforms requires substantial investment in infrastructure that is not easily moveable. Alternatively, trained tree climbers were used to collect zoonotic malaria vectors at canopy level in Malaysian Borneo [
23]. These human-baited methods allow people to record detailed data on when mosquitoes were collected to better characterise vector bionomics. However, both of these methods expose people to health and safety risks due to the need to climb trees and potential exposure to pathogens . Moreover, the use of humans and other species not typically found at canopy level as bait may alter the distribution and behaviour of vector and wildlife communities and therefore abundance estimates from human-baited traps may not be representative of vectors biting NHPs. While some vector species may be generalists, studies have identified vector species with much stronger attraction towards NHP odours compared to humans or other animals [
24].
Animal-Baited Traps in the Canopy
The ideal method of surveillance to understand sylvatic cycles is to use traps baited with the natural wildlife host, although this is biased towards vectors and pathogens associated with the chosen animals. For example, when using monkey-baited traps,
Anopheles cracens (a
Plasmodium knowlesi vector found in the forests of peninsular Malaysia) prefers feeding on NHP species at the canopy level, and on humans at the ground level[
25]. This identified potential biases in ground-based studies that have perhaps erroneously reported higher biting behaviour for humans, with important ramifications for understanding vectorial capacity [
8,
26]. Alternatively, early studies used CDC traps baited with non-host animals to examine vertical stratification and biting behaviours of
Culex and
Anopheles mosquitoes in Brazil [
27]. Despite the utility of these methods, animal-baited traps are rarely implemented in canopy level due to the expense, logistical challenges and ethics requirements around the use of NHPs and other animals in research.
Case Study: Canopy-Level Entomological Surveillance in Brazil: Insights and Operational Use
YFV is a vector-borne sylvatic disease that has a complex transmission cycle, and has a long history as a zoonotic disease with recurrent epidemics in rural regions of Africa and South America [
28]. Recently re-emerging in Minas Gerais and São Paulo states in Brazil in 2016, in the most significant epidemic in over 70 years [
29], YFV provides an interesting example of the application of surveillance strategies. As an arbovirus, control strategies for YFV, involve vector control measures for populations with high habitat specificity, with distinct mosquito species transmitting the virus in the three independent epidemiological cycles; jungle, savannah and urban cycles in Africa, and a sylvatic cycle in Brazil [
30]. Historically, the YFV urban transmission cycle has been interrupted by aggressive control of
Ae. aegypti vector populations, although these are often not economically feasible and are unable to control
Haemagogus spp. and
Sabethes spp. Vectors involved in enzootic transmission in forested landscapes in Brazil. The development of a highly successful vaccination program against YFV in the 1930s in Africa, foreshadowed these problems of sylvatic disease transmission [
31].
Canopy-level entomological trapping deployed in São Paulo State successfully sampled sylvatic YFV vectors [
32,
33]. Canopy-mounted platforms in the Atlantic Forest Biome were used to support a variety of entomological sampling methods that observed higher overall species diversity and higher YFV vector richness in the canopy. As a result of the successful demonstration of the importance of canopy-level surveillance, the São Paulo State Secretary of Health integrated canopy surveillance of YFV vectors into the Institutional Development Plan In Research and Technology for Vector Surveillance and Control [
34].
Mosquito specimens of epidemiological importance to YFV transmission, particularly
Haemagogus spp. and
Sabethes spp., were predominantly captured at a canopy height of 6–8 meters, compared to ground-level collections, in the Cantareira State Park within the Brazilian Atlantic Forest biome (
Figure 3A)[
32]. These collections took place in two areas between 10:00 a.m. and 3:00 p.m., using hand-held nets with mobile human bait and CDC-type automatic traps equipped with volatile chemical baits (dry ice and/or BG-Lure
® attractant). Platforms installed at 6–8 meters allowed simultaneous canopy and ground-level net collections in each area. A total of eight traps were deployed – four at ground level and four in the canopy – positioned 100 meters to the north, south, east, and west of the platform, which served as the central reference point. Although more mosquito specimens were captured on the ground (n=2,855) than in the canopy (n=715), the canopy exhibited greater species diversity and evenness, as well as lower species dominance, regardless of the collection method used (
Figure 3B)[
32].
In the Adolpho Ducke Forest Reserve, Manaus, located more than 2,500 km from Cantareira State Park in São Paulo, Brazil, researchers observed a similar pattern of vertical stratification in
Haemagogus spp. and
Sabethes spp. (
Figure 3C) [
22,
35]. BG-2 traps, baited with CO
2 (dry ice) and BG-Lure (synthetic attractant), were deployed at heights of 0, 5, 10, and 15 meters across three sites, each approximately 500 meters from the forest edge. Although
Haemagogus spp. and
Sabethes spp. were less frequently found at ground level compared to 5, 10, or 15 meters, their occurrence at ground level may increase when temperatures are higher and relative humidity is lower—a condition more common at forest edges [
35]. Furthermore, the study found no significant effect of trap height on the vertical stratification of mosquito community diversity, as measured by the Shannon-Wiener Diversity Index. However, a stronger effect was observed at two of the three sites sampled (
Figure 3D).
These studies used canopy collections at heights significantly lower than the actual canopy height of tropical rainforests [
22,
35]. In Cantareira State Park, an Atlantic rainforest habitat in Brazil, trees can reach heights of 20-25 meters, far exceeding the sampling heights of 6-8 meters [
32]. In the Adolpho Ducke Forest Reserve, canopy height ranges from 30 to 35 meters, with emergent trees reaching up to 50 meters [
35]. Thus, sampling at a maximum height of 15 meters introduces a clear limitation in vertical space coverage, potentially biasing results toward lower strata; this could be addressed through other deployment methods. Other researchers have addressed this challenge by documenting insect fauna variability using 6-meter Gressitt-style Malaise traps placed along a vertical canopy gradient (0-32 meters at 8-meter intervals) on a metal tower in a tropical forest north of Manaus, in the Central Amazon, Brazil [
36,
37,
38]. Certain fly families (Diptera) typically exhibit a two-peak pattern, particularly those that exploit very different resources—such as blood-feeding flies like mosquitoes (Culicidae) and biting midges (Ceratopogonidae). Mosquitoes were most frequently found at ground level (40%), with a secondary peak at 24 meters (20%), and at least 1% occurring at 32 meters.
Following a severe outbreak of a sylvatic arbovirus, which was unknown at the time but has since been identified as the Rocio Virus, in southeastern São Paulo, Brazil, several studies on mosquito ecology and systematics were conducted in the Ribeira River Valley, employing various methods commonly used at the time [
39]. CDC traps baited with pigeon (
Columba livia) and rodent (
Rattus norvegicus) were used to collect mosquitoes at both ground and canopy levels within the forest and edges and at ground level in open areas [
27]. For each baited trap in the forest environment, simultaneous collections were conducted without bait. Mosquito species associated with arboviral spillover from wildlife to humans, including those from the Culex (Melanoconion) subgenus—particularly
Cx. sacchettae—were prominently captured using CDC traps baited with pigeons or rodents. This method allowed researchers to identify
Cx. sacchettae as a species associated with ecotones at ground level, indicating its potential to transmit pathogens to humans if infected with the relevant pathogen. Together, this exemplifies a systematic approach for evaluating canopy-level trapping methods and the importance of monitoring across a vertical gradient.
Use of Mosquito Traps in the Canopy
These significant ethical and logistical considerations of live baited traps [
40], have resulted in the development of alternative trap models that use a combination of synthetic attractants to catch adult mosquitoes, and are easily transportable. Example traps include the Centers for Disease Control Light Trap (CDC-LT; BioQuip Products, Rancho Dominguez, California, USA;
http://www.bioquip.com ), BioGents Sentinel-2 and BioGents Pro traps (BioGents AG, Regensburg, Germany
https://eu.biogents.com), and the Mosquito Magnet (MosquitoMagnet, Lititz, Pennsylvania, USA;
http://www.mosquitomagnet.com), alongside a variety of variations in attractants and models used. These traps show variable efficacy targeting different mosquito genera in different settings and may not be directly comparable to HLCs or NHP baited traps [
41,
42,
43,
44]. However, there is limited data available to validate these methods for elevated canopy trapping. Whilst comparative efficacy studies of ground-based trap models are well represented geographically, very few studies have used elevated canopy trap designs to systematically compare trap efficacy against traps baited with the natural reservoirs. For example, at ground level, traps such as the CDC-LT have been observed to be more effective in sampling community richness and abundances [
18], whilst traps such as the BG-2 have been shown to be more effective at sampling target species such as
Aedes albopictus , Ae. aegypti and
Culex pipiens [
45]
, however both trap types show significant variation in efficacy across different contexts [
46,
47,
48].
Deployment of Mosquito Traps in the Canopy
Deploying traps in the canopy is significantly more logistically feasible than using traps with humans or NHPs, which may require either platforms or scaffold towers to be constructed. Previous studies have used ropes or other methods to lift traps into the canopy [
49]. However, depending on the trap type, weight and attractants used, there may be a need to construct different delivery systems. For example, if traps rely on large batteries or carbon dioxide tanks as attractants, these need to be either lifted with the trap or connected from ground-level. Additionally, common methods such as using a sling shot to throw a rope, may be difficult to deploy if there are complex vegetation structures or, at very high heights. Sling shot methods deploying ropes require field staff to be able to access the target location and may pose safety considerations, particularly if weights are used to deploy lines. Moreover, traps may be difficult to raise and lower without pulley systems and limit the ability to check hourly collections. Traps may be structurally damaged by wildlife such as NHPs and other arboreal vertebrates. Ants and other insects may also enter the traps and may predate mosquito catches; sticky substances are commonly applied to ropes to avoid this.
Among the few studies completed globally, significant vector community stratification has already been observed [
27]. Hendy et al. [
35] suspended BG-2 traps at intervals in the canopy of central Brazil to examine community composition of potential ZIKV vectors, and noted a positive correlation of two flavivirus mosquito vectors with increasing height above ground, and niche conditions including relative humidity and temperature. The use of Malaise traps suspended on specially constructed scaffold towers confirmed vertical stratification and higher diversity of
Diptera communities in the forest canopy [
38]. Alternatively, some studies have shown no differences in vector behaviour vertically. In Evangelista et al. [
50] mosquito vectors were collected using Nasci Aspirators [
51], Shannon Traps and CDC-LTs which were suspended at regular intervals in the canopy of conservation forests in São Paulo, following which blood meal analysis was conducted on visibly engorged specimens to determine relationships between vertical niches and biting behaviours. The study observed strong vertical abundance stratification in species such as
Culex nigripalpus and
Anopheles cruzii which showed no biting preference for hosts in this vegetation level and therefore concluded that biting was opportunistic and host preference was not constrained by height but rather host abundance. For studies aiming to assess vertical stratification of vector communities, there is a need to pair ground-based and canopy-level sampling to assess potential biases due to traps attracting mosquitoes from different heights.
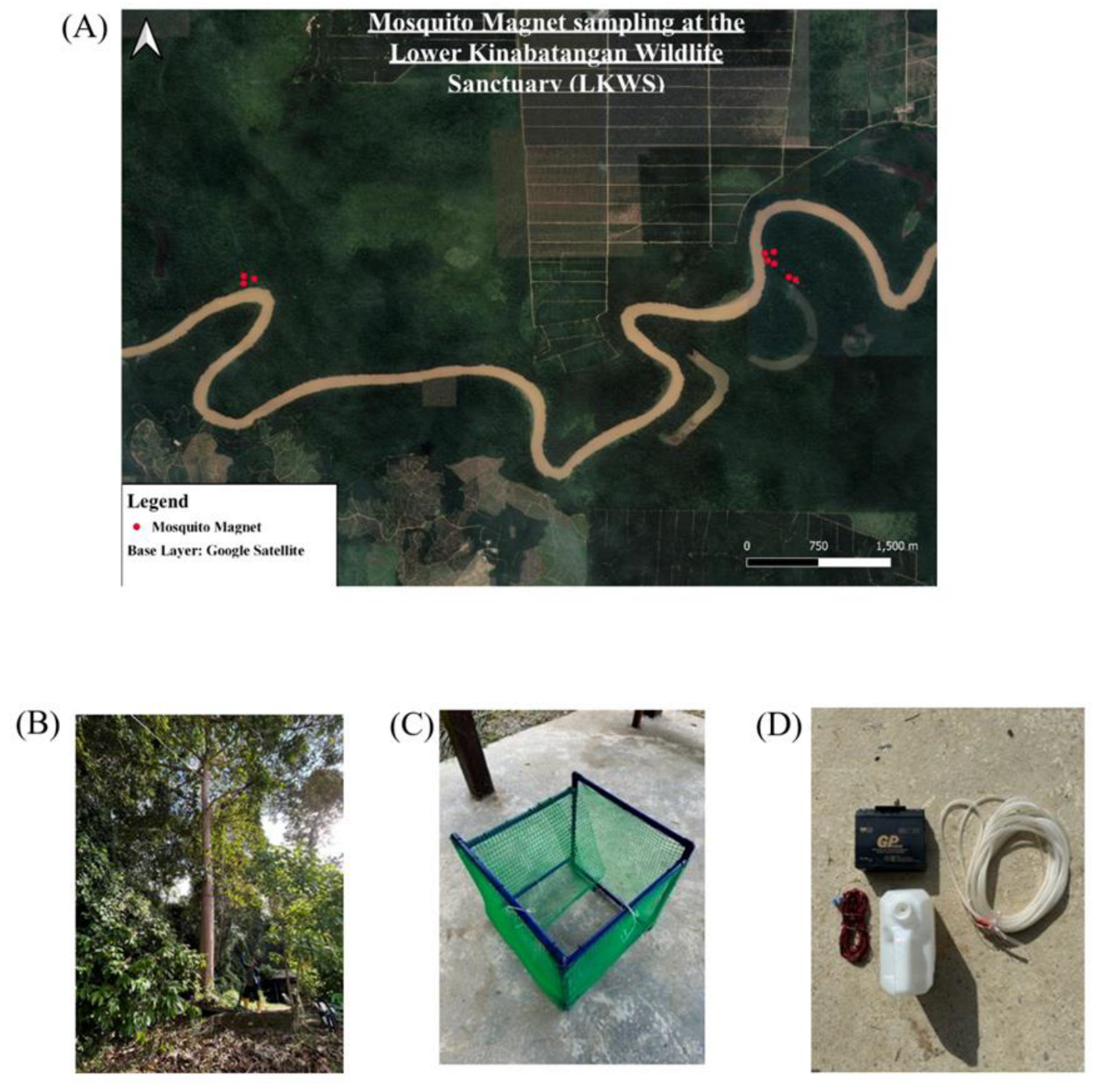
Case Study: Drone Deployment of Canopy-Level Entomological Traps in Sabah, Malaysia
In Sabah, Malaysia, deforestation has led to the emergence and transmission
Plasmodium knowlesi, a zoonotic malaria mostly involving macaques [
53]. Studies suggest macaque parasite prevalence is spatially heterogeneous and sylvatic cycles between macaques are driving spillover risks [see 54 for a review]. However, vector sampling in the majority of these studies was conducted near the ground. Deploying canopy-level mosquito traps poses significant challenges in tropical forest environments. However, drones seem to have the potential to deploy traps more efficiently and better target specific locations [
52]. Pilot studies using drones to deploy mosquito traps were conducted across a gradient of forest types in the Lower Kinabatangan Wildlife Sanctuary in Sabah, Malaysia (
Figure 4) (Taylor, S, MEng Thesis, University of Glasgow, 2024; Chan, J.H, BSc Thesis, University of Glasgow, 2024).
The BG-2 and Mosquito Magnet traps were used in these studies and deployed in the canopy at a height of ~15m. Traps deployed in the canopy required extensive design modifications (see
Figure 4) to ensure effective functioning, including a ‘slingshot’ method of deployment, piping of CO
2 to the trap, and major structural modifications. Within this site, there were considerable practical constraints. For example, traps and equipment may attract macaques, orangutans and other animals, requiring extra steps to prevent damage. This may include setting traps inside sturdy mesh cages (
Figure 4C). Suspended traps are also more vulnerable to weather conditions and may require sturdy fixation to tree branches, covering electrical connections with waterproof tape and preventing accumulation of rain.
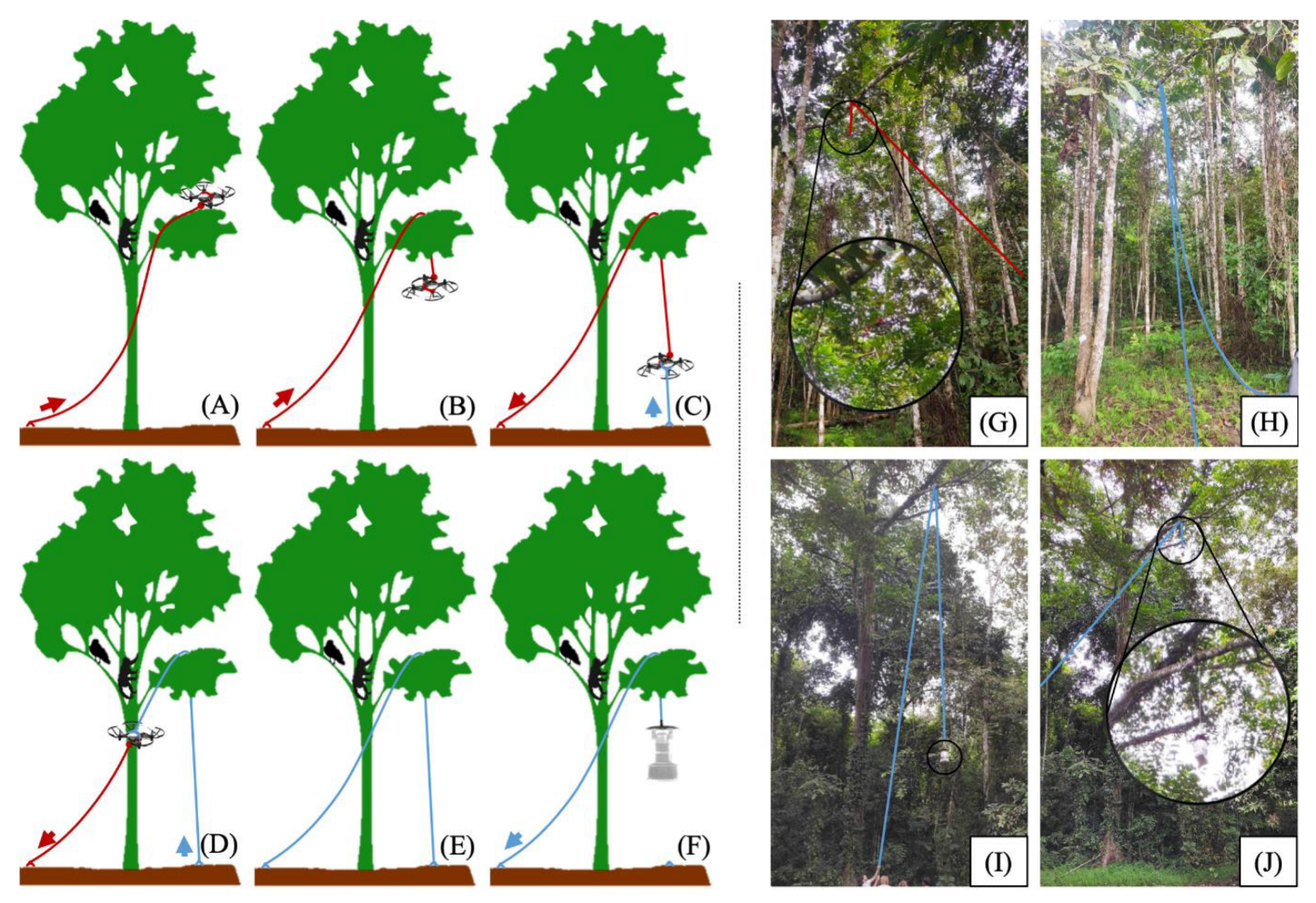
To enable more precise vertical deployment, drones were used to insert ropes at canopy level. Unlike the sling shot approach, the drones can navigate around branches to the desired altitude and location [
52]. However, to deploy an entire trap, such as the BG-2 with CO
2 tank and power supply, the drone would require an approximate payload capacity of 5 kg and be prohibitively large to operate within forests. In this study, a miniature, tethered drone (DJI Tello) used to insert a rope at canopy level for precise vertical deployment of the traps (
Figure 5)
The drone measures 10cm diagonally, weighs 87g and has a front-facing camera, enabling first-person-view controls through a smartphone app or computer API. For the initial phase of the line insertion in
Figure 5A-C, the drone manoeuvres around a target branch. Once the target branch is cleared, the drone shuts down causing it to get caught by the tether (fishing line in red). For the final trap deployment, the initial fishing line tether (red line) was retracted until the rope (blue line) could be anchored to the ground, as illustrated in
Figure 5D-E. Finally, the trap was hoisted into the canopy using the rope (
Figure 5F).
Figure 5G-J demonstrate the drone-based approach for the deployment of mosquito traps.
The difficulties associated with deploying traps with sufficient efficacy in suitable locations accessible by drones with high maximum payloads, are barriers that must be overcome to ensure scalability of surveillance strategies. There is significant international interest in developing sampling arrays that can be deployed in the canopy by drones, as demonstrated by the XPrize Foundation [
55]. Drones offer opportunities to deploy traps in very high or inaccessible canopy environments, where slingshots and other ground-based methods would be unlikely to reach.
Additionally, the known P. knowlesi vector, An. latens was detected in the canopy at two sites, but not on the ground. While further studies need to be conducted to understand the full vector bionomics in the region, the identification of P. knowlesi vectors only at canopy level highlights the need to consider these environments in future vector surveillance programmes.
Integration of Canopy-Level Surveillance with Xenosurveillance
As well as trapping host-seeking mosquitoes, combining canopy trapping methods with blood meal analysis will enable more accurate understanding of pathogens circulating in NHP populations as well as the importance of NHPs, humans and other species in sustaining vector populations and driving transmission dynamics [
56]. For example, canopy traps have been used for xenosurveillance of carrion flies biting different mammalian species to identify circulating pathogens in wildlife. Furthermore, mosquito vector species often rest on foliage after taking blood meals, analysis of which provides key insights into biting preferences and the importance of different wildlife and human populations in sustaining vector populations [
41]. Limited studies have attempted to use resting traps to sample canopy dwelling mosquitoes. Canopy-level deployment of resting traps in Malaysian Borneo collected blood-fed
Aedes and
Culex mosquitoes but no
Anopheles species [
57]. Other studies have used backpack aspirators on fixed canopy platforms to collect mosquitoes from surrounding foliage [
51]. Conventional methods, such as backpack aspiration, are unlikely to be feasible in the canopy without specialised equipment and tree climbers or fixed platforms and will not be able to survey wide areas. There is an acute need to develop more effective methods of trapping blood fed vectors in the canopy.
Outstanding Questions
How can deployable traps be designed for entomological surveillance in the forest canopy with equivalent efficacy to traditional ground-based entomological trapping methods?
How can technological advancements, such as drone-based methods and Artificial Intelligence for navigation and smart trap development, aid in deploying entomological traps in the canopy?
What survey designs most effectively integrate canopy surveillance with other data sources including xenosurveillance and routine health survey data to elucidate arboreal transmission cycles of vector-borne diseases?
How can this data be used most effectively to improve zoonotic disease surveillance and enhance risk factor monitoring for spillover events?
Funding
This work was supported by a National University of Singapore Start-Up grant awarded to KMF and a Sir Henry Dale fellowship jointly funded by the Wellcome Trust and Royal Society (Grant No. 221963/Z/20/Z).
Acknowledgements
We would like to acknowledge Clarissa Balinu, Addy Samsudin, Amaziasizamoria Jumail, Danau Girang Field Centre and Universiti Malaysia Sabah staff for their support for the Malaysian field studies.
Conflicts of Interest
The authors declare no competing interests.
Glossary
Acrodendrophilic mosquito: Species of mosquito which reside primarily in the forest canopy.
Anthropophilic mosquito: Referring to species of mosquito that exhibit a feeding preference for humans over other wildlife.
Bridge vector: Species of vector that are capable of transmitting pathogens between humans and wildlife; ‘bridging’ different transmission cycles.
Human Landing Catch (HLC): A technique of entomological trapping used in the context of anthropophilic vectors, in which human volunteers are used as bait to attract hematophagous vector species.
Non-human primates (NHPs): The taxonomic group of mammals known as primates, which may include both simians and prosimians.
Sylvatic disease cycle: refers to the stage of a pathogen transmission cycle between wild animals and vectors.
Xenosurveillance: A disease surveillance technique that involves the genomic analysis of hematophagous (blood-feeding) vector species to confirm the presence or absence of pathogen DNA/RNA.
Zoonotic disease: refers to diseases that are transmitted from wildlife to humans.
Zoophilic mosquito: Referring to species of mosquito that exhibit a feeding preference for wildlife rather than humans.
References
- Jiang, X. , et al. (2023) A review on zoonotic pathogens associated with non-human primates: Understanding the potential threats to humans. Microorganisms.
- Jones, K.E. , et al. (2008) Global trends in emerging infectious diseases. ( 2008) Global trends in emerging infectious diseases. Nature 451, 990–993.
- Valentine, M.J. , et al. (2019) Sylvatic cycles of arboviruses in non-human primates. Parasites & vectors.
- Weaver, S.C. and Barrett, A.D. (2004) Transmission cycles, host range, evolution and emergence of arboviral disease. D. ( evolution and emergence of arboviral disease. Nat Rev Microbiol 2, 789–801.
- Wolfe, N.D. , et al. (2007) Origins of major human infectious diseases. ( 2007) Origins of major human infectious diseases. Nature 447, 279–283.
- Gubler, D.J. (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev.
- W.H.O (1997) Dengue haemorrhagic fever: diagnosis, treatment, prevention and control.
- Fornace, K.M. , et al. (2023) Simian malaria: a narrative review on emergence, epidemiology and threat to global malaria elimination. The Lancet Infectious Diseases.
- Plowright, R.K. , et al. (2021) Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet Health.
- Hanley, K.A. , et al. (2013) Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol.
- Grubaugh, N.D. , et al. (2015) Xenosurveillance: a novel mosquito-based approach for examining the human-pathogen landscape. PLoS neglected tropical diseases.
- Ferguson, H.M. , et al. (2010) Ecology: a prerequisite for malaria elimination and eradication. PLoS Med.
- Jupp, P.G. , et al. (2002) The 2000 epidemic of Rift Valley fever in Saudi Arabia: mosquito vector studies. Med Vet Entomol.
- Farlow, R. , et al. (2020) Nextgen Vector Surveillance Tools: sensitive, specific, cost-effective and epidemiologically relevant. Malaria journal.
- Gao, Q. , et al. (2015) Comparison of mosquito population composition and dynamics between human-baited landing and CO2-baited trapping monitoring methods. Chin J Hyg Insect Equip.
- Rubio-Palis, Y. and Curtis, C. (1992) Evaluation of different methods of catching anopheline mosquitoes in western Venezuela. ( 1992) Evaluation of different methods of catching anopheline mosquitoes in western Venezuela. Journal of the American Mosquito Control Association 8, 261–267.
- Le Goff, G. , et al. (1997) Comparison of three sampling methods of man-biting anophelines in order to estimate the malaria transmission in a village of south Cameroon. Parasite.
- Lühken, R. , et al. (2014) Field evaluation of four widely used mosquito traps in Central Europe. Parasites & Vectors.
- Ngape, D. , et al. (2021) A comparison of BG Sentinel and CDC trap attractants for mosquito surveillance in urban and suburban areas of Montgomery and Prince George's Counties, Maryland, USA. Journal of Vector Ecology.
- Rapley, L. , et al. (2009) A lethal ovitrap-based mass trapping scheme for dengue control in Australia: II. Impact on populations of the mosquito Aedes aegypti. Medical and veterinary entomology.
- Barrera, R. (2022) New tools for Aedes control: mass trapping. Current opinion in insect science.
- Hendy, A. , et al. (2021) Microclimate and the vertical stratification of potential bridge vectors of mosquito-borne viruses captured by nets and ovitraps in a central Amazonian forest bordering Manaus, Brazil. Scientific reports.
- Brant, H.L. , et al. (2016) Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malaria journal.
- Bakker, J. , et al. (2020) Attraction of mosquitoes to primate odours and implications for zoonotic Plasmodium transmission. ( 2020) Attraction of mosquitoes to primate odours and implications for zoonotic Plasmodium transmission. Medical and veterinary entomology 34, 17–26.
- Warren, M. , et al. (1965) Ecology of simian malaria in the monsoon forests of the northern Malayan states. ( 1965) Ecology of simian malaria in the monsoon forests of the northern Malayan states. J Parasit 51, 17.
- Permana, D.H. , et al. (2023) The potential for zoonotic malaria transmission in five areas of Indonesia inhabited by non-human primates. Parasites & Vectors.
- Gomes, A.d.C. , et al. (1987) Composição e atividade de mosquitos Culicidae. Emprego de armadilha CDC no Vale do Ribeira, Estado de São Paulo, Brasil. Revista de saúde pública.
- Douam, F. and Ploss, A. (2018) Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends in microbiology.
- Saúde, M.d. (2018) Monitoramento do período sazonal da febre amarela: Brasil–2017/2018. Informe Epidemiológico–SUS–Ministério da Saúde.
- Barrett, A.D. and Higgs, S. (2007) Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol.
- Tomori, O. (2004) Yellow fever: the recurring plague. Crit Rev Clin Lab Sci.
- Deus, J.T.d. , et al. (2022) Evaluation of methods to collect diurnal culicidae (diptera) at canopy and ground strata, in the atlantic forest biome. ( in the atlantic forest biome. Insects 13, 202.
- Telles-de-Deus, J. , et al. (2024) COI DNA barcoding to differentiate Haemagogus janthinomys and Haemagogus capricornii (Diptera: Culicidae) mosquitoes. Acta Tropica.
- Saude, S.d. (2018) Institutional development plan in research and technology for vector surveillance and control of the Superintendência do Controle de Endemias - SUCEN (PDIp).
- Hendy, A. , et al. (2020) The vertical stratification of potential bridge vectors of mosquito-borne viruses in a central Amazonian forest bordering Manaus, Brazil. Sci Rep.
- Forattini, O.P. (1962) Parte geral: diptera; anophelini. Fac.
- Rafael, J.A. and Gorayeb, I.d.S. (1982) Tabanidae (Diptera) da Amazônia, I—Uma nova armadilha suspensa e primeiros registros de mutucas de copas de árvores. Acta amazonica.
- de Souza Amorim, D. , et al. (2022) Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. ( 2022) Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. Scientific Reports 12, 1734.
- Forattini, O.P. , et al. (1978) Estudos ecológicos sobre mosquitos Culicidae no sistema da Serra do Mar, Brasil: 1-Observações no ambiente extradomiciliar. Revista de Saúde Pública.
- Silver, J.B. (2007) Mosquito ecology: field sampling methods.
- Li, Y. , et al. (2016) Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasites & vectors.
- Gorsich, E.E. , et al. (2019) A comparative assessment of adult mosquito trapping methods to estimate spatial patterns of abundance and community composition in southern Africa. Parasites & vectors.
- Costantini, C. , et al. (1998) Relationship to human biting collections and influence of light and bednet in CDC light-trap catches of West African malaria vectors. Bulletin of Entomological Research.
- Goi, J. , et al. (2022) Comparison of different mosquito traps for zoonotic arbovirus vectors in Papua New Guinea. ( 2022) Comparison of different mosquito traps for zoonotic arbovirus vectors in Papua New Guinea. The American Journal of Tropical Medicine and Hygiene 106, 823.
- Meeraus, W.H. , et al. (2008) Field comparison of novel and gold standard traps for collecting Aedes albopictus in northern Virginia. ( 2008) Field comparison of novel and gold standard traps for collecting Aedes albopictus in northern Virginia. Journal of the American Mosquito Control Association 24, 244–248.
- Roiz, D. , et al. (2012) Efficacy of mosquito traps for collecting potential West Nile mosquito vectors in a natural Mediterranean wetland. ( 2012) Efficacy of mosquito traps for collecting potential West Nile mosquito vectors in a natural Mediterranean wetland. The American journal of tropical medicine and hygiene 86, 642.
- Drago, A. , et al. (2012) Looking for the gold standard: assessment of the effectiveness of four traps for monitoring mosquitoes in Italy. Journal of Vector Ecology.
- Hutchinson, R. , et al. (2007) Suitability of two carbon dioxide-baited traps for mosquito surveillance in the United Kingdom. Bulletin of entomological research.
- Brown, R. , et al. (2020) Human exposure to zoonotic malaria vectors in village, farm and forest habitats in Sabah, Malaysian Borneo. ( Malaysian Borneo. PLoS neglected tropical diseases 14, e0008617.
- Evangelista, E. , et al. (2021) Relationship between vertical stratification and feeding habits of mosquito (Diptera: Culicidae) assemblages collected in conservation units in the green belt of the city of São Paulo, Brazil. Acta Tropica.
- Nasci, R. (1981) A lightweight battery-powered aspirator for collecting resting mosquitoes in the field.
- Yong, K.E. , et al. (2023) Drone Navigation System for Autonomous Mosquito Sampling in Tree Canopies. In 2023 IEEE 9th World Forum on Internet of Things (WF-IoT), pp. -06.
- Fornace, K.M. , et al. (2016) Association between Landscape Factors and Spatial Patterns of Plasmodium knowlesi Infections in Sabah, Malaysia. ( Malaysia. Emerg Infect Dis 22, 201–208.
- Johnson, E. , et al. (2023) Landscape drives zoonotic malaria prevalence in non-human primates. eLife.
- Kirchgeorg, S. , et al. (2024) eProbe: Sampling of Environmental DNA within Tree Canopies with Drones. Environmental Science & Technology.
- Hoffmann, C. , et al. (2016) Assessing the feasibility of fly based surveillance of wildlife infectious diseases. ( 2016) Assessing the feasibility of fly based surveillance of wildlife infectious diseases. Scientific reports 6, 37952.
- Brown, R. , et al. (2018) Evaluation of resting traps to examine the behaviour and ecology of mosquito vectors in an area of rapidly changing land use in Sabah, Malaysian Borneo. ( Malaysian Borneo. Parasit Vectors 11, 346.
- Zhou, X. , et al. (2021) Ego-swarm: A fully autonomous and decentralized quadrotor swarm system in cluttered environments. In 2021 IEEE international conference on robotics and automation (ICRA), pp. 4101. [Google Scholar]
- Aucone, E. , et al. (2024) Synergistic morphology and feedback control for traversal of unknown compliant obstacles with aerial robots. ( 2024) Synergistic morphology and feedback control for traversal of unknown compliant obstacles with aerial robots. Nature Communications 15, 2646.
- Sharan, M. , et al. (2023) Surveillance and response strategies for zoonotic diseases: A comprehensive review. Science in One Health.
- Ribeiro, A.F. , et al. (2012) Mosquitoes in degraded and preserved areas of the Atlantic Forest and potential for vector-borne disease risk in the municipality of São Paulo, Brazil. Journal of Vector Ecology.
- Andreadis, T.G. and Armstrong, P.M. (2007) A two-year evaluation of elevated canopy trapping for Culex mosquitoes and West Nile virus in an operational surveillance program in the northeastern United States. J Am Mosq Control Assoc.
- de Carvalho, G.C. , et al. (2014) Blood meal sources of mosquitoes captured in municipal parks in São Paulo, Brazil. Journal of Vector Ecology.
- Taylor, S. (2024) Design, Build, and Test of a Drone Deployable Mosquito Trap for Canopy Level Collection. James Watt School of Engineering.
- Chan, J.H. (2024) Development of a Slingshot Drone. James Watt School of Engineering.
- Leão, P.d.O. , et al. (2020) Vertical stratification of sand fly diversity in relation to natural infections of Leishmania sp. and blood-meal sources in Jamari National Forest, Rondônia State, Brazil. Parasites & Vectors.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).