Submitted:
14 January 2025
Posted:
14 January 2025
You are already at the latest version
Abstract
Most brain development occurs in the "first 1000 days" a critical period from conception to a child's second birthday. Critical brain processes that occur during this time include synaptogenesis, myelination, neural pruning and formation of functioning neuronal circuits. Perturbations during the first 1000 days likely contribute to later-life neurodegenerative disease including sporadic amyotrophic lateral sclerosis (ALS). Neurodevelopment is determined by many events, including the maturation and colonization of the infant microbiome and its metabolites, specifically neurotransmitters, immune modulators, vitamins and short chain fatty acids. Successful microbiome maturation and gut-brain axis function depends on maternal factors (stress, exposure to toxins during pregnancy), mode of delivery, quality of the postnatal environment, diet after weaning from breast milk and nutritional deficiencies. While the neonatal microbiome is highly plastic, it remains prone to dysbiosis which, once established, may persist into adulthood, thereby inducing development of chronic inflammation and abnormal excitatory/inhibitory balance resulting in neural excitation. Both are recognized as key pathophysiological processes in the development of ALS.
Keywords:
1. Introduction
2. Maturation of the Microbiome
3. Brain Development and Gut Microbiome
4. Neonatal Gut Microbiome and Immunity
5. Neonatal Dysbiosis Induces Neurodegeneration
6. Neurotransmitters and the Excitatory/Inhibitory Balance
7. Changes in Brain Permeability
8. Mitochondrial Dysfunction and Microbiome
9. Future Considerations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Levison, L.S.; Blicher, J.U.; Andersen, H. Incidence and mortality of ALS: a 42-year population-based nationwide study. J Neurol 2024, 272, 44. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, R.; Peelo, C.; Galvin, M.; Heverin, M.; Hardiman, O. Epidemiologic Trends of Amyotrophic Lateral Sclerosis in Ireland, 1996-2021. Neurology 2023, 101, e1905–e1912. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat Rev Immunol 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Savelieff, M.G.; Jang, D.G.; Hur, J.; Feldman, E.L. The amyotrophic lateral sclerosis exposome: recent advances and future directions. Nat Rev Neurol 2023, 19, 617–634. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 2021, 17, 104–118. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Gonzalez, A.; Fullaondo, A.; Odriozola, A. Impact of evolution on lifestyle in microbiome. Adv Genet 2024, 111, 149–198. [Google Scholar] [CrossRef]
- Mitra, S.; Banik, A.; Saurabh, S.; Maulik, M.; Khatri, S.N. Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders. J Neurosci 2022, 42, 1888–1907. [Google Scholar] [CrossRef]
- Manske, S. Lifestyle Medicine and the Microbiome: Holistic Prevention and Treatment. Integr Med (Encinitas) 2024, 23, 10–14. [Google Scholar]
- Kwao-Zigah, G.; Bediako-Bowan, A.; Boateng, P.A.; Aryee, G.K.; Abbang, S.M.; Atampugbire, G.; Quaye, O.; Tagoe, E.A. Microbiome Dysbiosis, Dietary Intake and Lifestyle-Associated Factors Involve in Epigenetic Modulations in Colorectal Cancer: A Narrative Review. Cancer Control 2024, 31, 10732748241263650. [Google Scholar] [CrossRef]
- Kelsen, J.R.; Wu, G.D. The gut microbiota, environment and diseases of modern society. Gut Microbes 2012, 3, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O. Non-bacteria microbiome (virus, fungi, and archaea) in gastrointestinal cancer. J Gastroenterol Hepatol 2022, 37, 256–262. [Google Scholar] [CrossRef]
- Kawano-Sugaya, T.; Arikawa, K.; Saeki, T.; Endoh, T.; Kamata, K.; Matsuhashi, A.; Hosokawa, M. A single amplified genome catalog reveals the dynamics of mobilome and resistome in the human microbiome. Microbiome 2024, 12, 188. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: a brief review. Front Pediatr 2015, 3, 17. [Google Scholar] [CrossRef]
- Brines, J.; Rigourd, V.; Billeaud, C. The First 1000 Days of Infant. Healthcare (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging - relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Khalil, M.; Di Ciaula, A.; Mahdi, L.; Jaber, N.; Di Palo, D.M.; Graziani, A.; Baffy, G.; Portincasa, P. Unraveling the Role of the Human Gut Microbiome in Health and Diseases. Microorganisms 2024, 12. [Google Scholar] [CrossRef]
- Keshet, A.; Segal, E. Identification of gut microbiome features associated with host metabolic health in a large population-based cohort. Nat Commun 2024, 15, 9358. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Kandpal, M.; Varshney, N.; Rawal, K.S.; Jha, H.C. Gut dysbiosis and neurological modalities: An engineering approach via proteomic analysis of gut-brain axis. Adv Protein Chem Struct Biol 2024, 140, 199–248. [Google Scholar] [CrossRef] [PubMed]
- Pan, I.; Issac, P.K.; Rahman, M.M.; Guru, A.; Arockiaraj, J. Gut-Brain Axis a Key Player to Control Gut Dysbiosis in Neurological Diseases. Mol Neurobiol 2024, 61, 9873–9891. [Google Scholar] [CrossRef] [PubMed]
- Cavaleri, F. Paradigm shift redefining molecular, metabolic and structural events in Alzheimer's disease involves a proposed contribution by transition metals. Defined lengthy preclinical stage provides new hope to circumvent advancement of disease- and age-related neurodegeneration. Med Hypotheses 2015, 84, 460–469. [Google Scholar] [CrossRef]
- Poewe, W. Parkinson's disease and the quest for preclinical diagnosis: an interview with Professor Werner Poewe. Neurodegener Dis Manag 2017, 7, 273–277. [Google Scholar] [CrossRef]
- Eisen, A.; Kiernan, M.; Mitsumoto, H.; Swash, M. Amyotrophic lateral sclerosis: a long preclinical period? J Neurol Neurosurg Psychiatry 2014, 85, 1232–1238. [Google Scholar] [CrossRef]
- Aparicio, R.; Schmid, E.T.; Walker, D.W. Gut mitochondrial defects drive neurodegeneration. Nat Aging 2022, 2, 277–279. [Google Scholar] [CrossRef]
- Park, K.J.; Gao, Y. Gut-brain axis and neurodegeneration: mechanisms and therapeutic potentials. Front Neurosci 2024, 18, 1481390. [Google Scholar] [CrossRef]
- Cuffaro, F.; Lamminpaa, I.; Niccolai, E.; Amedei, A. Nutritional and Microbiota-Based Approaches in Amyotrophic Lateral Sclerosis: From Prevention to Treatment. Nutrients 2024, 17. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Ziemann, U.; Eisen, A. Amyotrophic lateral sclerosis: Origins traced to impaired balance between neural excitation and inhibition in the neonatal period. Muscle Nerve 2019, 60, 232–235. [Google Scholar] [CrossRef]
- Collado, M.C.; Devkota, S.; Ghosh, T.S. Gut microbiome: a biomedical revolution. Nat Rev Gastroenterol Hepatol 2024, 21, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Taddei, C.R.; Neu, J. Editorial: Microbiome in the first 1000 days: multi-omic interactions, physiological effects, and clinical implications. Front Cell Infect Microbiol 2023, 13, 1242626. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth - First 1000 Days and Beyond. Trends Microbiol 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.t.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Willyard, C. How gut microbes could drive brain disorders. Nature 2021, 590, 22–25. [Google Scholar] [CrossRef]
- Rykalo, N.; Riehl, L.; Kress, M. The gut microbiome and the brain. Curr Opin Support Palliat Care 2024. [Google Scholar] [CrossRef]
- Molinero, N.; Anton-Fernandez, A.; Hernandez, F.; Avila, J.; Bartolome, B.; Moreno-Arribas, M.V. Gut Microbiota, an Additional Hallmark of Human Aging and Neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef]
- Kargbo, R.B. Microbiome-Gut-Brain Axis Modulation: New Approaches in Treatment of Parkinson's Disease and Amyotrophic Lateral Sclerosis. ACS Med Chem Lett 2023, 14, 886–888. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med 2021, 19, 13. [Google Scholar] [CrossRef]
- Hertzberg, V.S.; Singh, H.; Fournier, C.N.; Moustafa, A.; Polak, M.; Kuelbs, C.A.; Torralba, M.G.; Tansey, M.G.; Nelson, K.E.; Glass, J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph Lateral Scler Frontotemporal Degener 2022, 23, 91–99. [Google Scholar] [CrossRef]
- Sun, J.; Huang, T.; Debelius, J.W.; Fang, F. Gut microbiome and amyotrophic lateral sclerosis: A systematic review of current evidence. J Intern Med 2021, 290, 758–788. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Sarnelli, G.; Esposito, G. Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson's disease. Neural Regen Res 2020, 15, 1037–1038. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association With Neurodevelopmental Psychiatric Disorders. Front Cell Dev Biol 2022, 10, 880544. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Cohen, D.G.; Wingert, R.A. Forever young by Alpha(diversity)ville: restricting intestinal microbiome maturation stunts immune system development and increases susceptibility to infection. Tissue Barriers 2024, 12, 2281209. [Google Scholar] [CrossRef]
- Simon, D.A.; Kellermayer, R. Disturbed Pediatric Gut Microbiome Maturation in the Developmental Origins of Subsequent Chronic Disease. J Pediatr Gastroenterol Nutr 2023, 76, 123–127. [Google Scholar] [CrossRef]
- Leech, S.M.; Borg, D.J.; Rae, K.M.; Kumar, S.; Clifton, V.L.; Dekker Nitert, M. Delivery mode is a larger determinant of infant gut microbiome composition at 6 weeks than exposure to peripartum antibiotics. Microb Genom 2024, 10. [Google Scholar] [CrossRef]
- Aagaard, K.M. Mode of delivery and pondering potential sources of the neonatal microbiome. EBioMedicine 2020, 51, 102554. [Google Scholar] [CrossRef]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; A, D.L.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016, 8, 343ra382. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N.; Roux, P.F.; Boireau-Adamezyk, E.; Lboukili, I.; Oddos, T. Skin maturation from birth to 10 years of age: Structure, function, composition and microbiome. Exp Dermatol 2023, 32, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Monteiro-Silva, F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent Res J (Isfahan) 2014, 11, 291–301. [Google Scholar]
- Xiao, S.; Zhou, W.; Caldwell, R.; Decker, S.; Oh, J.; Milstone, A.M. Association of Neonatal and Maternal Nasal Microbiome Among Neonates in the Intensive Care Unit. Open Forum Infect Dis 2024, 11, ofae644. [Google Scholar] [CrossRef]
- Jaspan, H.B.; Mitchell, C.M.; Happel, A.U. The vagina question: Can maternal vaginal fluid impact the infant gut microbiome and neurodevelopment? Cell Host Microbe 2023, 31, 1084–1086. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Barman, D.; Tripathi, D.; Dutta, S.; Bhattacharya, C.; Alam, M.; Choudhury, P.; Devi, U.; Mahanta, J.; Rasaily, R.; et al. Influence of Maternal Breast Milk and Vaginal Microbiome on Neonatal Gut Microbiome: a Longitudinal Study during the First Year. Microbiol Spectr 2023, 11, e0496722. [Google Scholar] [CrossRef]
- Zemanick, E.T.; Rosas-Salazar, C. The Role of the Microbiome in Pediatric Respiratory Diseases. Clin Chest Med 2024, 45, 587–597. [Google Scholar] [CrossRef]
- Kloepfer, K.M.; Kennedy, J.L. Childhood respiratory viral infections and the microbiome. J Allergy Clin Immunol 2023, 152, 827–834. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O'Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Tarrazo, M.; Garcia-Mantrana, I.; Gomez-Gallego, C.; Salminen, S.; Collado, M.C. Shaping Microbiota During the First 1000 Days of Life. Adv Exp Med Biol 2019, 1125, 3–24. [Google Scholar] [CrossRef]
- Beharry, K.D.; Latkowska, M.; Valencia, A.M.; Allana, A.; Soto, J.; Cai, C.L.; Golombek, S.; Hand, I.; Aranda, J.V. Factors Influencing Neonatal Gut Microbiome and Health with a Focus on Necrotizing Enterocolitis. Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; de Goffau, M.C.; Perez-Munoz, M.E.; Arrieta, M.C.; Backhed, F.; Bork, P.; Braun, T.; Bushman, F.D.; Dore, J.; de Vos, W.M.; et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 2023, 613, 639–649. [Google Scholar] [CrossRef]
- Briana, D.D.; Papaevangelou, V.; Malamitsi-Puchner, A. The jury is still out on the existence of a placental microbiome. Acta Paediatr 2021, 110, 2958–2963. [Google Scholar] [CrossRef]
- Stupak, A.; Geca, T.; Kwasniewska, A.; Mlak, R.; Piwowarczyk, P.; Nawrot, R.; Gozdzicka-Jozefiak, A.; Kwasniewski, W. Comparative Analysis of the Placental Microbiome in Pregnancies with Late Fetal Growth Restriction versus Physiological Pregnancies. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Zakis, D.R.; Paulissen, E.; Kornete, L.; Kaan, A.M.M.; Nicu, E.A.; Zaura, E. The evidence for placental microbiome and its composition in healthy pregnancies: A systematic review. J Reprod Immunol 2022, 149, 103455. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Jarvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J Allergy Clin Immunol 2022, 150, 523–534. [Google Scholar] [CrossRef]
- Xu, D.; Wan, F. Breastfeeding and infant gut microbiota: influence of bioactive components. Gut Microbes 2025, 17, 2446403. [Google Scholar] [CrossRef]
- Duman, H.; Bechelany, M.; Karav, S. Human Milk Oligosaccharides: Decoding Their Structural Variability, Health Benefits, and the Evolution of Infant Nutrition. Nutrients 2024, 17. [Google Scholar] [CrossRef]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front Cell Neurosci 2017, 11, 25. [Google Scholar] [CrossRef]
- Tapiainen, T.; Paalanne, N.; Tejesvi, M.V.; Koivusaari, P.; Korpela, K.; Pokka, T.; Salo, J.; Kaukola, T.; Pirttila, A.M.; Uhari, M.; Renko, M. Maternal influence on the fetal microbiome in a population-based study of the first-pass meconium. Pediatr Res 2018, 84, 371–379. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Coley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef]
- Samarra, A.; Flores, E.; Bernabeu, M.; Cabrera-Rubio, R.; Bauerl, C.; Selma-Royo, M.; Collado, M.C. Shaping Microbiota During the First 1000 Days of Life. Adv Exp Med Biol 2024, 1449, 1–28. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Dejardin, F.; Sparwasser, T.; Berard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288. [Google Scholar] [CrossRef]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of Human Milk Bioactives on Infants' Gut and Immune Health. Front Immunol 2021, 12, 604080. [Google Scholar] [CrossRef]
- Notarbartolo, V.; Carta, M.; Accomando, S.; Giuffre, M. The First 1000 Days of Life: How Changes in the Microbiota Can Influence Food Allergy Onset in Children. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Sun, J. The First 1000 Days: Assembly of the Neonatal Microbiome and Its Impact on Health Outcomes. Newborn (Clarksville) 2022, 1, 219–226. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Nandwana, V.; Nandwana, N.K.; Das, Y.; Saito, M.; Panda, T.; Das, S.; Almaguel, F.; Hosmane, N.S.; Das, B.C. The Role of Microbiome in Brain Development and Neurodegenerative Diseases. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Frerichs, N.M.; de Meij, T.G.J.; Niemarkt, H.J. Microbiome and its impact on fetal and neonatal brain development: current opinion in pediatrics. Curr Opin Clin Nutr Metab Care 2024, 27, 297–303. [Google Scholar] [CrossRef]
- Dubey, H.; Roychoudhury, R.; Alex, A.; Best, C.; Liu, S.; White, A.; Carlson, A.; Azcarate-Peril, M.A.; Mansfield, L.S.; Knickmeyer, R. Effect of Human Infant Gut Microbiota on Mouse Behavior, Dendritic Complexity, and Myelination. bioRxiv 2023. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp Biol Med (Maywood) 2018, 243, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Amir, M.; Ananthanarayanan, A.; Singaraju, A.; Shiland, N.B.; Hong, H.S.; Kamada, N.; Inohara, N.; Nunez, G.; Zeng, M.Y. Maternal gut microbiome-induced IgG regulates neonatal gut microbiome and immunity. Sci Immunol 2022, 7, eabh3816. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; Mazmanian, S.K. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Peng, W.; Mao, X. Glial polarization in neurological diseases: molecular mechanisms and therapeutic opportunities. Ageing Res Rev 2024, 102638. [Google Scholar] [CrossRef]
- Lopez-Ortiz, A.O.; Eyo, U.B. Astrocytes and microglia in the coordination of CNS development and homeostasis. J Neurochem 2024, 168, 3599–3614. [Google Scholar] [CrossRef]
- Pereira-Iglesias, M.; Maldonado-Teixido, J.; Melero, A.; Piriz, J.; Galea, E.; Ransohoff, R.M.; Sierra, A. Microglia as hunters or gatherers of brain synapses. Nat Neurosci 2024. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Eyo, U.; Molofsky, A.V. Defining microglial-synapse interactions. Science 2023, 381, 1155–1156. [Google Scholar] [CrossRef]
- Underwood, M.A.; Mukhopadhyay, S.; Lakshminrusimha, S.; Bevins, C.L. Neonatal intestinal dysbiosis. J Perinatol 2020, 40, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Apostol, A.C.; Jensen, K.D.C.; Beaudin, A.E. Training the Fetal Immune System Through Maternal Inflammation-A Layered Hygiene Hypothesis. Front Immunol 2020, 11, 123. [Google Scholar] [CrossRef]
- Jeurink, P.V.; Knipping, K.; Wiens, F.; Baranska, K.; Stahl, B.; Garssen, J.; Krolak-Olejnik, B. Importance of maternal diet in the training of the infant's immune system during gestation and lactation. Crit Rev Food Sci Nutr 2019, 59, 1311–1319. [Google Scholar] [CrossRef]
- Batty, G.D.; Kivimaki, M.; Frank, P.; Gale, C.R.; Wright, L. Systemic inflammation and subsequent risk of amyotrophic lateral sclerosis: Prospective cohort study. Brain Behav Immun 2023, 114, 46–51. [Google Scholar] [CrossRef]
- Appel, S.H.; Beers, D.R.; Zhao, W. Amyotrophic lateral sclerosis is a systemic disease: peripheral contributions to inflammation-mediated neurodegeneration. Curr Opin Neurol 2021, 34, 765–772. [Google Scholar] [CrossRef]
- Beland, L.C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: blurred lines between excessive inflammation and inefficient immune responses. Brain Commun 2020, 2, fcaa124. [Google Scholar] [CrossRef]
- Eisen, A.; Pioro, E.P.; Goutman, S.A.; Kiernan, M.C. Nanoplastics and Neurodegeneration in ALS. Brain Sci 2024, 14. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; Kril, J.J. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 2019, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Henderson, R.D.; Mathers, S.; Needham, M.; Schultz, D.; Kiernan, M.C.; group, T.s. Safety and efficacy of dimethyl fumarate in ALS: randomised controlled study. Ann Clin Transl Neurol 2021, 8, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Del Tredici, K.; Braak, H. Neuropathology and neuroanatomy of TDP-43 amyotrophic lateral sclerosis. Curr Opin Neurol 2022, 35, 660–671. [Google Scholar] [CrossRef]
- Pongracova, E.; Buratti, E.; Romano, M. Prion-like Spreading of Disease in TDP-43 Proteinopathies. Brain Sci 2024, 14. [Google Scholar] [CrossRef]
- Bright, F.; Chan, G.; van Hummel, A.; Ittner, L.M.; Ke, Y.D. TDP-43 and Inflammation: Implications for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Tamaki, Y.; Urushitani, M. Molecular Dissection of TDP-43 as a Leading Cause of ALS/FTLD. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Park, S.B. Hyperexcitability, neurodegeneration, and disease progression in amyotrophic lateral sclerosis. Muscle Nerve 2023, 68, 103–105. [Google Scholar] [CrossRef]
- Vucic, S.; Cheah, B.C.; Kiernan, M.C. Defining the mechanisms that underlie cortical hyperexcitability in amyotrophic lateral sclerosis. Exp Neurol 2009, 220, 177–182. [Google Scholar] [CrossRef]
- Odierna, G.L.; Vucic, S.; Dyer, M.; Dickson, T.; Woodhouse, A.; Blizzard, C. How do we get from hyperexcitability to excitotoxicity in amyotrophic lateral sclerosis? Brain 2024, 147, 1610–1621. [Google Scholar] [CrossRef]
- Odierna, G.L.; Vucic, S.; Dyer, M.; Dickson, T.; Woodhouse, A.; Blizzard, C. How do we get from hyperexcitability to excitotoxicity in amyotrophic lateral sclerosis? Brain 2024. [Google Scholar] [CrossRef] [PubMed]
- Fogal, B.; Hewett, S.J. Interleukin-1beta: a bridge between inflammation and excitotoxicity? J Neurochem 2008, 106, 1–23. [Google Scholar] [CrossRef]
- Zamudio, F.; Loon, A.R.; Smeltzer, S.; Benyamine, K.; Navalpur Shanmugam, N.K.; Stewart, N.J.F.; Lee, D.C.; Nash, K.; Selenica, M.B. TDP-43 mediated blood-brain barrier permeability and leukocyte infiltration promote neurodegeneration in a low-grade systemic inflammation mouse model. J Neuroinflammation 2020, 17, 283. [Google Scholar] [CrossRef]
- Compare, D.; Sgamato, C.; Rocco, A.; Coccoli, P.; Ambrosio, C.; Nardone, G. The Leaky Gut and Human Diseases: "Can't Fill the Cup if You Don't Plug the Holes First". Dig Dis 2024, 42, 548–566. [Google Scholar] [CrossRef]
- Camilleri, M. What is the leaky gut? Clinical considerations in humans. Curr Opin Clin Nutr Metab Care 2021, 24, 473–482. [Google Scholar] [CrossRef]
- Escalante, J.; Artaiz, O.; Diwakarla, S.; McQuade, R.M. Leaky gut in systemic inflammation: exploring the link between gastrointestinal disorders and age-related diseases. Geroscience 2024. [Google Scholar] [CrossRef]
- Zeng, M.; Peng, M.; Liang, J.; Sun, H. The Role of Gut Microbiota in Blood-Brain Barrier Disruption after Stroke. Mol Neurobiol 2024, 61, 9735–9755. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J.; Wan, M.L.Y.; Monteiro, A.R.; Nutt, D.J.; Swinny, J.D. From bugs to brain: unravelling the GABA signalling networks in the brain-gut-microbiome axis. Brain 2024. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front Physiol 2023, 14, 1077821. [Google Scholar] [CrossRef]
- Kern, L.; Mastandrea, I.; Melekhova, A.; Elinav, E. Mechanisms by which microbiome-derived metabolites exert their impacts on neurodegeneration. Cell Chem Biol 2024. [Google Scholar] [CrossRef]
- Bayer, S.; Jellali, A.; Crenner, F.; Aunis, D.; Angel, F. Functional evidence for a role of GABA receptors in modulating nerve activities of circular smooth muscle from rat colon in vitro. Life Sci 2003, 72, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Uliana, D.L.; Lisboa, J.R.F.; Gomes, F.V.; Grace, A.A. The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia. Biochem Pharmacol 2024, 228, 116298. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Lin, Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol Med 2011, 17, 452–462. [Google Scholar] [CrossRef]
- Taube, W.; Lauber, B. Changes in the cortical GABAergic inhibitory system with ageing and ageing-related neurodegenerative diseases. J Physiol 2024. [Google Scholar] [CrossRef]
- Wang, D.D.; Kriegstein, A.R. Defining the role of GABA in cortical development. J Physiol 2009, 587, 1873–1879. [Google Scholar] [CrossRef]
- Kilb, W. Development of the GABAergic system from birth to adolescence. Neuroscientist 2012, 18, 613–630. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O'Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- McKeon, S.D.; Perica, M.I.; Parr, A.C.; Calabro, F.J.; Foran, W.; Hetherington, H.; Moon, C.H.; Luna, B. Aperiodic EEG and 7T MRSI evidence for maturation of E/I balance supporting the development of working memory through adolescence. Dev Cogn Neurosci 2024, 66, 101373. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis. Eur J Neurol 2014, 21, 1451–1457. [Google Scholar] [CrossRef]
- Menon, P.; Geevasinga, N.; van den Bos, M.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis. Eur J Neurol 2017, 24, 816–824. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Zhang, Y.; Xia, C.; Lai, Q.; Dong, Z.; Kuang, W.; Yang, C.; Su, D.; Li, H.; Zhong, Z. Potential effects of antibiotic-induced gut microbiome alteration on blood-brain barrier permeability compromise in rhesus monkeys. Ann N Y Acad Sci 2020, 1470, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci 2003, 23, 5816–5826. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Mentor, S. Are claudin-5 tight-junction proteins in the blood-brain barrier porous? Neural Regen Res 2020, 15, 1838–1839. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: far more than claudin-5. Cell Mol Life Sci 2019, 76, 1987–2002. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6. [Google Scholar] [CrossRef]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci Lett 2019, 710, 132933. [Google Scholar] [CrossRef] [PubMed]
- Zachos, K.A.; Gamboa, J.A.; Dewji, A.S.; Lee, J.; Brijbassi, S.; Andreazza, A.C. The interplay between mitochondria, the gut microbiome and metabolites and their therapeutic potential in primary mitochondrial disease. Front Pharmacol 2024, 15, 1428242. [Google Scholar] [CrossRef]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the gut microbiome and ROS. Cell Signal 2020, 75, 109737. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer's Disease. J Gerontol A Biol Sci Med Sci 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci Signal 2019, 12. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Lyon, M.S.; Caress, J.; Milligan, C. Inflammation, immunity, and amyotrophic lateral sclerosis: II. immune-modulating therapies. Muscle Nerve 2019, 59, 23–33. [Google Scholar] [CrossRef]
- Eisen, A.; Vucic, S.; Kiernan, M.C. Amyotrophic lateral sclerosis represents corticomotoneuronal system failure. Muscle Nerve 2024. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther 2024, 9, 37. [Google Scholar] [CrossRef]
- Al-Ali, D.; Ahmed, A.; Shafiq, A.; McVeigh, C.; Chaari, A.; Zakaria, D.; Bendriss, G. Fecal microbiota transplants: A review of emerging clinical data on applications, efficacy, and risks (2015-2020). Qatar Med J 2021, 2021, 5. [Google Scholar] [CrossRef]
- Abrahamsson, T. We need more evidence about the risks and benefits of giving children faecal microbiota transplants. Acta Paediatr 2024, 113, 1987–1988. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.B.; Hsiao, E.Y. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef]
- Boccuto, L.; Tack, J.; Ianiro, G.; Abenavoli, L.; Scarpellini, E. Human Genes Involved in the Interaction between Host and Gut Microbiome: Regulation and Pathogenic Mechanisms. Genes (Basel) 2023, 14. [Google Scholar] [CrossRef]
- Diener, C.; Dai, C.L.; Wilmanski, T.; Baloni, P.; Smith, B.; Rappaport, N.; Hood, L.; Magis, A.T.; Gibbons, S.M. Genome-microbiome interplay provides insight into the determinants of the human blood metabolome. Nat Metab 2022, 4, 1560–1572. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. The microbiome extends host evolutionary potential. Nat Commun 2021, 12, 5141. [Google Scholar] [CrossRef]
- Hurst, G.D.D. Extended genomes: symbiosis and evolution. Interface Focus 2017, 7, 20170001. [Google Scholar] [CrossRef]
- Wilde, J.; Slack, E.; Foster, K.R. Host control of the microbiome: Mechanisms, evolution, and disease. Science 2024, 385, eadi3338. [Google Scholar] [CrossRef]
- Obeng, N.; Czerwinski, A.; Schutz, D.; Michels, J.; Leipert, J.; Bansept, F.; Garcia Garcia, M.J.; Schultheiss, T.; Kemlein, M.; Fuss, J.; et al. Bacterial c-di-GMP has a key role in establishing host-microbe symbiosis. Nat Microbiol 2023, 8, 1809–1819. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Savidge, T.C. Leveraging human microbiomes for disease prediction and treatment. Trends Pharmacol Sci 2024. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Mazzini, L.; D'Alfonso, S.; Corrado, L.; Canosa, A.; Moglia, C.; Manera, U.; Bersano, E.; Brunetti, M.; Barberis, M.; et al. The multistep hypothesis of ALS revisited: The role of genetic mutations. Neurology 2018, 91, e635–e642. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Calvo, A.; Chio, A.; Colville, S.; Ellis, C.M.; Hardiman, O.; Heverin, M.; Howard, R.S.; Huisman, M.H.B.; Keren, N.; et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 2014, 13, 1108–1113. [Google Scholar] [CrossRef]
- Vucic, S.; Westeneng, H.J.; Al-Chalabi, A.; Van Den Berg, L.H.; Talman, P.; Kiernan, M.C. Amyotrophic lateral sclerosis as a multi-step process: an Australia population study. Amyotroph Lateral Scler Frontotemporal Degener 2019, 20, 532–537. [Google Scholar] [CrossRef]
- Vucic, S.; Higashihara, M.; Sobue, G.; Atsuta, N.; Doi, Y.; Kuwabara, S.; Kim, S.H.; Kim, I.; Oh, K.W.; Park, J.; et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 2020, 94, e1657–e1663. [Google Scholar] [CrossRef]
- Ziser, L.; van Eijk, R.P.A.; Kiernan, M.C.; McRae, A.; Henderson, R.D.; Schultz, D.; Needham, M.; Mathers, S.; McCombe, P.; Talman, P.; Vucic, S. Amyotrophic lateral sclerosis established as a multistep process across phenotypes. Eur J Neurol 2025, 32, e16532. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Kaul, M.; Mukherjee, D.; Weiner, H.L.; Cox, L.M. Gut microbiota immune cross-talk in amyotrophic lateral sclerosis. Neurotherapeutics 2024, 21, e00469. [Google Scholar] [CrossRef]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M.; et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol 2020, 131, 1975–1978. [Google Scholar] [CrossRef]
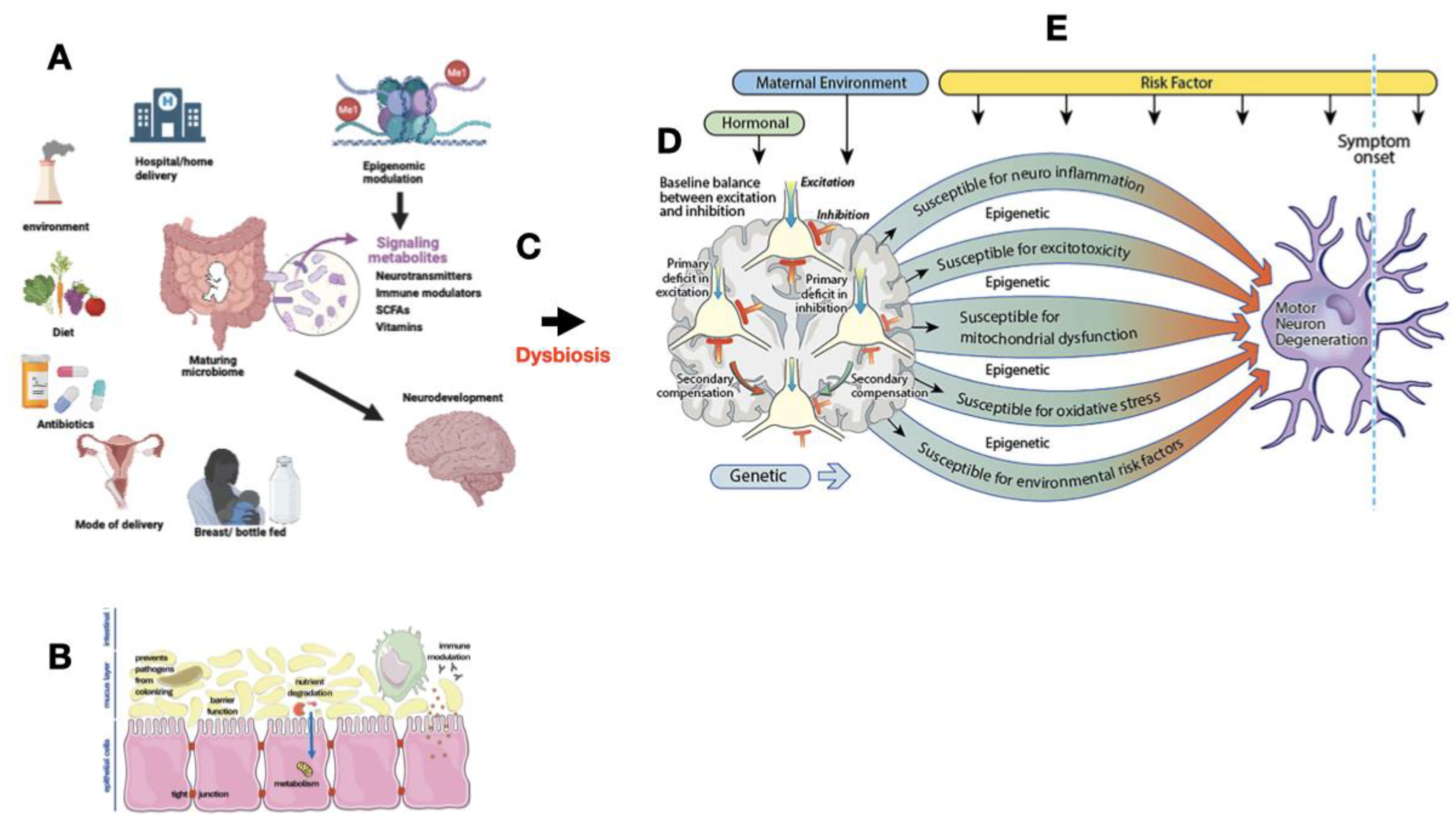
| 1 | Impaired immune system programming |
| 2 | Miscommunication through the Gut-Brain Axis |
| 3 | Metabolite toxicity |
| 4 | Blood brain barrier breakdown |
| 5 | Epigenetic modulation |
| 6 | Mitochondrial dysfunction |
| 7 | Misfolded protein aggregation |
| 8 | Dysregulation of hypothalamic-pituitary-adrenal axis |
| 9 | Altered neurotransmitter production |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





