1. Introduction
1.1. Background & Significance
The mainstream medical treatment of androgenetic alopecia (AGA), also known as common pattern hair loss, consists primarily of FDA approved drugs such as minoxidil and finasteride, each with demonstrated efficacy but each also linked to negative side-effects. Recent reports of cell based or biologic therapies, such as platelet rich plasma (PRP) or mesenchymal stem cell derived exosomes have also shown inconsistent results [
1]. Due to its polygenic etiology, it has been suggested that AGA is unlikely to respond optimally to a single therapeutic agent or approach.
1.2. Botanical Therapy
To optimize medicant preparations, various combinations of active ingredients plus delivery-enhancing methods have been investigated [
2]. Recent studies [
3] report the utility of naturally derived botanical preparations with enhanced efficacy using improved oral and topical delivery formulations. As the hair follicle constitutes an ectodermal appendage that is amenable to both local and systemic therapy, our group has focused on developing concomitant oral and topical treatment strategies [
4]. We previously tested topical and oral botanicals against the benchmark drug, finasteride, and demonstrated positive outcomes [
5]. Subsequently, we reported the use of a novel oral and topical concomitant botanical formula dosed in two subjects with AGA, who demonstrated visible reversal of phenotype evident upon the conclusion of the study at 270 days [[
6,13,25].
1.3. Cryo-Carboxy
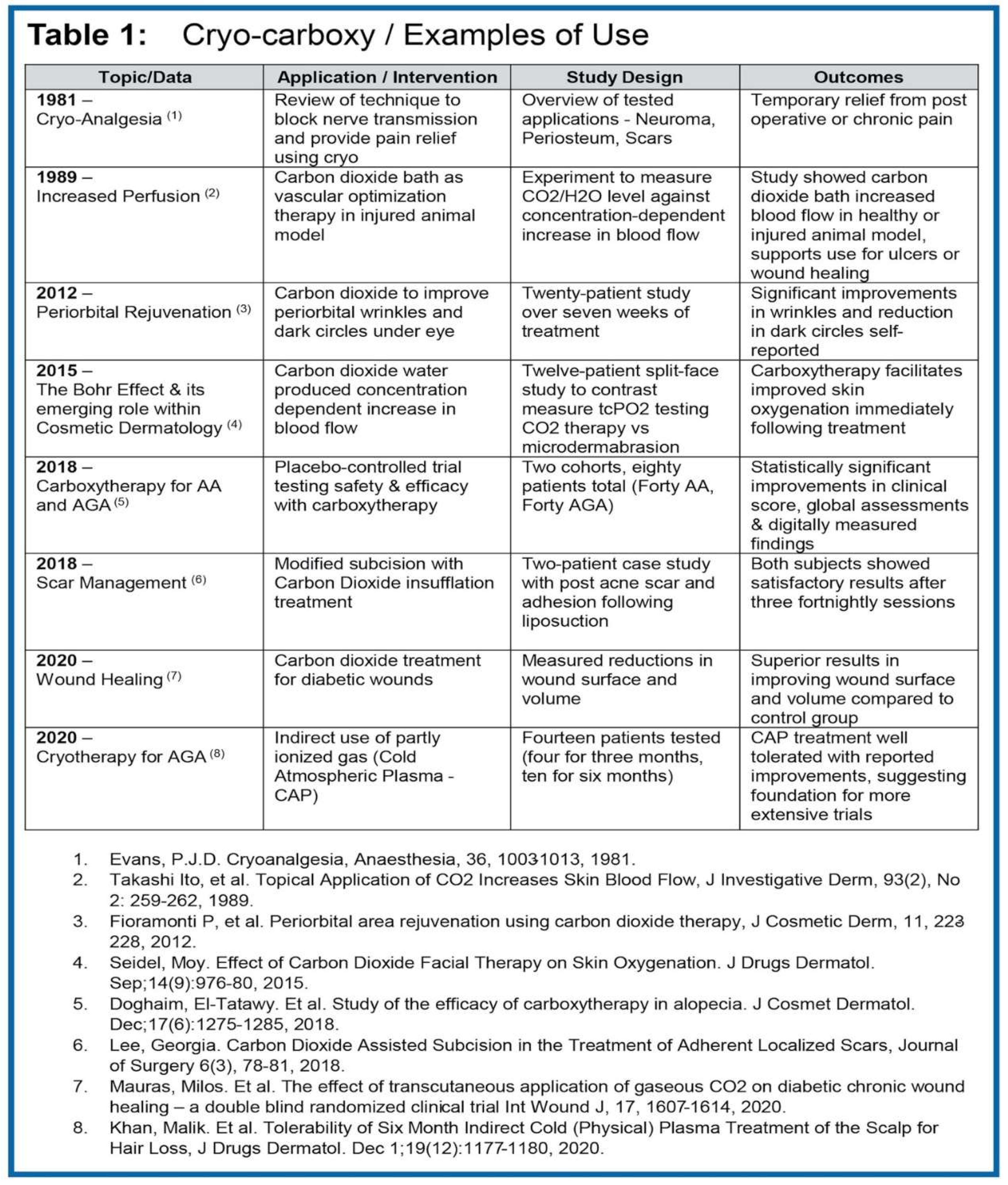
Both cold therapy (cryo) and carbon dioxide therapy (CO
2 / carboxy) have long been used to treat multiple disease states. Several lines of evidence have emerged supporting the therapeutic benefit of cryo as well as CO
2 as a method of enhancing topical penetration of active agents into the skin (
Table 1).
Other studies have shown that cryotherapy has an inhibitory effect on reactive oxygen species (ROS), while simultaneously stimulating the production of antioxidants in the skin [
9]. Therapeutic improvement has been observed in several different hair disorders and reported within a series of clinical studies. For example, a clinical experiment interrogating cryotherapy using cold atmospheric plasma (CAP), reported broad clinical benefit in AGA patients - wherein fourteen patients were treated using the indirect CAP method for three months (four patients) and six months (ten patients). The indirect CAP treatment was well-tolerated, and most patients reported improvement, with the findings essentially replicated in another, similarly structured study [
10].
Tests trialing therapeutic CO2 in hair loss models have shown similarly encouraging results. For example, in one placebo-controlled carboxytherapy trial, enrolling both AGA and AA patients, positive outcomes were observed across both cohorts. This randomized study was included eighty patients divided into two groups; Group one included forty AA patients, and Group two included forty AGA patients (Group One-A and Two-A received carboxytherapy while Groups Two-A and Two-B control groups received placebo).
All cohorts were followed up monthly for three months. Results were evaluated clinically (by assessment of Severity of Alopecia Tool (SALT) score in group One, and Sinclair scale and Norwood-Hamilton scale in group Two, respectively), by investigator assessment and dermoscopy. Notably, the AA carboxy treated ‘Group One-A’ patients showed modest clinical improvement in SALT score after carboxytherapy - while AA placebo ‘Group One-B’ patients remained unchanged. At study endpoint, AGA Group Two-A patients also showed significant clinical improvement after carboxytherapy with marked increase in hair density. While some regression of observed positive results was documented during the follow-up period, hair density was still judged to be significantly improved when compared to status before treatment [
11].
The mechanisms of enhanced penetration from cryotherapy and carboxytherapy have been attributed historically to the Bohr’s effect and possibly vascular rebound following thermal shock [
7]. In this regard, it has been shown that topically applied CO
2 induces an oxygen deficit, triggering an increase in blood flow and the release of growth factors such as vascular endothelial growth factor (VEGF). This, in turn, promotes the generation of new blood vessels, an improvement in cutaneous microcirculation, as well as the augmentation of capillary blood flow [
8].
When measured against mainstream standard-of-care AA treatment options cryotherapy has likewise produced noteworthy data. For example, in a trial comparing against intralesional corticosteroid injection, cryotherapy treated patients evinced lower relapse rates [
12]. Although cryotherapy and carboxytherapy each appear to lead to improvement in hair disorders, no studies, to our knowledge, have been reported while testing both modalities in combination. With high relevance to the work reported herein, neither cryotherapy nor carboxytherapy appear to have been studied either separately or together with botanically derived actives in hair loss affected patients.
2. Study Design, Materials and Methods
2.1. Study Participants
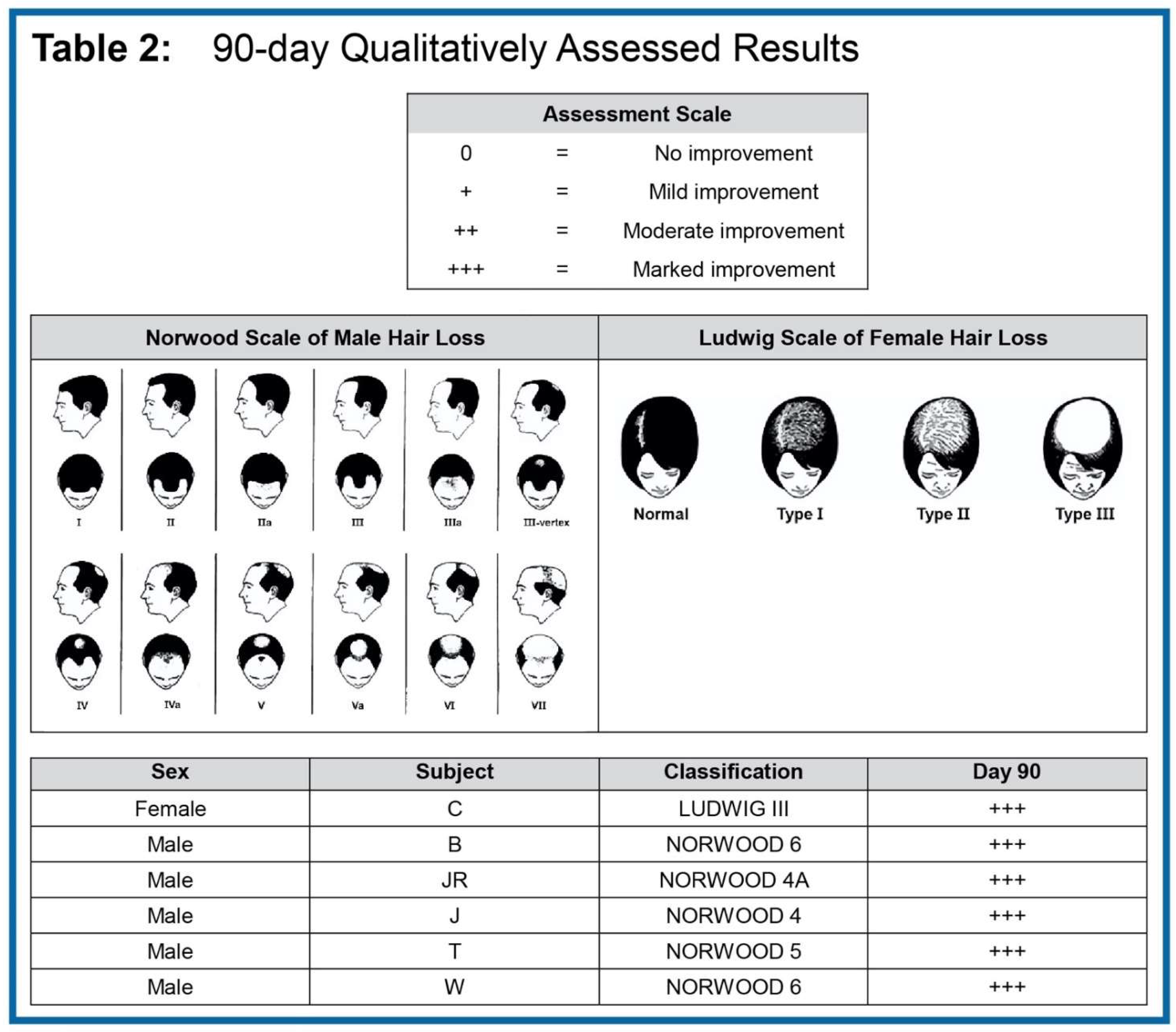
To investigate the therapeutic efficacy of this novel approach, we enrolled five male participants and one female participant with moderate to advanced stage AGA. Enrolled males were classified from moderate to advanced stage Norwood Class 4 - 6 and the female volunteer was determined to be an advanced-stage Ludwig Grade 3. Prior to initiation, informed consent was provided and executed by all subjects (
Supplementary Materials).
2.2. Formulations
Each of the three test formulations (topical, oral and hydrogel) contained the same set of botanically-derived compounds previously trialed in 2020 [
6,
13,
25]. The base phytochemical formula was composed of γ linolenic acid, β-Sitosterol, epigallocatechin gallate, genistein, curcumin and piperine -- with each constituting unique mechanistic actives. All versions were produced with analytical standard grade betasitosterol (≥95% purity/GC; CAS Number: 83-46-5), γ linolenic acid (≥ 98.5% purity/GC; CAS Number: 506-26-3), epigallocatechin gallate (≥ 95.0% purity/HPLC; CAS Number: 989-51-5), genistein (≥ 97.0% purity/HPLC; CAS Number: 446-72-0), curcumin (≥ 97.0% purity/HPLC; CAS Number: 458-37-7) and piperine (≥ 95.0% purity/HPLC; CAS Number: 94-62-2).
To optimize for absorption, potency and stability, the botanical actives were nano-encapsulated within a 2-hydroxypropylbetacyclodextrin vehicle using classic co-dissolution procedures. Analytical grade β-cyclodextrin (CAS Number: 7585-39-9; ≥ 97% purity/MS) selected for this purpose, was likewise obtained from Sigma-Aldrich (St. Louis, MO, USA). Chromatographic characterization of the β-cyclodextrin inclusion complex demonstrated a 1:1 host-to-guest stoichiometry. During production, the host–guest inclusion complex of β-cyclodextrin vehicle and each bioactive test substance was reduced to a fine granular powder via coprecipitation, kneading, grinding, air drying and freeze drying. The resulting admixture was characterized by differential scanning [HPLC] and initially solubilized into a 1:1 water-formula inclusion complex.
2.3. The Test Protocol
Daily therapy consisted of a single tablet (375 mg. total actives) oral bolus combined with a single application of topical formula (5 mL) to the AGA-affected scalp. Accordingly, the point-of-service cryo-carboxy delivery device, styled ‘CryoTouch® MD’, represented the key innovative facet of this experiment.
2.4. Cryo / Carboxy Device Preparation & Treatment Application
To optimize for delivery via the cryo-carboxy dispensing device, the botanical complex was further solubilized within an ultra-pure 2-hydroxyethyl methacrylate construct as the hydrogel component (Aquasonic™, Parker Laboratories, Inc.) pH 6.5 – 6.95, viscosity 130,000 – 195,000 cps. The resulting mixture was then stabilized via simple sonication. After being loaded into a polystyrene barrel cartridge, the test formula was ready for use.
Designed to hold a 20 oz pre-loaded canister of medical grade carbon dioxide, the device is therefore a self-contained dispensing instrument. For topical application, the attachable barrel cartridge may hold and deliver up to 8mL - although for this protocol, each cartridge was loaded with 5 mL of formula. Each cartridge is labeled with an RFID tag which is programed for the temperature and dispense speed. Temperature, during dispense and application, is controlled, using ambient heat within the unit to maintain the temperature above -20oC. This ensures that treated patients experience a cold but comfortable sensation at the surface of the skin. During treatment, within the applicator tip, the temperature-controlled carbon dioxide mixes with cartridge-held formula.
Functionally, when held against the skin, a slight vibration at the tip facilitates efficient application. At the same time, a small amount of pressure builds between the applicator tip and the surface of the scalp -- thus transcutaneously delivering the ‘chilled C02 plus botanical formula’ directly into the target hair follicles. Each weekly cryo-carboxy treatment session was administered with the participant comfortably seated in the full Fowler’s position.
As shown in
Figure 1, during therapeutic delivery the operator dispenses formula onto the scalp, working in rows while moving the applicator tip in small circles. Immediately post treatment, the scalp is then lightly blotted to remove any residual material. Trial participants were directed to refrain from aggressively rinsing their scalp for at least 30 minutes post-treatment -- however most allowed the formula to remain in situ for up to 24 hours. Point-of-service treatment consumed an average of five minutes - start to finish.
2.5. Measurement of Results
In order to qualitatively assess and quantitatively validate the clinical results, a number of measurements were utilized. At test day zero, the collection of gross (1x) macroscopic photos represented a primary acquisition set of baseline data, with interval progress photo capture planned consecutively -- each to occur at three 90-day time points -- throughout the initially anticipated 270-day duration of the study. Based on zone of hair loss, standardized images were focused upon the midfrontal vertex and / or occipital parietal scalp, with the head positioned to best visualize the AGA-affected areas. Several subjects in the study had grey or lightly pigmented hair that was not quantifiably evaluable using the operative analytic methodology, due to low pixel-capture contrast between hair and scalp. Fortuitously, two subjects in the trial had darkly pigmented hair that was evaluable by ImageJ (Male Subject J. and Female Subject C.). In these two subjects, both 1x and 10x photos were captured and quantified using the proprietary FIJI / ImageJ digital analysis platform [
14] -- and these are presented herein as representative examples of test subject response.
To quantify the differential hair density variance over time, histogram throughput, thusly obtained, was computed to display mean, modal, minimum and maximum shade value -- i.e., wherein greyscale variance across the x-axis is simultaneously charted against total pixel count derived from the y-axis. Once processed, and to graphically represent the differential values derived from the captured 8-bit images, the range of greyscale pixel intensity was interpolated by ImageJ analytics, sub-plotted and divided within 255 bins. Numerical value was calculated using an Excel standard mean variance algorithm [
15]. A decrease in ImageJ value denotes an increase in pixel density, thereby corresponding to increased hair coverage.
3. Results
3.1. Open Label Clinical Study Patient Experience
Patient adherence with injection-based therapy has often induced compliance hesitancy, such as when measured against less invasive treatment options [
16]. Moreover, long term safety concerns, particularly associated with microneedle-delivered exosome therapy, are reflected in several documented reports -- including a recently published FDA warning [
17,
18]. Conversely, study subjects expressed no such hesitation or concerns during this protocol, with most remarking upon the ease and lack of discomfort associated with all facets of the regimen. Overall, tested participants reported the once-weekly, cryo/carboxy therapy application process to be quick, relaxing and pleasant.
3.2. Rapid Clinical Improvement led to Early Study Conclusion
This test-of-innovation was initially planned as a 270-day open label clinical, primarily based on the nine-month response-to-therapy gestation, as observed within our previously discussed clinical study testing the same botanical composition [
6,
13,
19]. Quite unexpectedly, the incorporation of the once-weekly point-of-service cryo/carboxy augmentation appeared to bring about a markedly accelerated rate of regrowth that was observed across all six enrolled participants by the first evaluation time point at treatment day-90. Therefore, at day-90 the study was concluded, study participants were released, and the data was collected for analysis. No adverse events occurred during the abbreviated test period and no participant suffered the incidence of a negative side effect. In
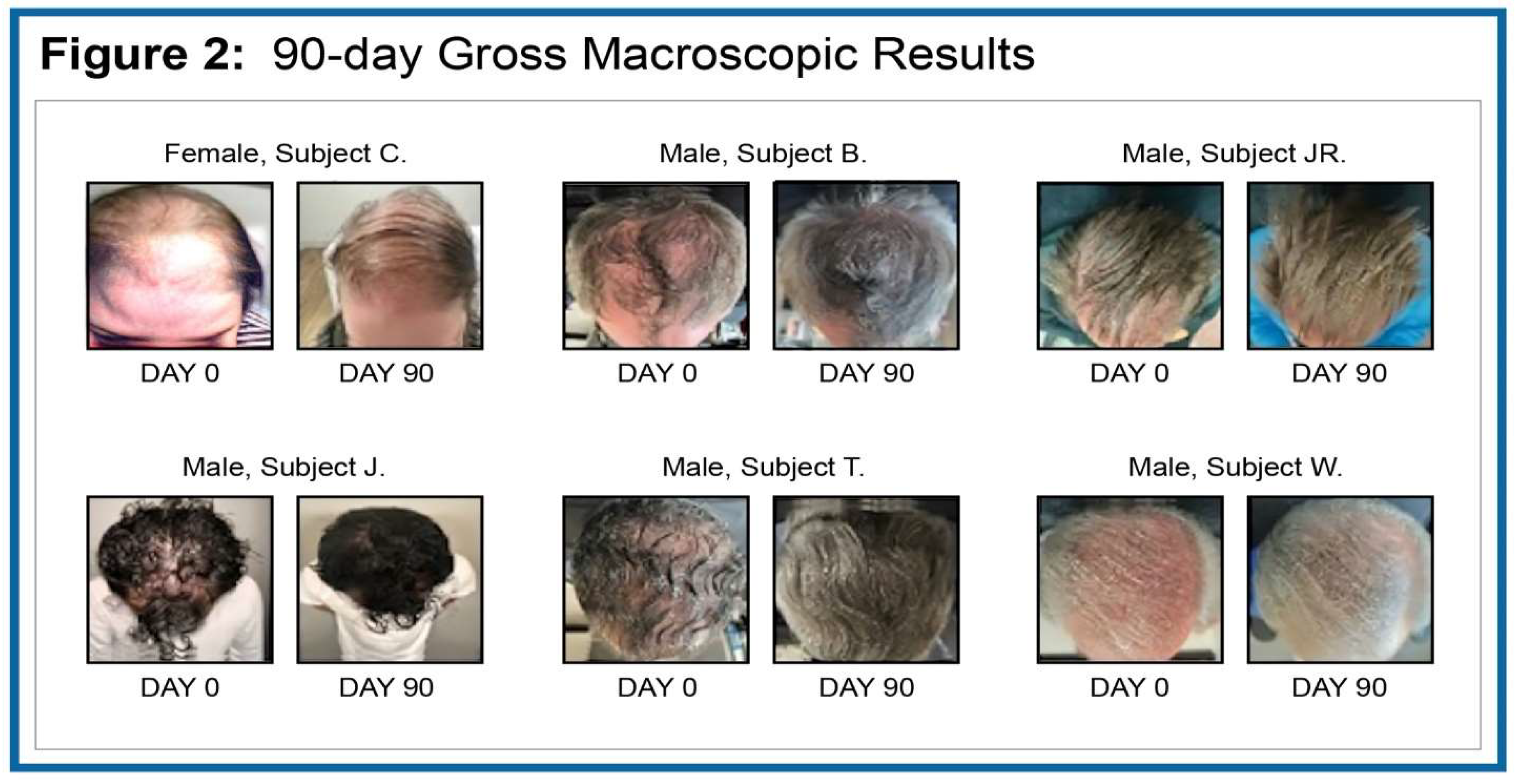
Figure 2 we show ‘before and after’ gross macroscopic changes over the 90-day test period.
3.3. Qualitative Assessment of Hair Growth
In the reported work we were able to capture and show both qualitative and quantitative changes. As noted, several subjects had grey hair that was not evaluable using the quantitative methodology due to low grey-scale pixel contrast across the X and Y axes. In
Table 2 we grade qualitative assessment in all six subjects scaled according to observed positive improvement. Scaling was assessed with a grade of 0 = no improvement to +++ = significant improvement. Notwithstanding the abbreviated test period, we found that all enrolled subjects showed +++ significant improvement.
As noted, two patients in our study had hair ‘color to scalp contrast’ that was evaluable by the FIJI / ImageJ photo analysis processing system [
20]. Therefore, we show quantitative changes in those subjects. In
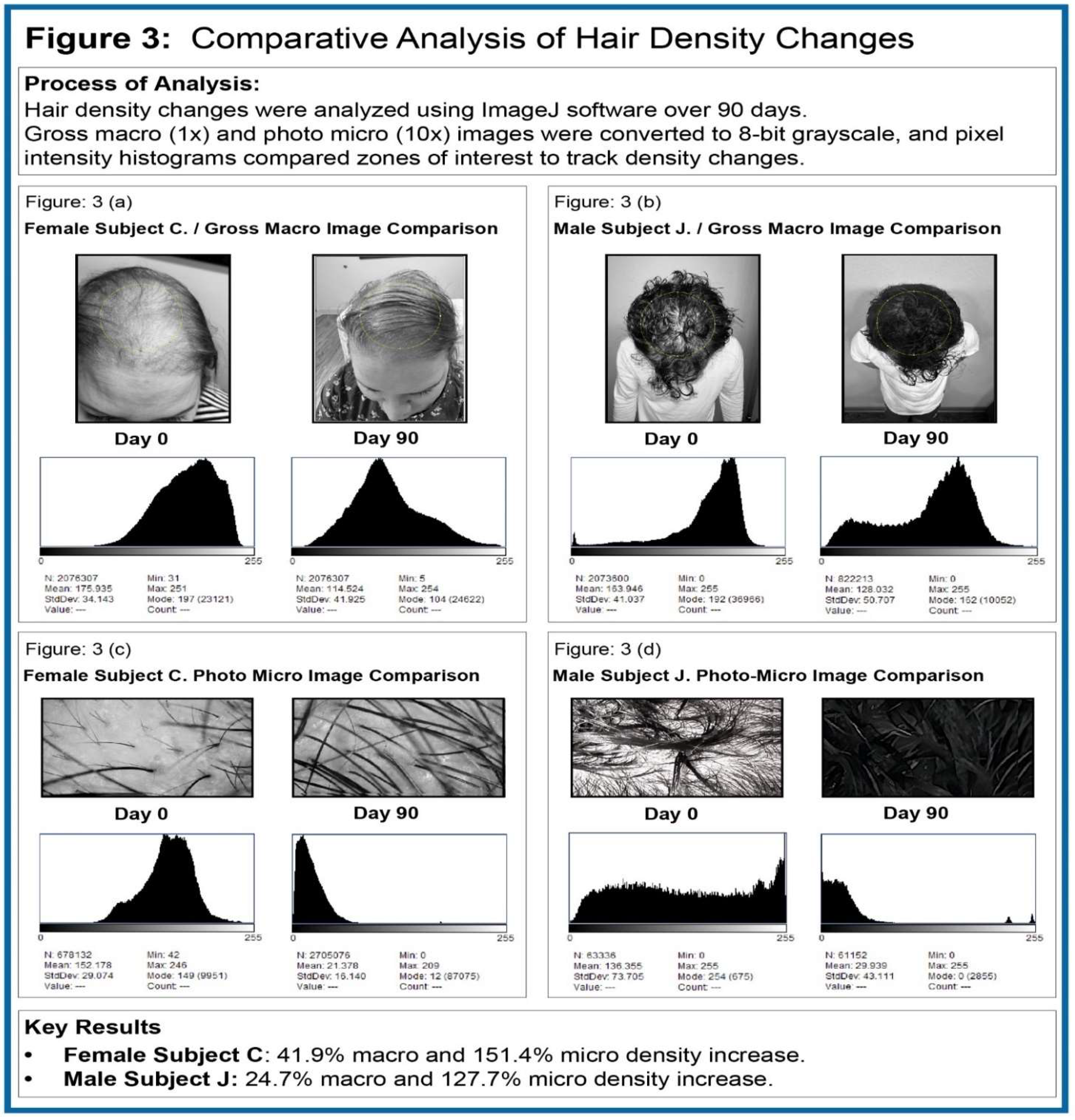
Figure 3 (3a thru 3d) these differential changes are quantified and displayed. Global photo-macroscopic images reflect overall 90-day change at 1x magnification - while photo-micro capture contrasts 10x focal amplification of positive density change from test inception day-0 to test conclusion day-90.
As reflected in the analyzed data, male Subject J. showed a 24.6% global macroscopic density increase at day-90 and a 127.7% photo-micro density increase over the same period. From day-0 to day-90, female Subject C. showed a 41.9% global macroscopic density increase and a 151.4% photo-micro density increase.
4. Discussion
It is now well-understood that the multi-factorial phenotype, androgenetic alopecia (AGA) involves complex interactions that encompass genetic susceptibility, hormonal influence and chronobiology. From a clinical perspective, as when measured against monotherapeutic small molecules, several key paradigms converge in favor of a multi-faceted, local and systemic, botanically based approach. First, as ectodermal appendages, scalp hair follicles are amenable to concomitant therapy via oral and local delivery [
21]. Second, naturally based compounds have been linked to fewer negative side effects. Third, botanically derived chemicals may be combined to concurrently amplify clinical benefit [
22].
Decades of published, well-designed, basic science studies report upon the activity of botanically based candidate hair growth formulations. Yet bridging the divide between in vitro potential and clinical utility has remained an elusive goal -- with a number of factors contributing to the challenge. Phytochemicals are intrinsically limited by poor aqueous solubility, weak permeation, low systemic bioavailability, structural instability and deactivation during first pass metabolism. Further, many promising candidate naturally-derived actives tested in vitro have proven highly susceptible to volatile interaction with solubilizers, excipients, buffers, co-actives and other components necessary to the development of safe, stable, efficacious, clinically appropriate medicinal agents [
23].
These findings suggest that without protective mechanisms, hair growth formulas built upon a foundation of active botanicals, especially those prone to rapid oxidation, tend to be denatured, converted, inactivated, poorly absorbed, or simply excreted via the kidneys and /or the liver [
24]. Previously, we postulated that complex, pleiotropic, phytochemically-based formulations may better survive in vivo metabolic challenge when first protectively sequestered. In 2020, we reported positive outcomes arising from a 270-day, clinical study of a nano-enabled (hydroxypropylbetacyclodextrin invaginated), botanically-based, oral and topical concomitant regimen trialed in two male subjects with AGA [
6,
13,
25]. This work led the Authors to hypothesize as to whether further therapeutic amplification mechanisms might be possible.
Cryotherapy and carboxytherapy each appear to have demonstrated published examples of safety and utility in hair disorders, yet no studies, to our knowledge, have thus far, tested both in combination -- nor either or both in concert with botanically derived actives. Accordingly, a heterogenous AGA-affected cohort consisting of five males and one female -- were recruited for the purpose of evaluating cryo-carboxy as an innovative therapeutic augmentation to the previously tested botanical-actives approach.
While initially planned to mirror the previous 270-day trial, at day-90 a strikingly accelerated rate of regrowth was observed across all enrolled participants. In light of the unexpected, but highly positive response, the test was concluded 180 days earlier than originally planned -- as visible results eclipsed the predicted best-case-scenario outcomes. Accelerated regrowth was surprisingly consistent in all enrolled volunteers -- and most notably in those initially presenting with advanced stage phenotype --as illustrated by [male Subject T. / Norwood Class 5] and [female Subject C. / Ludwig Stage 3].
Given the unexpectedly rapid, consistently robust response to therapy attendant to this trial, a reasonable explanation is called for to resolve the chasm between [anticipated and observed] findings. Here, one may perhaps conjecture that the transcutaneous delivery of cryo-carboxy plus multi-faceted, pleiotropic botanicals so improves uptake efficiency to the target tissue that time-compressed, highly positive outcomes consistently occur. Inasmuch as AGA remains a disorder with many unknowns, this is certainly a testable hypothesis – and one worthy of consideration.
It is also possible that well-designed, interrogative analyses may lead to alternative explanations. For example, it may be interesting to interrogate known as well as novel gene markers to assay for therapy-induced modulation. Accordingly, to further explore and critically assess the potential utility of the described approach, organized clinical trials – concurrent with thoughtfully conceived basic science experiments -- are suggested as rational, next step follow on.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Informed Consent (attached).
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation by S.N. Writing—review and editing, visualization, supervision and project administration by G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research described herein received no external funding.
Ethics Statement
The innovative case series described herein followed the ethical principles outlined in the World Medical Association’s Declaration of Helsinki for medical research involving human subjects.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the work.
Data Availability Statement
All data relevant to this work has been provided herein. .
Acknowledgments
The Authors would like to express their sincere gratitude to KC Aestheticare for generously providing access to their facilities and staff, whose support and cooperation was essential to the successful completion of this work. .
Conflicts of Interest
The authors declare no conflict of interest. .
References
- Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. M. Nestor, et al. J Cosmet Dermatol. 2021 Dec;20(12):3759-3781.
- Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. K. Khezri, et al. Biomed Pharmacother. 2018 Oct:106:1499-1505.
- Plant-derived natural products for drug discovery: current approaches and prospects. N. Nasim, et al. Nucleus (Calcutta). 2022 Oct 18;65(3):399–411.
- Inhibition of inflammatory gene expression in keratinocytes using a composition containing carnitine, thioctic acid and saw palmetto extract. S. Chittur, et al. Evid Based Complement Alternat Med. 2011:2011:985345.
- Blockade of Androgen Markers Using a Novel Betasitosterol, Thioctic Acid and Carnitine-containing Compound in Prostate and Hair Follicle Cell-based Assays. L. Chen, et al. Phytother Res. 2016 Jun;30(6):1016-20.
- An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopcia- Affected Male Subjects. G. Marcovici & A. Bauman. Cosmetics 2020, 7(3), 65.
- Effect of emotional stress, anesthesia, and death on body temperature of mice exposed to cold. R. G. Bartlett, et al. Proc Soc Exp Biol Med. 1953 May;83(1):4-5.
- Carbon dioxide therapy in the treatment of localized adiposities: clinical study and histopathological correlations. C. Brandi, et al. Aesthetic Plast Surg. 2001 May-Jun;25(3):170-4.
- The Effect of Repeated Whole-Body Cryotherapy on Sirt1 and Sirt3 Concentrations and Oxidative Status in Older and Young Men Performing Different Levels of Physical Activity. G. Wojciak, et al. Antioxidants (Basel). 2020 Dec 31;10(1):37. [CrossRef]
- Tolerability of Six Months Indirect Cold (Physical) Plasma Treatment of the Scalp for Hair Loss. A. Kahn, et al. J Drugs Dmatol. 2020 Dec 1;19(12):1177-1180.
- Study of the efficacy of carboxytherapy in alopecia. N. N. Dogheim, et al. J Cos Derm. Dec. 2018. Vol 17, (6) 945-1294.
- Review of Superficial Cryotherapy for the Treatment of Alopecia Areata. M. Kaiser, et al. JDD. 2023 Aug 1; 22(8) 802.
- An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopcia- Affected Male Subjects. G. Marcovici & A. Bauman. Cosmetics 2020, 7(3), 65. [CrossRef]
- https://imagej.net/software/fiji/.
- https://imagej.net/plugins/excel-functions.
- Medication Adherence Measures: An Overview. W. Yin Lam & P. Fresco. Biomed Res Int. 2015 Oct 11;2015:217047.
- Exosomes: The Good, The Bad, and The Ugly. A. Hanyu-Deutmeyer, et al. ASRA Pain Medicine News 2023;48.
- https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/consumer-alert-regenerative-medicine-products- incling- stem-cells-and-exosomes.
- An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopcia- Affected Male Subjects. G. Marcovici & A. Bauman. Cosmetics 2020, 7(3), 65. [CrossRef]
- https://imagej.net/learn/.
- Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. S. Brito, et al. Pharmaceutics. 2024 Oct 31;16(11).
- Revolutionizing Cosmetic Ingredients: Harnessing the Power of Antioxidants, Probiotics, Plant Extracts, and Peptides in Personal and Skin Care Products. H. Yung Choi, et al. Cosmetics 2024, 11(5), 157. [CrossRef]
- Possible Combinatorial Utilization of Phytochemicals and Extracellular Vesicles for Wound Healing and Regeneration. S Koyama, et al. Int J Mol Sci. 2024 Sep 26;25(19):10353.
- Bioavailability of phytochemicals and its enhancement by drug delivery systems. F. Aqil, et al. Cancer Lett. 2013 Feb 19;334(1):133–141.
- An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopcia- Affected Male Subjects. G. Marcovici & A. Bauman. Cosmetics 2020, 7(3), 65. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).