Submitted:
09 January 2025
Posted:
10 January 2025
Read the latest preprint version here
Abstract
The controversy surrounding measles, mumps, and rubella (MMR) vaccination and autism has been ongoing for over 30 years. It is rooted in the gaslit, parent-led, grassroots movements of the 1990s and was further fueled by a case-series clinical study in 1998 by Wakefield et al., which hypothesized a causal link between MMR vaccination, gut inflammation, and autism. This controversy cascaded through numerous observational studies and reports by the US Institute of Medicine (IoM), culminating in 2019 with a population-based observational study by Hviid et al. This study was hailed at the time by the US media and medical establishment as conclusive proof that the MMR vaccine does not increase the risk of autism, even among “genetically susceptible children”. However, as detailed in this critical review, Hviid et al. did not faithfully intend or interpret the data to test this hypothesis and, therefore, cannot possibly have falsified it. We elucidate methodological flaws, discrepancies, irreproducibility, and conflicts of interest for Hviid et al. We further conjecture that researchers who faithfully serve the status quo of a vaccine orthodoxy know how to design studies to produce the desired results. In addition, we further illustrate that the conclusion from Hviid et al. cannot be generalized to the CDC childhood vaccination schedule, salient features of which have remained oblivious to so many opinion leaders, regulators, mainstream media, and professional associations in the USA. Looking at the broader picture, in the post-COVID-19 era, stereotyping, social stigma, shunning, condescension, and polarization of parents who choose not to vaccinate their children have only been exacerbated and intensified. We would retort that health freedom, parental autonomy, and open, frank, and honest scientific debate, not consensus or censorship, are the only pathways to foster real advancements for true service to our children, families, and the wider society. On this basis, we would propose a moratorium on the stigmatization and dichotomization of the unvaccinated, the vaccine-injured, and vaccine critics, as well as an end to mandates for childhood vaccines for school entry.
Keywords:
1. Introduction
2. Wakefield et al. 1998
3. Hviid et al. 2019
- A CNN headline declared, “MMR vaccine does not cause autism, another study confirms.” Emphasizing that the “biggest contribution of the study was the inclusion of children at risk of autism”, CNN reported that vaccines do “not increase the risk of autism and does not trigger autism in children who are at risk” [67].
- A headline from National Public Radio (NPR) similarly declared, “A Large Study Provides More Evidence That MMR Vaccines Don’t Cause Autism.” This article quoted lead author Anders Hviid, conclusively stating that “MMR does not cause autism.” The study, according to NPR, “found no increased risk among subgroups of children who might be unusually susceptible to autism, such as those with a brother or sister with the disorder” [68].
- The headline of a LiveScience article about the study stated, “Confirmed: No Link Between Autism and Measles Vaccine, even for ‘At Risk’ Kids” [69].
- A headline in the New York Times trumpeted, “One More Time, With Big Data: Measles Vaccine Doesn’t Cause Autism” [70].
- “Another Massive Study Finds Measles Vaccine Doesn’t Cause Autism”, said the headline of a Healthline article that quoted coauthor Mads Melbye saying, “It’s time to bury the hypothesis that MMR causes autism” [71].
- MedicalNewsToday reported, “MMR vaccine does not cause autism, even in those most at risk” [72].
- “Study Again Confirms No Link Between MMR Vaccine and Autism”, read the headline of a Psychiatry Advisor article claiming that the study showed the vaccine “does not trigger autism in children who are susceptible to the disorder” [73].
- The New Yorker magazine stated, “The science on this point is settled, to the extent that any science ever is, in the pursuit of proving a negative” [74].
4. Study Overview
4.1. Aims
4.2. Study Design, Methodology & Demographic, and Conclusions
5. Study Design Flaws
5.1. Misleading Definition of “Genetic Susceptibility”, Exclusion of Children with High Susceptibility & Inadequate Sample Size
5.2. Failure to Control for “Healthy User Bias”
5.2.1. Jain et al.
5.2.2. Hviid et al.
5.3. Failure to Consider All Vaccines Routinely Recommended for Children in Denmark
5.4. Failure to Account for MMR Formulation Change
5.5. Children Too Young for Autism Diagnosis
5.6. Failure to Consider a Change of Recommended age for 2nd MMR Dose
5.7. Failure to Consider Maternal Vaccination
5.8. Exclusion of Immigrants
5.9. Potential Misclassification of Study Subjects
- 1.
- Discrepancies in Autism Rate in the Study Group vs. Danish Population
- 2.
- Irreproducible Findings
- 3.
- Unexplained Risk of Autism Incidence for Boys and Girls with Genetic Susceptibility
- 4.
- Non-Generalizability to the Us Childhood Population
- 5.
- Conflicts of Interest
5.10. Hviid et al.
5.11. Annals of Internal Medicine
6. Discussion
7. Closing Remarks
Supplementary Materials
Funding
Data Availability
Acknowledgments
Competing Interests
Credit Authorship Contribution Statement
References
- Centers for Disease Control and Prevention (CDC). Mumps. (2024) [cited 2024 Aug 15]. Mumps Symptoms and Complications. Available online: https://www.cdc.gov/mumps/signs-symptoms/index.html.
- Centers for Disease Control and Prevention (CDC). Rubella (German Measles, Three-Day Measles). (2024) [cited 2024 Aug 15]. About Rubella. Available online: https://www.cdc.gov/rubella/about/index.html.
- DeStefano, F., & Shimabukuro, T. T. (2019). The MMR vaccine and autism. Annual Review of Virology, 6(1), 585-600. [CrossRef]
- Mathis, A. D. (2024). Measles—United States, January 1, 2020–March 28, 2024. MMWR. Morbidity and Mortality Weekly Report, 73. Available online: https://www.cdc.gov/mmwr/volumes/73/wr/mm7314a1.htm?ftag=MSF0951a18.
- Centers for Disease Control and Prevention (CDC). (2005). Elimination of rubella and congenital rubella syndrome--United States, 1969-2004. MMWR. Morbidity & Mortality Weekly Report, 54(11), 279-282. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5411a5.htm.
- Papania, M. J., Wallace, G. S., Rota, P. A., Icenogle, J. P., Fiebelkorn, A. P., Armstrong, G. L., ... & Seward, J. F. (2014). Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: The US experience. JAMA Pediatrics, 168(2), 148-155. [CrossRef]
- Tappe, J., Leung, J., Mathis, A. D., Oliver, S. E., & Masters, N. B. (2024). Characteristics of reported mumps cases in the United States: 2018–2023. Vaccine, 42(25), 126143. [CrossRef]
- Barskey, A. E., Glasser, J. W., & LeBaron, C. W. (2009). Mumps resurgences in the United States: A historical perspective on unexpected elements. Vaccine, 27(44), 6186-6195. [CrossRef]
- De Serres, G., Markowski, F., Toth, E., Landry, M., Auger, D., Mercier, M., ... & Skowronski, D. M. (2013). Largest measles epidemic in North America in a decade—Quebec, Canada, 2011: Contribution of susceptibility, serendipity, and superspreading events. The Journal of Infectious Diseases, 207(6), 990-998. [CrossRef]
- Dayan, G. H., Rubin, S., & Plotkin, S. (2008). Mumps outbreaks in vaccinated populations: Are available mumps vaccines effective enough to prevent outbreaks? Clinical Infectious Diseases, 47(11), 1458-1467. [CrossRef]
- Poland, G. A., & Jacobson, R. M. (1994). Failure to reach the goal of measles elimination: Apparent paradox of measles infections in immunized persons. Archives of Internal Medicine, 154(16), 1815-1820. [CrossRef]
- Maenner, M. J., Warren, Z., Williams, A. R., Amoakohene, E., Bakian, A. V., Bilder, D. A., ... & Shaw, K. A. (2023). Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2020. MMWR Surveillance Summaries, 72(2), 1.
- Li, Y., Yan, X., Li, Q., Li, Q., Xu, G., Lu, J., & Yang, W. (2023). Prevalence and trends in diagnosed ADHD among us children and adolescents, 2017-2022. JAMA Network Open, 6(10), e2336872-e2336872. [CrossRef]
- Zahran, H. S., Bailey, C. M., Damon, S. A., Garbe, P. L., & Breysse, P. N. (2018). Vital signs: Asthma in children—United States, 2001–2016. Morbidity & Mortality Weekly Report, 67(5), 149.
- Zablotsky, B., Black, L. I., & Akinbami, L. J. (2023). Diagnosed allergic conditions in children aged 0-17 years: United States, 2021. NCHS Data Brief. No. 459. National Center for Health Statistics. Available online: https://www.cdc.gov/nchs/data/databriefs/db459.pdf.
- Ullah, F., & Kaelber, D. C. (2021). Using large aggregated de-identified electronic health record data to determine the prevalence of common chronic diseases in pediatric patients who visited primary care clinics. Academic Pediatrics, 21(6), 1084-1093. [CrossRef]
- Killeen, R. M. (2007). Vaccines—one of the greatest medical advances of modern times. Canadian Pharmacists Journal/Revue des Pharmaciens du Canada, 140(2_suppl), S2-S2. [CrossRef]
- Guyer, B., Freedman, M. A., Strobino, D. M., & Sondik, E. J. (2000). Annual summary of vital statistics: Tcrends in the health of Americans during the 20th century. Pediatrics, 106(6), 1307-1317. [CrossRef]
- Bianchi, F. P., Mascipinto, S., Stefanizzi, P., De Nitto, S., Germinario, C., & Tafuri, S. (2021). Long-term immunogenicity after measles vaccine vs. wild infection: An Italian retrospective cohort study. Human Vaccines & Immunotherapeutics, 17(7), 2078-2084. [CrossRef]
- Horstmann, D. M., Schluederberg, A., Emmons, J. E., Evans, B. K., Randolph, M. F., & Andiman, W. A. (1985). Persistence of vaccine-induced immune responses to rubella: comparison with natural infection. Clinical Infectious Diseases, 7(Supplement_1), S80-S85. [CrossRef]
- Amanna, I. J., Carlson, N. E., & Slifka, M. K. (2007). Duration of humoral immunity to common viral and vaccine antigens. New England Journal of Medicine, 357(19), 1903-1915. [CrossRef]
- Papania, M., Baughman, A. L., Lee, S., Cheek, J. E., Atkinson, W., Redd, S. C., ... & Markowitz, L. (1999). Increased susceptibility to measles in infants in the United States. Pediatrics, 104(5), e59-e59. [CrossRef]
- Mandomando, I. M., Naniche, D., Pasetti, M. F., Vallès, X., Cuberos, L., Nhacolo, A., ... & Alonso, P. (2008). Measles-specific neutralizing antibodies in rural Mozambique: Seroprevalence and presence in breast milk. American Journal of Tropical Medicine and Hygiene, 79(5), 787-792. [CrossRef]
- Waaijenborg, S., Hahné, S. J., Mollema, L., Smits, G. P., Berbers, G. A., van der Klis, F. R., ... & Wallinga, J. (2013). Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. The Journal of Infectious Diseases, 208(1), 10-16. [CrossRef]
- Sasco, A. J., & Paffenbarger JR, R. S. (1985). Measles infection and Parkinson's disease. American Journal of Epidemiology, 122(6), 1017-1031. [CrossRef]
- Parodi, S., Crosignani, P., Miligi, L., Nanni, O., Ramazzotti, V., Rodella, S., ... & Stagnaro, E. (2013). Childhood infectious diseases and risk of leukaemia in an adult population. International Journal of Cancer, 133(8), 1892-1899. [CrossRef]
- Kubota, Y., Iso, H., Tamakoshi, A., & JACC Study Group. (2015). Association of measles and mumps with cardiovascular disease: The Japan Collaborative Cohort (JACC) study. Atherosclerosis, 241(2), 682-686. [CrossRef]
- Montella, M., Dal Maso, L., Crispo, A., Talamini, R., Bidoli, E., Grimaldi, M., ... & Franceschi, S. (2006). Do childhood diseases affect NHL and HL risk? A case-control study from northern and southern Italy. Leukemia Research, 30(8), 917-922. [CrossRef]
- Rosenlund, H., Bergström, A., Alm, J. S., Swartz, J., Scheynius, A., van Hage, M., ... & PARSIFAL Study Group. (2009). Allergic disease and atopic sensitization in children in relation to measles vaccination and measles infection. Pediatrics, 123(3), 771-778. [CrossRef]
- Shaheen, S. O., Barker, D. J. P., Heyes, C. B., Shiell, A. W., Aaby, P., Hall, A. J., & Goudiaby, A. (1996). Measles and atopy in Guinea-Bissau. The Lancet, 347(9018), 1792-1796. [CrossRef]
- Benn, C. S., Amenyogbe, N., Björkman, A., Domínguez-Andrés, J., Fish, E. N., Flanagan, K. L., ... & Aaby, P. (2023). Implications of non-specific effects for testing, approving, and regulating vaccines. Drug Safety, 46(5), 439-448. [CrossRef]
- Demicheli, V., Rivetti, A., Debalini, M. G., & Di Pietrantonj, C. (2013). Vaccines for measles, mumps and rubella in children. Evidence-Based Child Health: A Cochrane Review Journal, 8(6), 2076-2238. [CrossRef]
- Miller, N. Z. (2021). Vaccines and sudden infant death: An analysis of the VAERS database 1990–2019 and review of the medical literature. Toxicology Reports, 8, 1324-1335.
- Centers for Disease Control and Prevention (CDC). CDC vaccine safety. 2024 [cited 2024 Jul 14]. Autism and Vaccines. Available online: https://www.cdc.gov/vaccine-safety/about/autism.html?CDC_AAref_Val=https://www.cdc.gov/vaccinesafety/concerns/autism.html.
- Board on Health Promotion, Disease Prevention, & Immunization Safety Review Committee. (2004). Immunization safety review: Vaccines and autism. Washington (DC): National Academies Press (US). [CrossRef]
- Committee to Review Adverse Effects of Vaccines; Institute of Medicine. Adverse effects of vaccines: Evidence and causality. Clayton, E. W., Rusch, E., Ford, A., & Stratton, K. (Eds.). (2012). Adverse effects of vaccines: Evidence and causality. Washington (DC): National Academies Press (US). [CrossRef]
- Board on Population Health, Public Health Practice, & Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule. (2013). The childhood immunization schedule and safety: Stakeholder concerns, scientific evidence, and future studies. Washington (DC): National Academies Press (US). [CrossRef]
- Wakefield, A. J., Murch, S. H., Anthony, A., Linnell, J., Casson, D. M., Malik, M., ... & Walker-Smith, J. A. (1998). RETRACTED: Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. The Lancet, 351(9103), 637-641. [CrossRef]
- Murch, S. H., Anthony, A., Casson, D. H., Malik, M., Berelowitz, M., Dhillon, A. P., ... & Walker-Smith, J. A. (2004). Retraction of an interpretation. The Lancet, 363(9411), 750. [CrossRef]
- Deer, B. (2010). Wakefield’s “autistic enterocolitis” under the microscope. BMJ, 340. [CrossRef]
- Deer, B. (2011). How the vaccine crisis was meant to make money. BMJ, 342. [CrossRef]
- Deer, B. (2011). How the case against the MMR vaccine was fixed. BMJ, 342. [CrossRef]
- Ekbom, A., Adami, H. O., Wakefield, A., & Zack, M. J. T. L. (1994). Perinatal measles infection and subsequent Crohn's disease. The Lancet, 344(8921), 508-510. [CrossRef]
- Wakefield, A. J., Ekbom, A., Dhillon, A. P., Pittilo, R. M., & Pounder, R. E. (1995). Crohn's disease: Pathogenesis and persistent measles virus infection. Gastroenterology, 108(3), 911-916. [CrossRef]
- Ekbom, A., Daszak, P., Kraaz, W., & Wakefield, A. J. (1996). Crohn's disease after in-utero measles virus exposure. The Lancet, 348(9026), 515-517. [CrossRef]
- Thompson, N. P., Pounder, R. E., Wakefield, A. J., & Montgomery, S. M. (1995). Is measles vaccination a risk factor for inflammatory bowel disease? The Lancet, 345(8957), 1071-1074. [CrossRef]
- Wakefield, A. J., Puleston, J. M., Montgomery, S. M., Anthony, A., O'leary, J. J., & Murch, S. H. (2002). The concept of entero-colonic encephalopathy, autism and opioid receptor ligands. Alimentary Pharmacology & Therapeutics, 16(4), 663-674. [CrossRef]
- Wakefield, A. J., Ashwood, P., & Collins, I. (2005). The gut-brain axis in childhood developmental disorders: Viruses and vaccines. Neuropsychiatric Disorders & Infection, 198. [CrossRef]
- Varia, J., Herbert, M., & Hooker, B. (2024). The neuroimmunology of autism. [CrossRef]
- Turville, C., & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Buie, T., Campbell, D. B., Fuchs III, G. J., Furuta, G. T., Levy, J., VandeWater, J., ... & Winter, H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASD: A consensus report. Pediatrics, 125(Supplement_1), S1-S18. [CrossRef]
- Casiday, R. E. (2007). Children's health and the social theory of risk: Insights from the British measles, mumps and rubella (MMR) controversy. Social Science & Medicine, 65(5), 1059-1070. [CrossRef]
- Shapiro, D., & Hayburn, A. (2024). Medical gaslighting as a mechanism for medical trauma: Case studies and analysis. Current Psychology, 1-14. [CrossRef]
- Williams, S. J., & Calnan, M. (1996). The ‘limits’ of medicalization? Modern medicine and the lay populace in ‘late’ modernity. Social Science & Medicine, 42(12), 1609-1620. [CrossRef]
- Bennett, P., Celik, F., Winstanley, J., Hunt, B. J., & Pavord, S. (2023). Living with vaccine-induced immune thrombocytopenia and thrombosis: A qualitative study. BMJ Open, 13(7), e072658. [CrossRef]
- Fagen, J. L., Shelton, J. A., & Luché-Thayer, J. (2023, December). Medical gaslighting and Lyme disease: The patient experience. In Healthcare (Vol. 12, No. 1, p. 78). MDPI. [CrossRef]
- Leach, M. (2005). MMR mobilisation: Citizens and science in a British vaccine controversy. The Institute of Development Studies & Partner Organizations. Available online: https://hdl.handle.net/20.500.12413/4111.
- Angell, M. (2009). Drug companies & doctors: A story of corruption. The New York Review of Books, 56(1), 8-12. Available online: http://www.nybooks.com/articles/22237.
- Mello, M. M., Abiola, S., & Colgrove, J. (2012). Pharmaceutical companies’ role in state vaccination policymaking: The case of human papillomavirus vaccination. American Journal of Public Health, 102(5), 893-898. [CrossRef]
- Lewis, D. L. (2012). Apparent egregious ethical misconduct by British Medical Journal, Brian Deer. UK Research Integrity Office (UKRIO), Reference, (2011-060). Available online: https://www.junkscience.com/wp-content/uploads/2012/01/david-lewis-bmj.pdf.
- Sharav, V. (2017). L’Affaire Wakefield: Shades of Dreyfus and BMJ’s descent into tabloid science. Alliance for Human Research Protection. Available online: https://ahrp.org/wp-content/uploads/2017/10/LAffaire-Wakefield-Shades-of-Dreyfus-BMJs-Descent-to-Tabloid-Journalism-Vera-Sharav-2017.pdf.
- Mohammed, S. A., Rajashekar, S., Ravindran, S. G., Kakarla, M., Gambo, M. A., Salama, M. Y., ... & Hamid, P. (2022). Does vaccination increase the risk of autism spectrum disorder? Cureus, 14(8). [CrossRef]
- Turville, C., & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Poltorak, M., Leach, M., Fairhead, J., & Cassell, J. (2005). ‘MMR talk’ and vaccination choices: An ethnographic study in Brighton. Social Science & Medicine, 61(3), 709-719. [CrossRef]
- Hviid, A., Hansen, J. V., Frisch, M., & Melbye, M. (2019). Measles, mumps, rubella vaccination and autism: a nationwide cohort study. Annals of Internal Medicine, 170(8), 513-520. [CrossRef]
- Jenco, M. AAP News. (2024) [cited 2024 Sep 9]. Study: MMR vaccine not linked to increased autism risk. Available online: https://publications.aap.org/aapnews/news/13925.
- Bracho-Sanchez, E. CNN. (2019) [cited 2024 Aug 1]. MMR vaccine does not cause autism, another study confirms. Available online: https://www.cnn.com/2019/03/04/health/mmr-vaccine-autism-study/index.html.
- Stein, R. NPR. (2019) [cited 2024 Aug 15]. A large study provides more evidence that MMR vaccines don’t cause autism. Available online: https://www.npr.org/sections/health-shots/2019/03/04/699997613/a-large-study-provides-more-evidence-that-mmr-vaccines-dont-cause-autism.
- Geggel, L. Live Science. (2019) [cited 2024 Aug 15]. Confirmed: No link between autism and measles vaccine, even for “at risk” kids. Available online: https://www.livescience.com/64909-measles-vaccine-not-linked-to-autism.html.
- Hoffman, J. New York Times. (2019) [cited 2024 Aug 1]. One more time, with big data: Measles vaccine doesn’t cause autism. Available online: https://www.nytimes.com/2019/03/05/health/measles-vaccine-autism.html.
- Mammoser, G. Healthline. (2024) [cited 2024 Aug 10]. Another massive study finds measles vaccine doesn’t cause autism. Available from: https://web.archive.org/web/20190307053507/https://www.healthline.com/health-news/no-link-found-between-mmr-vaccine-and-autism.
- MedicalNewsToday. Transcripts. (2019) [cited 2024 Aug 1]. MMR vaccine does not cause autism, even in those most at risk. Available online: https://www.medicalnewstoday.com/articles/324619.
- May, B. Psychiatry Advisor. (2019) [cited 2024 Aug 1]. Study again confirms no link between MMR vaccine and autism. Available online: https://www.psychiatryadvisor.com/home/topics/autism-spectrum-disorders/study-again-confirms-no-link-between-mmr-vaccine-and-autismm.
- Paumgarten, N. The New Yorker. (2019) [cited 2024 Aug 1]. The message of measles. Available online: https://www.newyorker.com/magazine/2019/09/02/the-message-of-measles.
- Madsen, K. M., Hviid, A., Vestergaard, M., Schendel, D., Wohlfahrt, J., Thorsen, P., ... & Melbye, M. (2002). A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine, 347(19), 1477-1482. [CrossRef]
- Parner, E. T., Schendel, D. E., & Thorsen, P. (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics & Adolescent Medicine, 162(12), 1150-1156. [CrossRef]
- Kragh Andersen, P., Pohar Perme, M., van Houwelingen, H. C., Cook, R. J., Joly, P., Martinussen, T., ... & Therneau, T. M. (2021). Analysis of time-to-event for observational studies: Guidance to the use of intensity models. Statistics in Medicine, 40(1), 185-211. [CrossRef]
- Benkeser, D., Carone, M., & Gilbert, P. B. (2018). Improved estimation of the cumulative incidence of rare outcomes. Statistics in Medicine, 37(2), 280-293. [CrossRef]
- Abd ElHafeez, S., D’Arrigo, G., Leonardis, D., Fusaro, M., Tripepi, G., & Roumeliotis, S. (2021). Methods to analyze time-to-event data: The cox regression analysis. Oxidative Medicine & Cellular Longevity, 2021(1), 1302811. [CrossRef]
- Jain, A., Marshall, J., Buikema, A., Bancroft, T., Kelly, J. P., & Newschaffer, C. J. (2015). Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA, 313(15), 1534-1540. [CrossRef]
- Betancur, C. (2011). Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Research, 1380, 42-77. [CrossRef]
- Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., ... & Risch, N. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095-1102. [CrossRef]
- Gaugler, T., Klei, L., Sanders, S. J., Bodea, C. A., Goldberg, A. P., Lee, A. B., ... & Buxbaum, J. D. (2014). Most genetic risk for autism resides with common variation. Nature Genetics, 46(8), 881-885. [CrossRef]
- Hocking, M. C., Albee, M. V., Kim, M., Berman, J. I., Fisher, M. J., Roberts, T. P. L., & Blaskey, L. (2024). Social challenges, autism spectrum disorder, and attention deficit/hyperactivity disorder in youth with neurofibromatosis type I. Applied Neuropsychology: Child, 1-9. [CrossRef]
- Wiznitzer, M. (2004). Autism and tuberous sclerosis. Journal of Child Neurology, 19(9), 675-679. [CrossRef]
- Peters, S. U., Beaudet, A. L., Madduri, N., & Bacino, C. A. (2004). Autism in Angelman syndrome: Implications for autism research. Clinical Genetics, 66(6), 530-536. [CrossRef]
- Abbeduto, L., McDuffie, A., & Thurman, A. J. (2014). The fragile X syndrome–autism comorbidity: What do we really know? Frontiers in Genetics, 5, 355. [CrossRef]
- Bennett, J. A., Germani, T., Haqq, A. M., & Zwaigenbaum, L. (2015). Autism spectrum disorder in Prader–Willi syndrome: A systematic review. American Journal of Medical Genetics Part A, 167(12), 2936-2944. [CrossRef]
- Reilly, C. (2009). Autism spectrum disorders in Down syndrome: A review. Research in Autism Spectrum Disorders, 3(4), 829-839. [CrossRef]
- Vorstman, J. A., Morcus, M. E., Duijff, S. N., Klaassen, P. W., Beemer, F. A., Swaab, H., ... & van Engeland, Herman. (2006). The 22q11. 2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 45(9), 1104-1113. [CrossRef]
- Bjørklund, G., Mkhitaryan, M., Sahakyan, E., Fereshetyan, K., Meguid, N. A., Hemimi, M., ... & Yenkoyan, K. (2024). Linking environmental chemicals to neuroinflammation and autism spectrum disorder: Mechanisms and implications for prevention. Molecular Neurobiology, 1-13. [CrossRef]
- Park, S. K., Tao, Y., Meeker, J. D., Harlow, S. D., & Mukherjee, B. (2014). Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES study using serum lipid levels. PloS One, 9(6), e98632. [CrossRef]
- Liu, J., & Lewis, G. (2014). Environmental toxicity and poor cognitive outcomes in children and adults. Journal of Environmental Health, 76(6), 130. Available online: https://pubmed.ncbi.nlm.nih.gov/24645424/.
- Maitre, L., Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C. H. E., Siskos, A. P., ... & Vrijheid, M. (2022). Multi-omics signatures of the human early life exposome. Nature Communications, 13(1), 7024. [CrossRef]
- Palmer, R. F., Kattari, D., Rincon, R., & Miller, C. S. (2024). Assessing chemical intolerance in parents predicts the risk of autism and ADHD in their children. Journal of Xenobiotics, 14(1), 350-367. [CrossRef]
- Merriam-Webster. Definition of the verb “lie”, meaning number two. (2024) [cited 2014 Aug 19]. Available online: https://www.merriam-webster.com/dictionary/lie.
- Glickman, G., Harrison, E., & Dobkins, K. (2017). Vaccination rates among younger siblings of children with autism. New England Journal of Medicine, 377(11), 1099-1101. [CrossRef]
- Zerbo, O., Modaressi, S., Goddard, K., Lewis, E., Fireman, B. H., Daley, M. F., ... & Klein, N. P. (2018). Vaccination patterns in children after autism spectrum disorder diagnosis and in their younger siblings. JAMA Pediatrics, 172(5), 469-475. [CrossRef]
- Kuwaik, G. A., Roberts, W., Zwaigenbaum, L., Bryson, S., Smith, I. M., Szatmari, P., ... & Brian, J. (2014). Immunization uptake in younger siblings of children with autism spectrum disorder. Autism, 18(2), 148-155. [CrossRef]
- Jain, A., Marshall, J., Buikema, A., Bancroft, T., Kelly, J. P., & Newschaffer, C. J. (2015). Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA, 313(15), 1534-1540. [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). ECDC vaccine scheduler. (2024) [cited 2024 Jul 12]. Denmark: Recommended vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByCountry?SelectedCountryId=58&IncludeChildAgeGroup=true&IncludeAdultAgeGroup=true&SelectedVersionId=60.
- Statens Serum Institut. Danish childhood vaccination program vaccination offers protection. 13th ed. Sundhedsstyrelsen. (2024) [cited 2024 Jul 2]. Available online: https://www.sst.dk/-/media/Udgivelser/2023/Boernevaccination/Pjecer/Boernevaccinationsprogrammet-i-Danmark-2022-Engelsk.ashx.
- Ingels, H., Rasmussen, J., Andersen, P. H., Harboe, Z. B., Glismann, S., Konradsen, H., ... & Lambertsen, L. (2012). Impact of pneumococcal vaccination in Denmark during the first 3 years after PCV introduction in the childhood immunization programme. Vaccine, 30(26), 3944-3950. [CrossRef]
- Harboe, Z. B., Dalby, T., Weinberger, D. M., Benfield, T., Mølbak, K., Slotved, H. C., ... & Valentiner-Branth, P. (2014). Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clinical Infectious Diseases, 59(8), 1066-1073. [CrossRef]
- Wójcik, O. P., Simonsen, J., Mølbak, K., & Valentiner-Branth, P. (2013). Validation of the 5-year tetanus, diphtheria, pertussis and polio booster vaccination in the Danish childhood vaccination database. Vaccine, 31(6), 955-959. [CrossRef]
- Harder, K. M., Cowan, S., Eriksen, M. B., Krarup, H. B., & Christensen, P. B. (2011). Universal screening for hepatitis B among pregnant women led to 96% vaccination coverage among newborns of HBsAg positive mothers in Denmark. Vaccine, 29(50), 9303-9307. [CrossRef]
- Centers for Disease Control and Prevention (CDC). CDC Vaccines & immunizations. (2013). Recommended immunization schedule for persons age 0 through 18 years. Available online: https://web.archive.org/web/20130203110629/https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html.
- U.S. Food & Drug Administration (FDA). (2023) [cited 2024 Sep 15]. Gardasil. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil.
- World Health Organization (WHO). WHO International. (2018) [cited 2024 Mar 18]. Human papillomavirus (HPV). Available online: https://web.archive.org/web/20190724085659/https://www.ssi.dk/vaccinationer/boernevaccination/vaccination-mod-livmoderhalskraeft.
- Statens Serum Institut. (2018) [cited 2024 Aug 19]. Vaccination against cervical cancer (HPV). Available online: https://www.ssi.dk/vaccinationer/boernevaccination/vaccination-mod-livmoderhalskraeft.
- Turville, C., & Golden, I. (2015). Autism and vaccination: The value of the evidence base of a recent meta-analysis. Vaccine, 33(42), 5494-5496. [CrossRef]
- Sørup, S., Jensen, A. K., Aaby, P., & Benn, C. S. (2019). Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: A Danish register-based cohort study. Clinical Infectious Diseases, 68(2), 282-290. [CrossRef]
- Sørup, S., Jensen, A. K., Aaby, P., & Benn, C. S. (2019). Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: A Danish register-based cohort study. Clinical Infectious Diseases, 68(2), 282-290. [CrossRef]
- Davidkin, I., Peltola, H., Leinikki, P., & Valle, M. (2000). Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine, 18(27), 3106-3112. [CrossRef]
- Paunio, M., Heinonen, O. P., Virtanen, M., Leinikki, P., Patja, A., & Peltola, H. (2000). Measles history and atopic diseases: A population-based cross-sectional study. JAMA, 283(3), 343-346. [CrossRef]
- U.S. Food & Drug Administration (FDA). U.S. Food & Drug Administration (FDA). (2022) [cited 2024 Sep 15]. GlaxoSmithKline Biologicals SA, Priorix Package Insert. Available online: https://web.archive.org/web/20240713033807/https://www.fda.gov/media/151707/download.
- Sørup, S., Jensen, A. K., Aaby, P., & Benn, C. S. (2019). Revaccination with measles-mumps-rubella vaccine and infectious disease morbidity: A Danish register-based cohort study. Clinical Infectious Diseases, 68(2), 282-290. [CrossRef]
- Gillet, Y., Habermehl, P., Thomas, S., Eymin, C., & Fiquet, A. (2009). Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (MM-RvaxPro®) and a varicella vaccine (VARIVAX®) by intramuscular or subcutaneous routes at separate injection sites: A randomised clinical trial. BMC Medicine, 7, 1-11. [CrossRef]
- European Commission. Sanofi Pasteur, M-M-RVAXPRO Package Insert. (2024) [cited 2024 Aug 19]. Available online: https://ec.europa.eu/health/documents/community-register/2010/2010090688568/anx_88568_en.pdf.
- Statens Serum Institut. (2024) [cited 2024 Aug 19]. Uge 24 – 2013. Available online: https://www.ssi.dk/aktuelt/nyhedsbreve/epi-nyt/2013/uge-24---2013.
- Benn, C. S. (MD, PhD) Chair of Health Sciences, Department of Clinical Research, Danish Institute for Advanced Study (DIAS) University of Southern Denmark (personal communication, August 19, 2024).
- Statens Serum Institut. (2008) [cited 2024 Aug 19]. Pneumokokvaccine I Det Danske Børnevaccinationsprogram. Available online: https://www.ssi.dk/-/media/arkiv/dk/aktuelt/nyhedsbreve/epi-nyt/2008/2008-pdf/epi-nyt---2008---uge-38.pdf.
- Parner, E. T., Schendel, D. E., & Thorsen, P. (2008). Autism prevalence trends over time in Denmark: Changes in prevalence and age at diagnosis. Archives of Pediatrics & Adolescent Medicine, 162(12), 1150-1156. [CrossRef]
- Statens Serum Institut. (2008) [cited 2024 Aug 19]. MMR 2 Vaccination Advanced to 4 Years. Available online: https://web.archive.org/web/20240816180533/https://en.ssi.dk/-/media/arkiv/uk/news/epi-news/2008/pdf/epi-news---2008---no-9.pdf.
- Knuesel, I., Chicha, L., Britschgi, M., Schobel, S. A., Bodmer, M., Hellings, J. A., ... & Prinssen, E. P. (2014). Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology, 10(11), 643-660. [CrossRef]
- Wu, W. L., Hsiao, E. Y., Yan, Z., Mazmanian, S. K., & Patterson, P. H. (2017). The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, Behavior, & Immunity, 62, 11-23. [CrossRef]
- Zerbo, O., Qian, Y., Yoshida, C., Fireman, B. H., Klein, N. P., & Croen, L. A. (2017). Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatrics, 171(1), e163609-e163609. [CrossRef]
- Hooker, B. S. (2017). Influenza vaccination in the first trimester of pregnancy and risk of autism spectrum disorder. JAMA Pediatrics, 171(6), 600-600. [CrossRef]
- Jorgensen, P., Mereckiene, J., Cotter, S., Johansen, K., Tsolova, S., & Brown, C. (2018). How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine, 36(4), 442-452. [CrossRef]
- Mølgaard-Nielsen, D., Fischer, T. K., Krause, T. G., & Hviid, A. (2019). Effectiveness of maternal immunization with trivalent inactivated influenza vaccine in pregnant women and their infants. Journal of Internal Medicine, 286(4), 469-480. [CrossRef]
- Nakken, C. S., Skovdal, M., Nellums, L. B., Friedland, J. S., Hargreaves, S., & Norredam, M. (2018). Vaccination status and needs of asylum-seeking children in Denmark: A retrospective data analysis. Public Health, 158, 110-116. [CrossRef]
- Cherri, Z., Lau, K., Nellums, L. B., Himmels, J., Deal, A., McGuire, E., ... & Hargreaves, S. (2024). The immune status of migrant populations in Europe and implications for vaccine-preventable disease control: A systematic review and meta-analysis. Journal of Travel Medicine, taae033. [CrossRef]
- Holt, N., Mygind, A., & Bro, F. (2017). Danish MMR vaccination coverage is considerably higher than reported. Danish Medical Journal, 64(2), A5345. Available online: https://ugeskriftet.dk/dmj/danish-mmr-vaccination-coverage-considerably-higher-reported.
- Schendel, D. E., & Thorsteinsson, E. (2018). Cumulative incidence of autism into adulthood for birth cohorts in Denmark, 1980-2012. JAMA, 320(17), 1811-1813. [CrossRef]
- Centers for Disease Control and Prevention (CDC). About Autism Spectrum Disorder (ASD). (2024) [cited 2024 Jul 15]. Data and Statistics on Autism Spectrum Disorder. Available online: https://www.cdc.gov/autism/data-research/index.html.
- Munafò, M. R., Nosek, B. A., Bishop, D. V., Button, K. S., Chambers, C. D., Percie du Sert, N., ... & Ioannidis, J. (2017). A manifesto for reproducible science. Nature Human Behavior, 1(1), 1-9. [CrossRef]
- Clarkson, E (PhD), Chief Statistician at the National Institute for Aviation Research (NIAR) and Senior Research Engineer and Chief Statistician for the National Center for Advanced Materials Performance (NCAMP). Wichita State University (personal communication, April 4, 2019).
- Centers for Disease Control and Prevention (CDC). CDC Vaccines & Immunizations. 2023. Child and Adolescent Immunization Schedule by Age. Available online: https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-age.html.
- Centers for Disease Control and Prevention (CDC). CDC Pregnancy and Vaccination. 2024 [cited 2024 Aug 15]. Vaccine recommendations before, during, and after pregnancy. Available online: https://www.cdc.gov/vaccines-pregnancy/recommended-vaccines/index.html.
- Bennett J. (2019) Shortcomings in the latest MMR vaccination and autism study: A healthcare administrator’s response. Science, Public Health Policy & the Law. 1, 2019-2024. Available online: https://publichealthpolicyjournal.com/shortcomings-in-the-latest-mmr-vaccination-and-autism-study-a-healthcare-administrators-response/.
- BioSpace. (2024) [cited 2024 Nov 2]. Biologics market size to hit around USD 1. 37 trillion by 2033. Available online: https://www.biospace.com/biologics-market-size-to-hit-around-usd-1-37-trillion-by-2033.
- Precedence Research. (2024) [cited 2024 Nov 2]. Vaccines market size, share, and trends 2024 to 2034. Available online: https://www.precedenceresearch.com/vaccines-market.
- Allied Market Research. (2022) [cited 2024 Nov 2]. Mumps vaccine market size, share, competitive landscape and trend analysis report, by age group, by distribution channel: Global opportunity analysis and Industry Forecast, 2021-2031. Available online: https://www.alliedmarketresearch.com/mumps-vaccine-market-A15401.
- Parker, E. M., Zhu, B. P., Li, Z., Puddy, R. W., Kelly, M. A., Scott, C., ... & Stephens, J. W. (2024). Domains of excellence: A CDC framework for developing high-quality, impact-driven public health science publications. Journal of Public Health Management & Practice, 30(1), 72-78. [CrossRef]
- Kern, J. K., Geier, D. A., Deth, R. C., Sykes, L. K., Hooker, B. S., Love, J. M., ... & Geier, M. R. (2017). Systematic assessment of research on autism spectrum disorder (ASD) and mercury reveals conflicts of interest and the need for transparency in autism research. Science & Engineering Ethics, 23, 1691-1718. [CrossRef]
- Lenzer, J. (2015). Centers for Disease Control and Prevention: Protecting the private good? BMJ, 350. [CrossRef]
- Huntoon, L. R. (2020). CDC: Bias and disturbing conflicts of interest. Journal of American Physicians & Surgeons, 23, 66-69. Available online: https://www.jpands.org/vol25no3/huntoon.pdf.
- Miller, N. Z., & Goldman, G. S. (2022). Case study: Varicella vaccine and the suppression of data. Journal of American Physicians and Surgeons, 27(1). Available online: https://jpands.org/vol27no1/miller.pdf.
- Hooker, B. S. (2018). Reanalysis of CDC data on autism incidence and time of first MMR vaccination. Journal of American Physicians & Surgeons, 23(4), 105-110. Available online: https://www.jpands.org/vol23no4/hooker.pdf.
- Hooker, B. S. (2017). CDC data manipulation exposed: Four years later. Journal of American Physicians & Surgeons, 22(4), 119-121. Available online: https://www.jpands.org/vol22no4/hooker.pdf.
- Nissen, S. E. (2017). Conflicts of interest and professional medical associations: Progress and remaining challenges. JAMA, 317(17), 1737-1738. [CrossRef]
- Deirdre, I. Fox News. (2010) [cited 2024 Nov 2]. Conflict of children’s interest inside the American academy of pediatrics. Available online: https://www.foxnews.com/health/conflict-of-childrens-interest-inside-the-american-academy-of-pediatrics.
- Steinbrook, R., Kassirer, J. P., & Angell, M. (2015). Justifying conflicts of interest in medical journals: A very bad idea. BMJ, 350. [CrossRef]
- Angell M, Relman A. S. New York Times. (2004) [cited 2024 Nov 1]. Physicians, educate yourselves. Available online: https://www.nytimes.com/2004/12/30/opinion/physicians-educate-yourselves-017566.html.
- Angell, M. (2009). Drug companies & doctors: A story of corruption. The New York Review of Books, 56(1), 8-12. Available online: https://www.nybooks.com/articles/2009/01/15/drug-companies-doctorsa-story-of-corruption/.
- Statens Serum Institut. (2022) [cited 2024 Jul 2]. Vaccine research. Available online: https://en.ssi.dk/research/center-for-vaccine-research.
- Statens Serum Institut. (2019) [cited 2024 Aug 19]. No Association between MMR vaccine and autism. Available online: https://en.ssi.dk/news/news/2019/no-association-between-mmr-vaccine-and-autism.
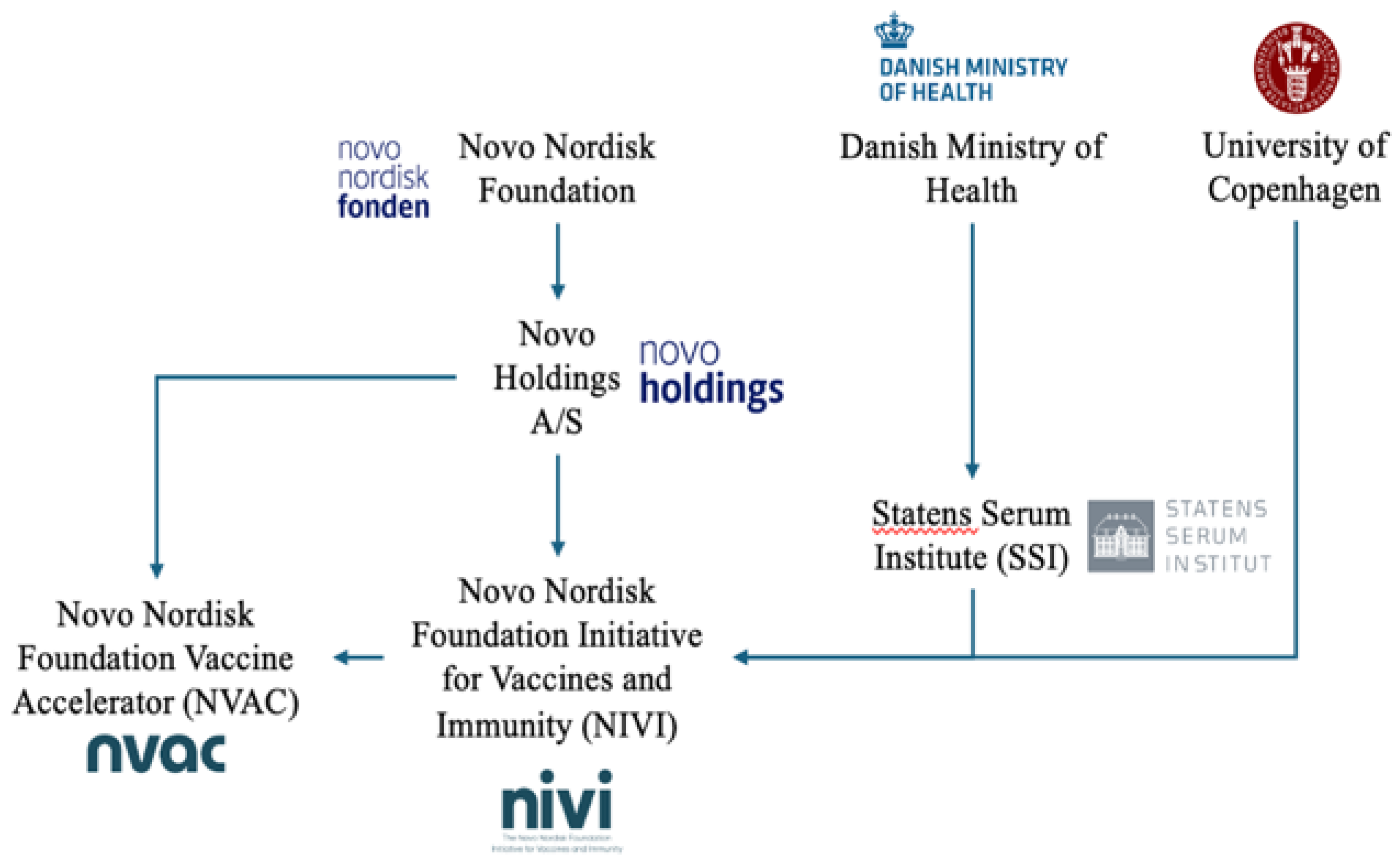
- Novo Nordisk Foundation. (2024) [cited 2024 Aug 2]. Who are we? Available online: https://novonordiskfonden.dk/en/who-we-are/.
- International Committee of Medical Journal Editors. ICMJE Form for disclosure of potential conflicts of interest. (2019) [cited 2024 Aug 1]. Available online: https://rmed.acponline.org/authors/conflictFormServlet/M18-2101/ICMJE/M18-2101-Conflicts.pdf.
- Indenrigs-OG Sundhedsministeriet (2022) [cited 2024 Aug 1]. Minister for the interior and health of Denmark. Available online: https://www.ism.dk/english.
- Novo Nordisk Foundation. (2022) [cited 2024 Aug 21]. Ownership. Available online: https://novonordiskfonden.dk/en/who-we-are/ownership/.
- Novo Nordisk A/S. (2024) [cited 2024 Aug 2]. Corporate governance. Available online: https://www.novonordisk.com/about/corporate-governance.html.
- CompaniesMarketCap. Global ranking. (2024) [cited 2024 Aug 1]. Market capitalization of Novo Nordisk (NVO). Available online: https://companiesmarketcap.com/novo-nordisk/marketcap/#google_vignette.
- Novo Holdings. (2024) [cited 2024 Aug 21]. Corporate governance. Available online: https://www.novonordisk.com/about/corporate-governance.html.
- Novo Holdings. (2021) [cited 2024 Aug 21]. Investments. Available online: https://novoholdings.dk/investments.
- Novo Holdings. (2021) [cited 2024 Aug 21]. Joining the fight against COVID-19 while running a life-saving business. Available online: https://novoholdings.dk/news/joining-the-fight-against-covid-19-while-running-a-life-saving-business.
- Novo Nordisk Foundation Initiative for Vaccines and Immunity (NIVI). (2023) [cited 2024 Aug 21]. Projekter og initiativer. Available online: https://novonordiskfonden.dk/projekter/novo-nordisk-foundation-initiative-for-vaccines-and-immunity-nivi.
- University of Copenhagen. (2023) [cited 2024 Aug 21]. New initiative puts Danish vaccine science on the global map. Available online: https://healthsciences.ku.dk/newsfaculty-news/2023/12/new-initiative-puts-danish-vaccine-science-on-the-global-map.
- NIVI Novo Nordisk Foundation Initiative for Vaccines and Immunity. (2024) [cited 2024 Aug 23]. About the initiative. Available online: https://nivi.ku.dk/about-nivi/.
- Novo Nordisk Foundation. (2024) [cited 2024 Aug 2]. The Novo Nordisk Foundation Vaccine Accelerator (NVAC). Available online: https://novonordiskfonden.dk/en/projects/the-novo-nordisk-foundation-vaccine-accelerator-nvac/.
- Statens Serum Institut. (2018) [cited 2024 Aug 19]. Vaccination against cervical cancer (HPV). Available online: https://www.ssi.dk/vaccinationer/boernevaccination/vaccination-mod-livmoderhalskraeft.
- Olsen, S. F., Halldorsson, T. I., Thorne-Lyman, A. L., Strøm, M., Gørtz, S., Granstrøm, C., ... & Zhou, W. (2018). Plasma concentrations of long chain N-3 fatty acids in early and mid-pregnancy and risk of early preterm birth. EBioMedicine, 35, 325-333. [CrossRef]
- Novo Nordisk Foundation. (2019) [cited 2024 Aug 2]. The Novo Nordisk Foundation awards grants to 12 excellent research leaders within bioscience and basic biomedicine. Available online: https://researchleaderprogramme.com/news/the-novo-nordisk-foundation-awards-grants-to-12-excellent-research-leaders-within-bioscience-and-basic-biomedicine.
- Statens Serum Institut. (2023) [cited 2024 Aug 19]. New research sheds light on genetics of placenta growth. Available online: https://en.ssi.dk/news/news/2023/new-research-sheds-light-on-genetics-of-placenta-growth.
- Statens Serum Institut. (2024) [cited 2024 Jul 2]. SSI researcher receives multi-million kroner grant from the Novo Nordisk Foundation. Available online: https://en.ssi.dk/news/news/2019/ssi-researcher-receives-multi-million-kroner-grant-from-the-novo-nordisk-foundation.
- Statens Serum Institut. (2024) [cited 2024 Jul 2]. Babies’ own genes influence when they are born. Available online: https://en.ssi.dk/news/news/2019/babies-own-genes-influence-when-they-are-born.
- Statens Serum Institut. (2023) [cited 2024 Aug 2]. The Danish national biobank. Available online: https://en.ssi.dk/research/the-danish-national-biobank.
- ngell, M., & Relman, A. S. (2002). Patents, profits & American medicine: Conflicts of interest in the testing & marketing of new drugs. Daedalus, 131(2), 102-111. Available online: https://www.jstor.org/stable/20027764.
- Kennedy Jr, R. F. (2015). Thimerosal: Let the science speak: The evidence supporting the immediate removal of mercury—A known neurotoxin—From vaccines. Simon & Schuster.
- Schuemie, M. J., Hripcsak, G., Ryan, P. B., Madigan, D., & Suchard, M. A. (2018). Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proceedings of the National Academy of Sciences, 115(11), 2571-2577. [CrossRef]
- Hviid, A., Stellfeld, M., Wohlfahrt, J., & Melbye, M. (2003). Association between thimerosal-containing vaccine and autism. JAMA, 290(13), 1763-1766. [CrossRef]
- Hviid, A., Stellfeld, M., Wohlfahrt, J., & Melbye, M. (2004). Childhood vaccination and type 1 diabetes. New England Journal of Medicine, 350(14), 1398-1404. [CrossRef]
- Vestergaard, M., Hviid, A., Madsen, K. M., Wohlfahrt, J., Thorsen, P., Schendel, D., ... & Olsen, J. (2004). MMR vaccination and febrile seizures: Evaluation of susceptible subgroups and long-term prognosis. JAMA, 292(3), 351-357. [CrossRef]
- Hviid, A., Melbye, M., & Pasternak, B. (2013). Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. New England Journal of Medicine, 369(25), 2406-2415. [CrossRef]
- Pasternak, B., Svanström, H., & Hviid, A. (2013). Ondansetron in pregnancy and risk of adverse fetal outcomes. New England Journal of Medicine, 368(9), 814-823. [CrossRef]
- Geier, D. A., King, P. G., Hooker, B. S., Dórea, J. G., Kern, J. K., Sykes, L. K., & Geier, M. R. (2015). Thimerosal: Clinical, epidemiologic and biochemical studies. Clinica Chimica acta, 444, 212-220. [CrossRef]
- Kern, J., Geier, D., Audhya, T., King, P., Sykes, L., & Geier, M. (2012). Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiologiae Experimentalis, 72(2), 113-153. [CrossRef]
- Mezzacappa, A., Lasica, P. A., Gianfagna, F., Cazas, O., Hardy, P., Falissard, B., ... & Gressier, F. (2017). Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: A systematic review and meta-analysis. JAMA Pediatrics, 171(6), 555-563. [CrossRef]
- Kennedy, D. (2016). Ondansetron and pregnancy: Understanding the data. Obstetric Medicine, 9(1), 28-33. [CrossRef]
- Kirby, D. (2008). The vaccine-autism court document every American should read. Huffington Post. Available online: https://www.jeremyrhammond.com/wp-content/uploads/2019/10/080226-Vaccine-Autism-Court-Document-Kirby-HuffPost.pdf.
- Transcripts. (2008) [cited 2024 Aug 1]. Unraveling the mystery of autism; talking with the CDC director; stories of children with autism; aging with autism. Available online: http://transcripts.cnn.com/TRANSCRIPTS/0803/29/hcsg.01.html.
- Reuters. Reuters. (2009) [cited 2024 Aug 21]. Former CDC head lands vaccine job at Merck. Available online: https://www.reuters.com/article/us-merck-gerberding/former-cdc-head-lands-vaccine-job-at-merck-idUSTRE5BK2K520091221/.
- Merck. (2022) [cited 2024 Aug 21]. Dr. Julie L. Gerberding to Retire from Merck. Available online: https://www.merck.com/news/dr-julie-l-gerberding-to-retire-from-merck/.
- Hallmayer, J., Cleveland, S., Torres, A., Phillips, J., Cohen, B., Torigoe, T., ... & Risch, N. (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry, 68(11), 1095-1102. [CrossRef]
- Bradstreet, J. (2004). Biological evidence of significant vaccine related side-effects resulting in neurodevelopmental disorders. Presentation to the Vaccine Safety Committee of the Institute of Medicine, The National Academies of Science, 9. Available online: https://www.amalgam-informationen.de /dokument/Bradstreet.pdf.
- Maitre, L., Bustamante, M., Hernández-Ferrer, C., Thiel, D., Lau, C. H. E., Siskos, A. P., ... & Vrijheid, M. (2022). Multi-omics signatures of the human early life exposome. Nature Communications, 13(1), 7024. [CrossRef]
- Laugesen, K., Mengel-From, J., Christensen, K., Olsen, J., Hougaard, D. M., Boding, L., ... & Sørensen, H. T. (2023). A review of major Danish biobanks: Advantages and possibilities of health research in Denmark. Clinical Epidemiology, 213-239. [CrossRef]
- Attkisson, S. (2018) [cited 2024 Aug 13]. CDC: “Possibility” that vaccines rarely trigger autism. Available online: https://sharylattkisson.com/2018/12/cdc-possibility-that-vaccines-rarely-trigger-autism/.
- Price, C. S., Thompson, W. W., Goodson, B., Weintraub, E. S., Croen, L. A., Hinrichsen, V. L., ... & DeStefano, F. (2010). Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics, 126(4), 656-664. [CrossRef]
- Janiaud, P., Agarwal, A., Tzoulaki, I., Theodoratou, E., Tsilidis, K. K., Evangelou, E., & Ioannidis, J. P. (2021). Validity of observational evidence on putative risk and protective factors: appraisal of 3744 meta-analyses on 57 topics. BMC Medicine, 19, 1-17. [CrossRef]
- Ioannidis, J. P. (2016). Why most clinical research is not useful. PLoS medicine, 13(6), e1002049. [CrossRef]
- Kennedy. R. F, Hooker B. (2023) Vax-Unvax: Let the science speak. Skyhorse Publishing.
- Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. J. H. W. (2019). Cochrane handbook for systematic reviews of interventions. Hoboken, Wiley. [CrossRef]
- Barnes, H. J., Ragnarsson, G., & Alván, G. (2009). Quality and safety considerations for recombinant biological medicines: A regulatory perspective. International Journal of Risk & Safety in Medicine, 21(1-2), 13-22. [CrossRef]
- Valiant, W. G., Cai, K., & Vallone, P. M. (2022). A history of adventitious agent contamination and the current methods to detect and remove them from pharmaceutical products. Biologicals, 80, 6-17. [CrossRef]
- Chooi, W. H., Ng, P. W., Hussain, Z., Ming, L. C., Ibrahim, B., & Koh, D. (2022). Vaccine contamination: Causes and control. Vaccine, 40(12), 1699. [CrossRef]
- Aranha, H., & Solutions, C. P. (2011). Virus safety of biopharmaceuticals. Contract Pharma, 13. Available online: https://www.contractpharma.com/virus-safety-of-biopharmaceuticals/.
- English, J. M. (1995). The rights and wrongs of measles vaccination. British Homoeopathic Journal, 84(3), 156-163. [CrossRef]
- Price, D., Jefferson, T., & Demicheli, V. (2004). Methodological issues arising from systematic reviews of the evidence of safety of vaccines. Vaccine, 22(15-16), 2080-2084. [CrossRef]
- Halvorsen, R. (2007). The Truth About Vaccines: How we are used as guinea pigs without knowing it. Gibson Square.
- McKinlay, J. B., & McKinlay, S. M. (1977). The questionable contribution of medical measures to the decline of mortality in the United States in the twentieth century. The Milbank Memorial Fund Quarterly. Health & Society, 405-428. Available online: http://ftp.columbia.edu/itc/hs/pubhealth/rosner/g8965/client_edit/readings/week_2/mckinlay.pdf.
- Armstrong, G. L., Conn, L. A., & Pinner, R. W. (1999). Trends in infectious disease mortality in the United States during the 20th century. JAMA, 281(1), 61-66. [CrossRef]
- Beck, M. A. (1996). The role of nutrition in viral disease. The Journal of Nutritional Biochemistry, 7(12), 683-690. [CrossRef]
- Hooker, B. S., & Miller, N. Z. (2020). Analysis of health outcomes in vaccinated and unvaccinated children: Developmental delays, asthma, ear infections and gastrointestinal disorders. SAGE Open Medicine, 8, 2050312120925344. [CrossRef]
- Miller, N. Z., & Goldman, G. S. (2023). Neonatal, infant, and under age five vaccine doses routinely given in developed nations and their association with mortality rates. Cureus, 15(7). [CrossRef]
- Light, D. W., Lexchin, J., & Darrow, J. J. (2013). Institutional corruption of pharmaceuticals and the myth of safe and effective drugs. Journal of Law, Medicine & Ethics, 41(3), 590-600. [CrossRef]
- Barosi, G., & Gale, R. P. (2018). Is there expert consensus on expert consensus? Bone Marrow Transplantation, 53(8), 1055-1060. [CrossRef]
- Djulbegovic, B., & Guyatt, G. (2019). Evidence vs consensus in clinical practice guidelines. JAMA, 322(8), 725-726. [CrossRef]
- Bragazzi, N. L., Watad, A., Amital, H., & Shoenfeld, Y. (2017). Debate on vaccines and autoimmunity: Do not attack the author, yet discuss it methodologically. Vaccine, 35(42), 5522-5526. [CrossRef]
- Martin, B. (2015). On the suppression of vaccination dissent. Science & Engineering Ethics, 21, 143-157. [CrossRef]
- Elisha, E., Guetzkow, J., Shir-Raz, Y., & Ronel, N. (2024). Suppressing scientific discourse on vaccines? Self-perceptions of researchers and practitioners. In Hec Forum (Vol. 36, No. 1, pp. 71-89). Dordrecht: Springer Netherlands. [CrossRef]
- Kempner, J. (2008). The chilling effect: how do researchers react to controversy? PLoS Medicine, 5(11), e222. [CrossRef]
- Martin, B. (1981). The scientific straightjacket: The power structure of science and the suppression of environmental scholarship. Ecologist, 11(1), 33-43. Available online: https://documents.uow.edu.au/~bmartin/pubs/81ecol.html.
- Larsson, A., Oxman, A. D., Carling, C., & Herrin, J. (2003). Medical messages in the media–barriers and solutions to improving medical journalism. Health Expectations, 6(4), 323-331. [CrossRef]
- Shuchman, M., & Wilkes, M. S. (1997). Medical scientists and health news reporting: A case of miscommunication. Annals of Internal Medicine, 126(12), 976-982. [CrossRef]
- Carroll, A. E. New York Times. (2018) [cited 2024 Aug 1]. Why it’s still worth getting a flu shot. Available online: https://www.nytimes.com/2018/01/11/upshot/flu-shot-risks-benefits-strain.html.
- Carroll, A. E. New York Times. (2017) [cited 2024 Aug 1]. A link between alcohol and cancer? It’s not nearly as scary as it seems. Available online: https://www.nytimes.com/2017/11/10/upshot/health-alcohol-cancer-research.html.
- Lipworth, W., Kerridge, I., Morrell, B., Bonfiglioli, C., & Forsyth, R. (2012). Medicine, the media and political interests. Journal of Medical Ethics, 38(12), 768-770. [CrossRef]
- Wiley, K. E., Leask, J., Attwell, K., Helps, C., Barclay, L., Ward, P. R., & Carter, S. M. (2021). Stigmatized for standing up for my child: A qualitative study of non-vaccinating parents in Australia. SSM-population Health, 16, 100926. [CrossRef]
- Kennedy Jr, R. F. (2022). Profiles of the vaccine-injured: "A lifetime price to pay." Simon & Schuster.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




