Submitted:
23 January 2025
Posted:
23 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Method
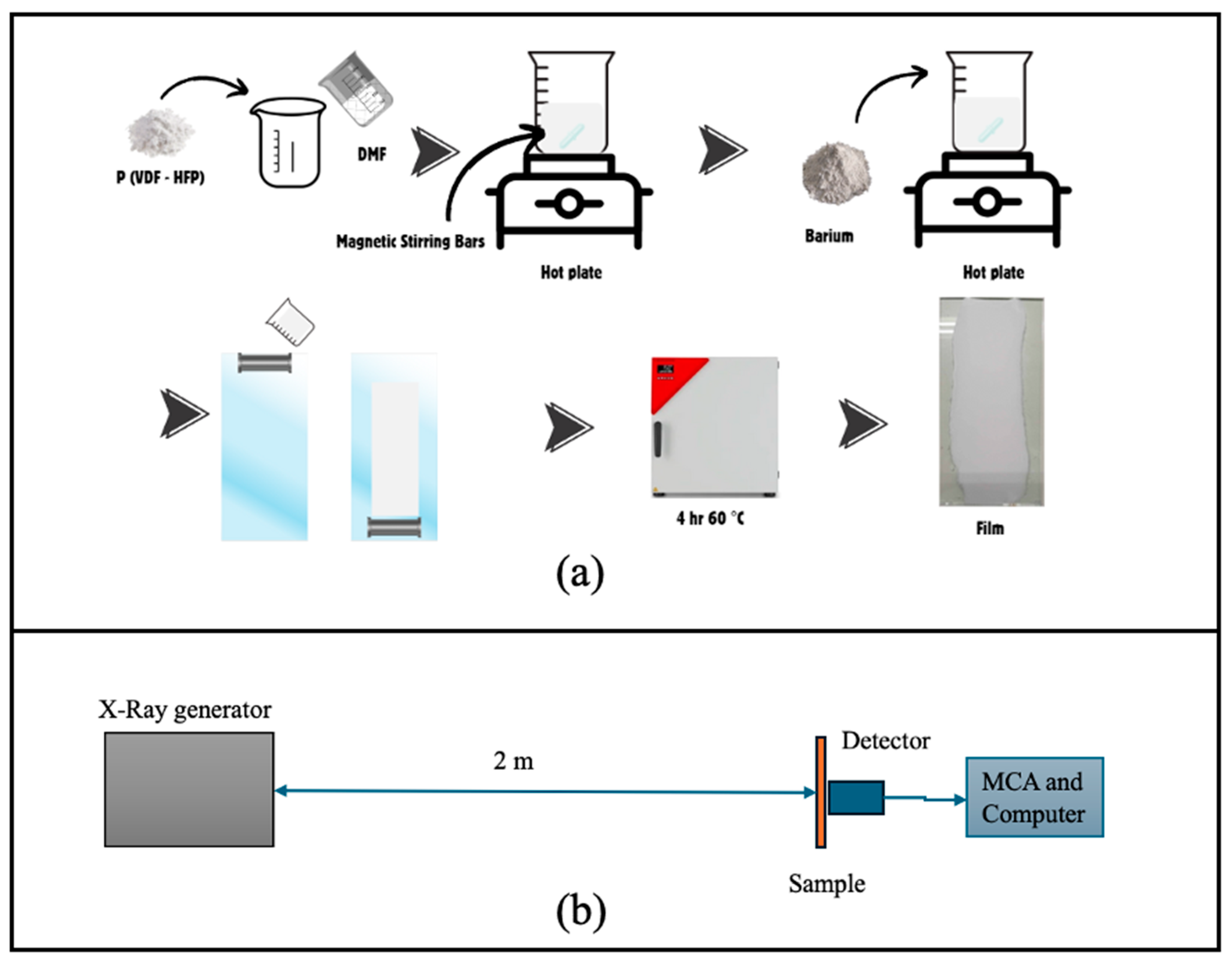
2.1. Materials and Film Preparation
- A.
-
Sample characterization
- 1.
- Surface Morphology
- 2.
- Hydrophobicity
- 3.
- Crystal Structures
- 4.
- Mechanical Property
- 5.
- Thermal Stability
- 6.
- Absorption performance
3. Results and Discussion
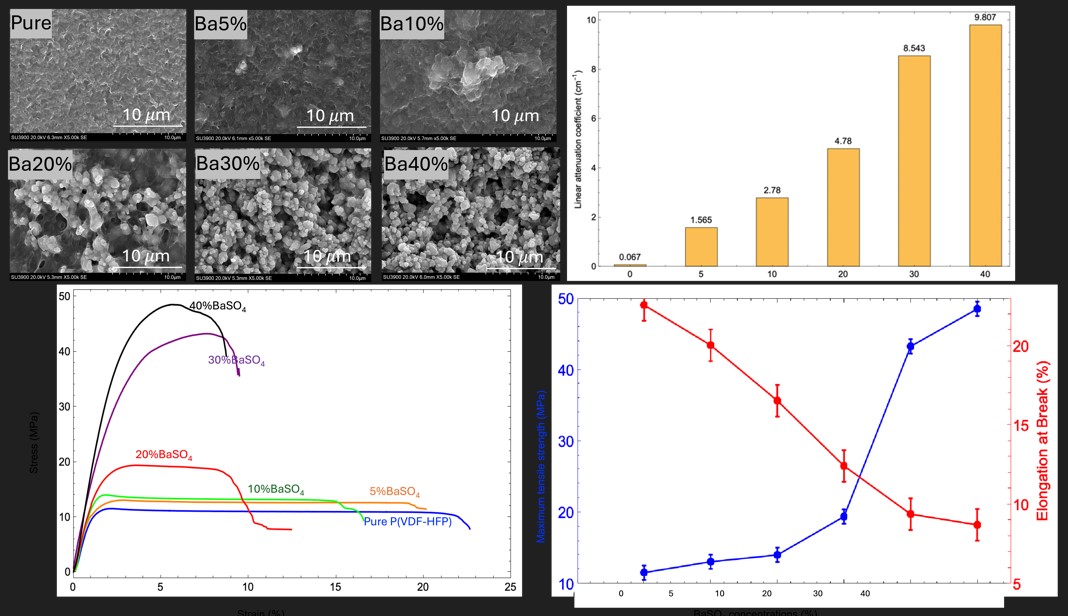
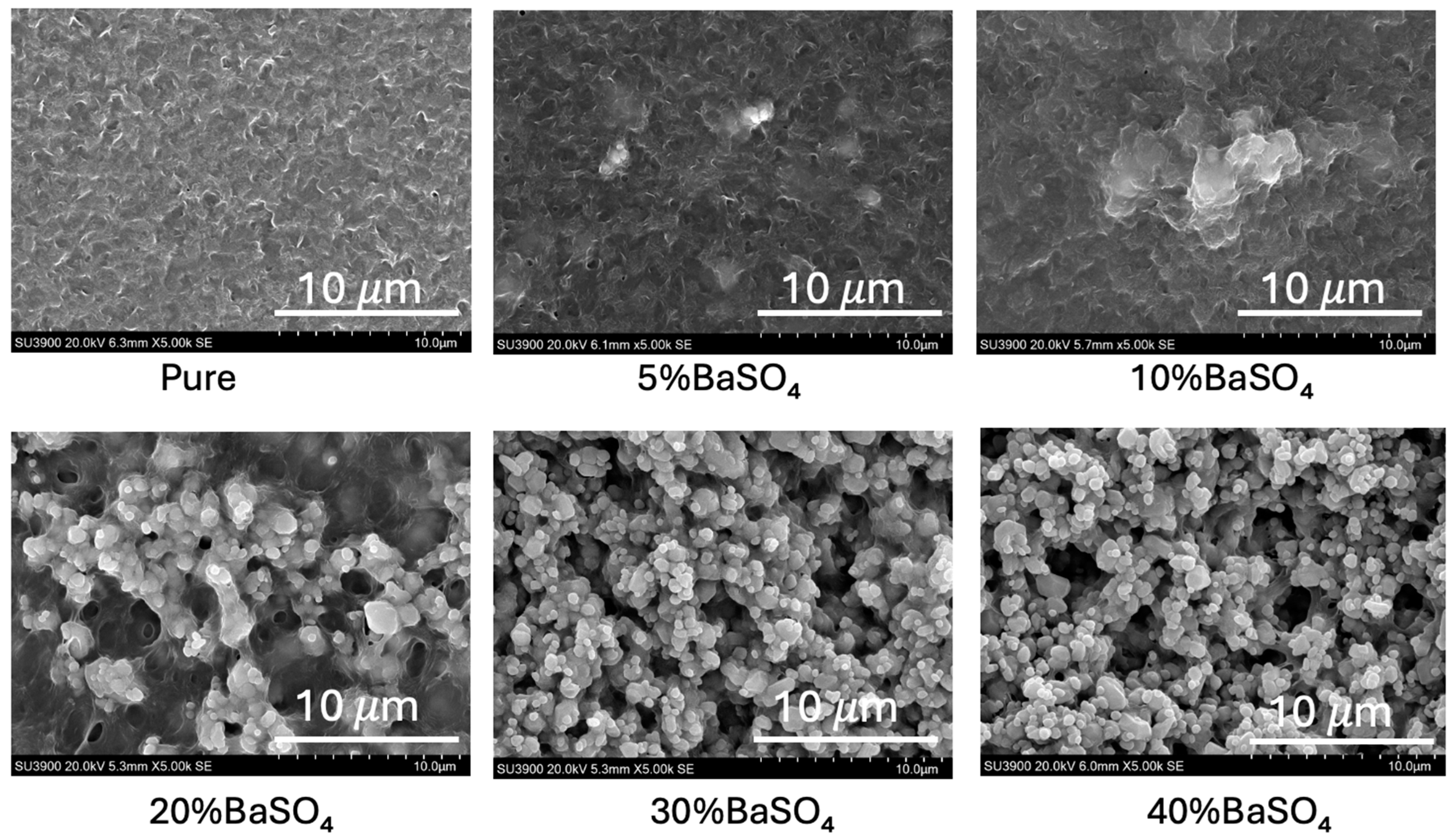
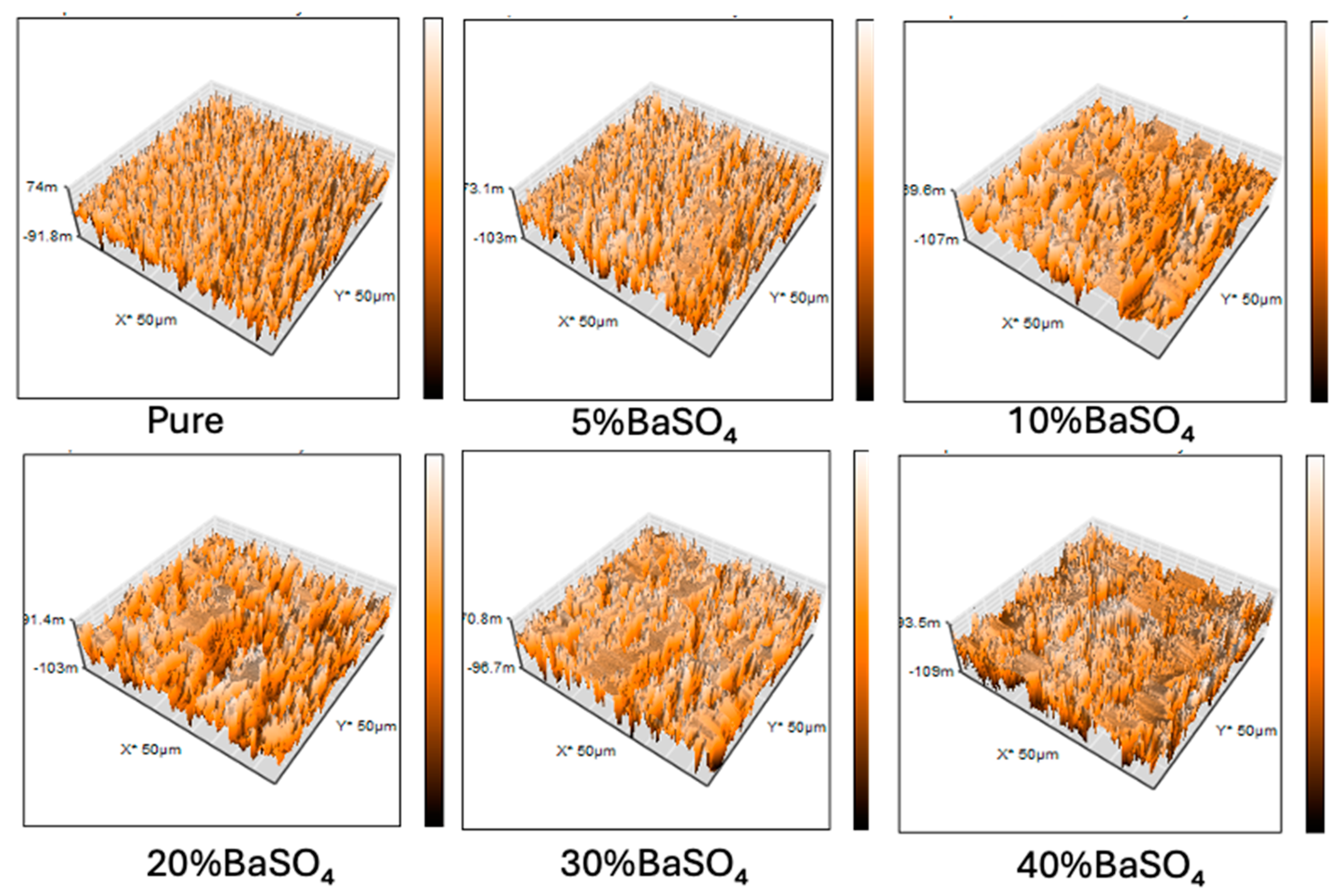
3.1. Surface Morphology
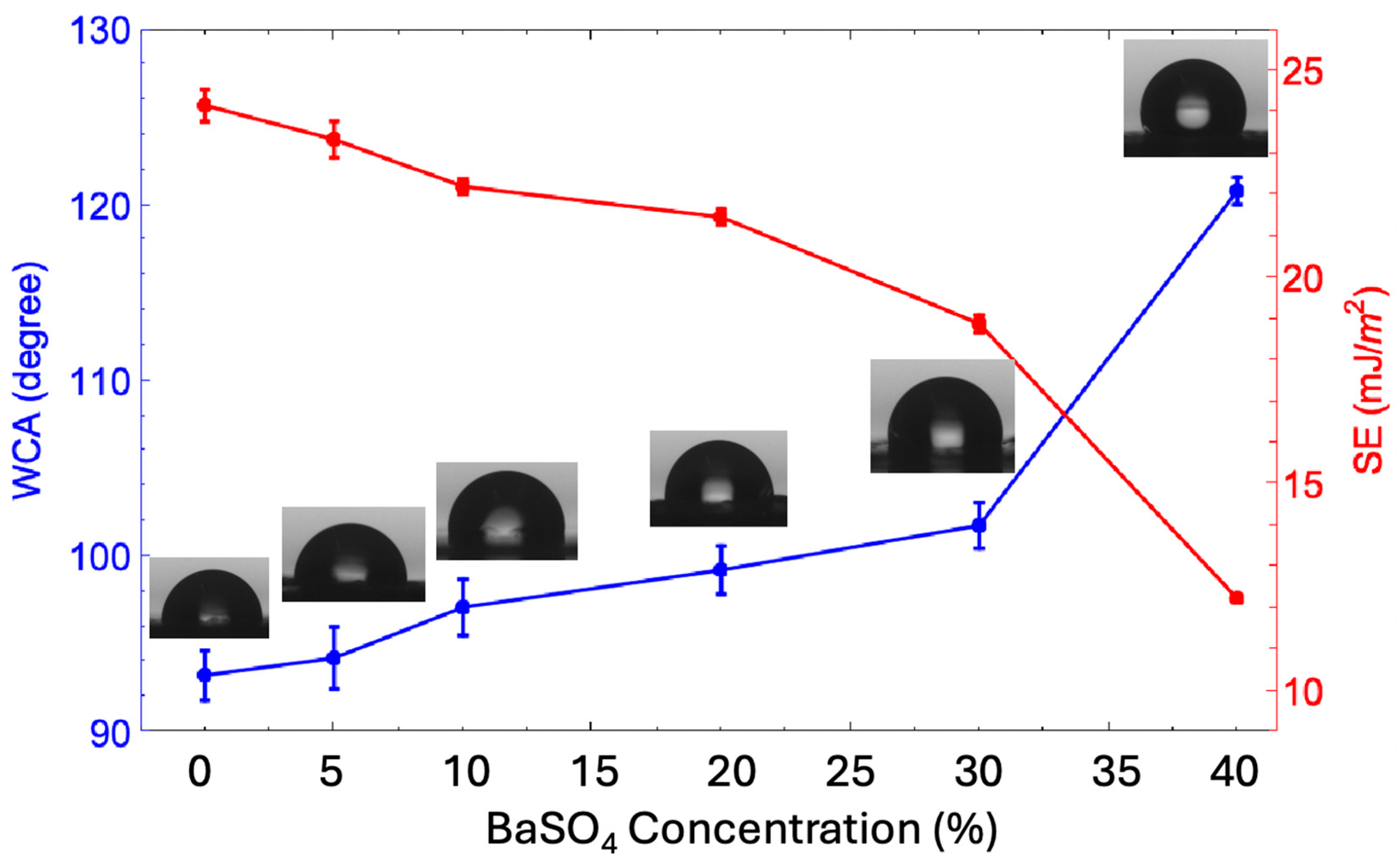
3.2. Hydrophobicity
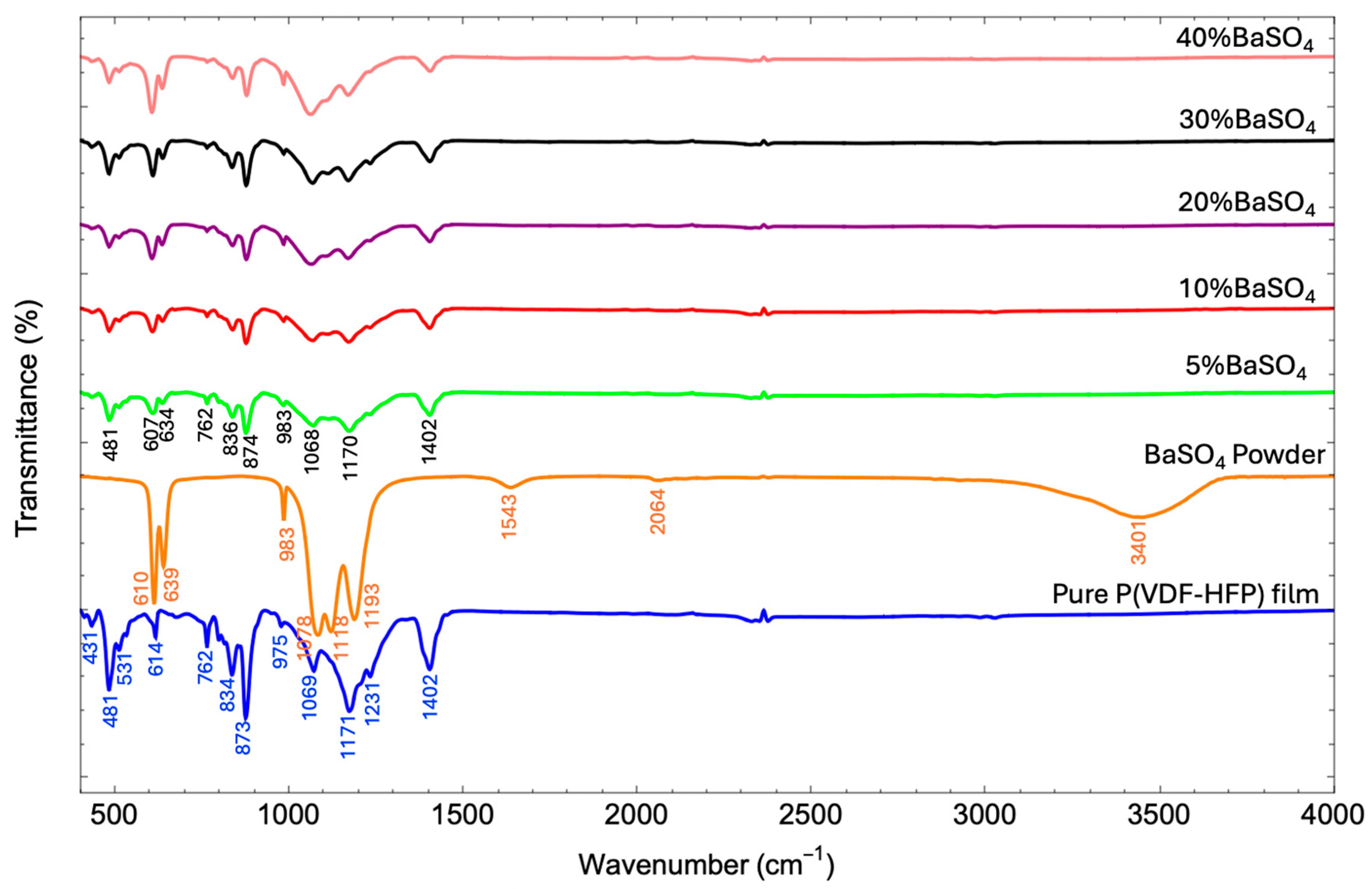
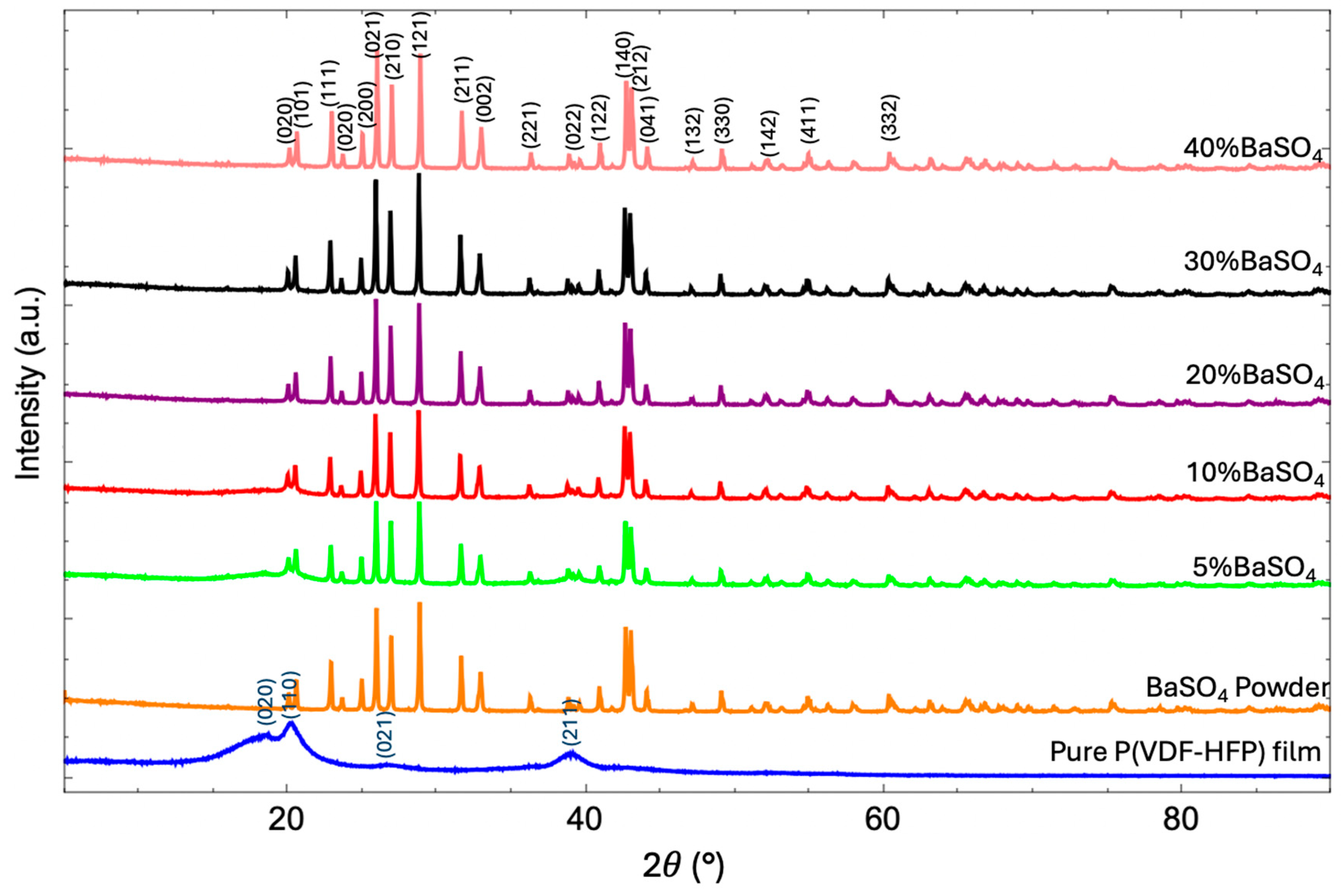
3.3. Crystal Structure
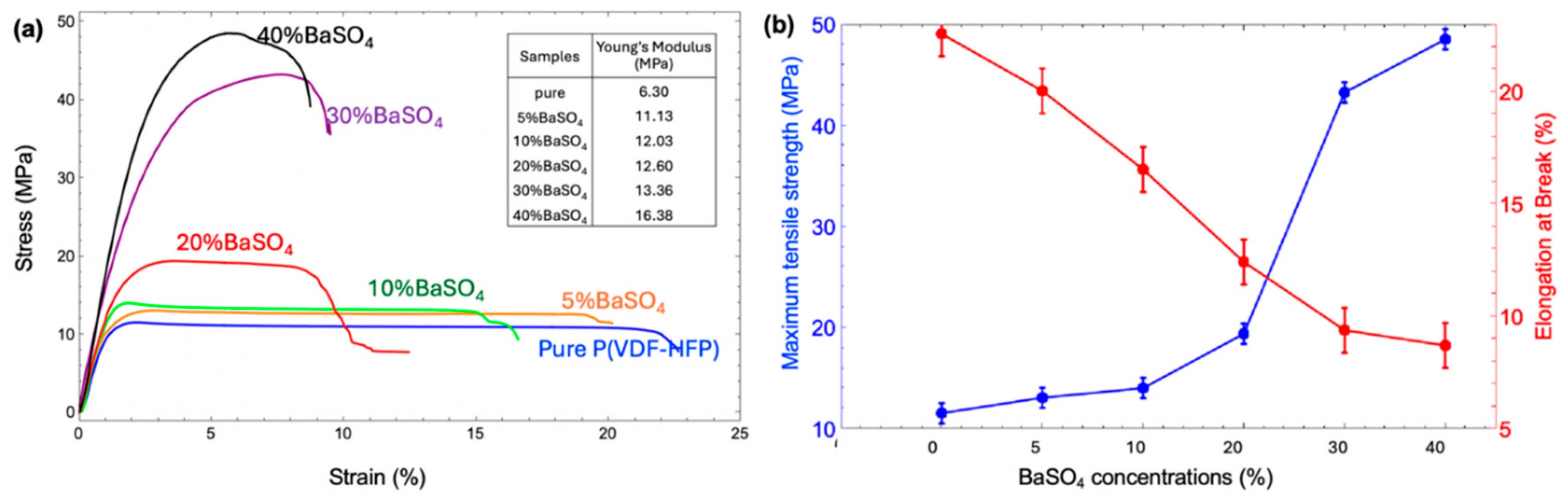
3.4. Mechanical Property
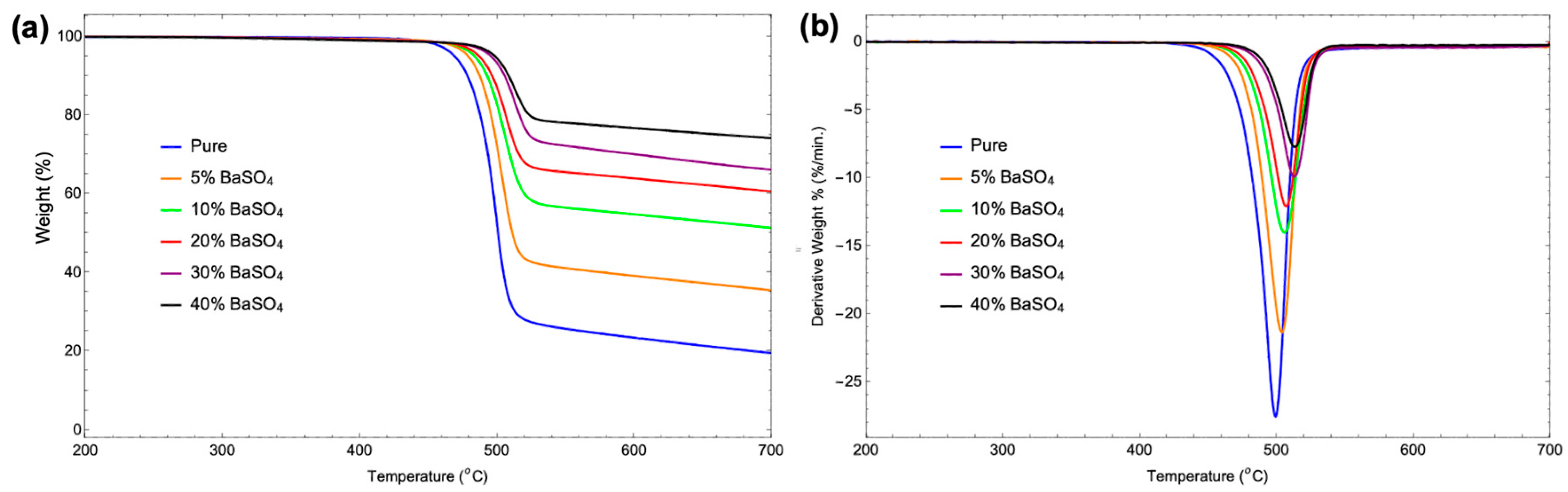
3.5. Thermal Stability
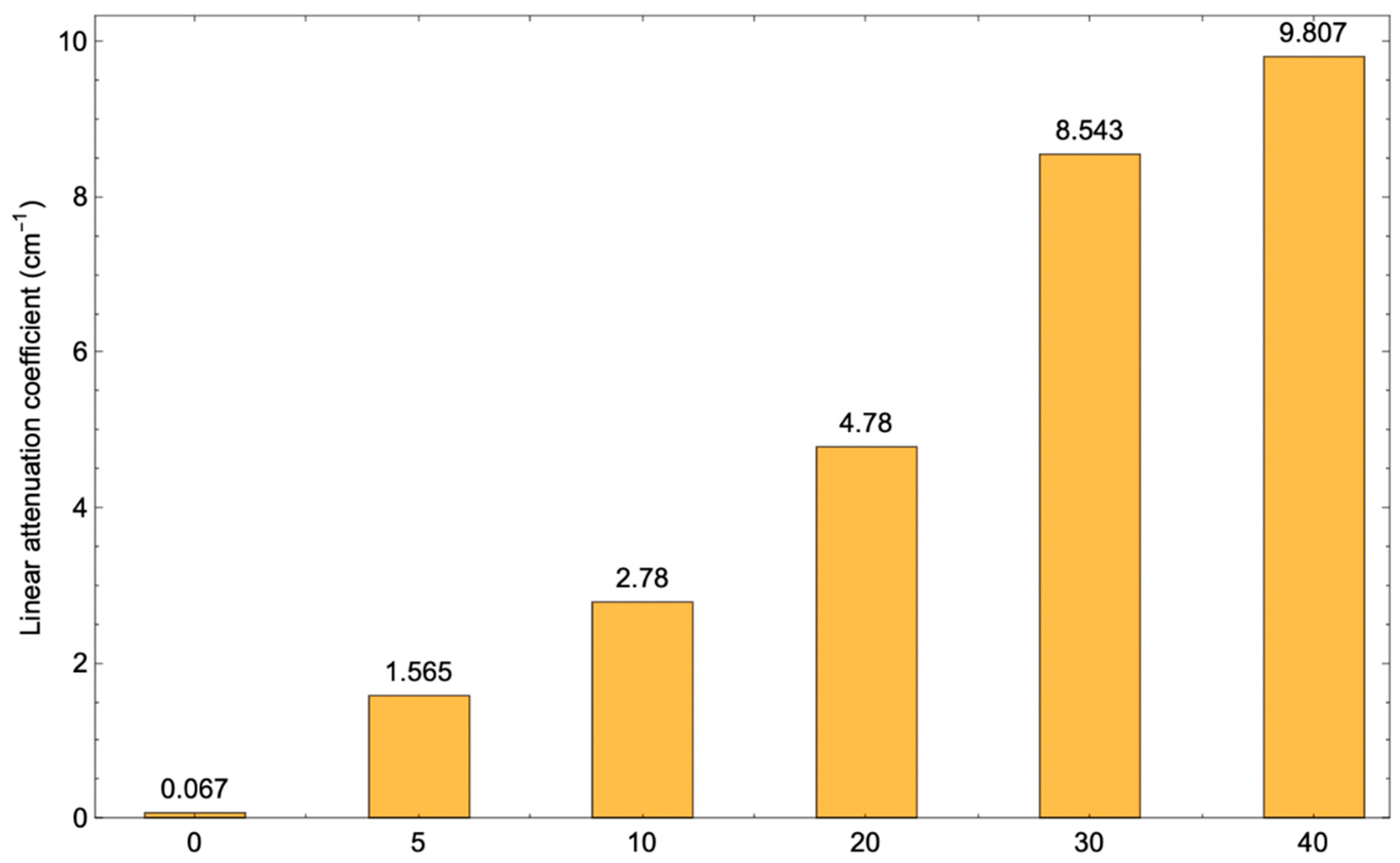
3.5. Absorption Performance
4. Conclusions
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghamdi et al., "Radiation Risk Awareness Among Health Care Professionals: An Online Survey," Journal of Radiology Nursing, vol. 39, pp. 132-138, 06/01 2020. [CrossRef]
- D. Miller and D. Schauer, "The ALARA principle in medical imaging," AAPM Newsletter, vol. 40, pp. 38-40, 01/01 2015.
- S. M. J. Mortazavi et al., "Lead-free, multilayered, and nanosized radiation shields in medical applications, industrial, and space research," 2024, pp. 305-322.
- K. Singh, R. K. Singh, B. Sharma, and A. K. Tyagi, "Characterization and biocompatibility studies of lead free X-ray shielding polymer composite for healthcare application," Radiation Physics and Chemistry (1993), pp. 9-15, 2017, doi: DOI:101016/jradphyschem201704016.
- L. Yu, P. L. Yap, A. Santos, D. Tran, and D. Losic, "Lightweight polyester fabric with elastomeric bismuth titanate composite for high-performing lead-free X-ray shielding," Radiation Physics and Chemistry, vol. 205, p. 110726, 12/01 2022. [CrossRef]
- S. Palanisami et al., "Lead-free X-Ray shielding aprons using Zn-doped SnO2 epoxy nanocomposite: A promising alternative to traditional heavy and lead-based materials," Optical Materials, vol. 145, p. 114496, 2023/11/01/ 2023. [CrossRef]
- S. Jayakumar, T. Saravanan, and J. Philip, "A review on polymer nanocomposites as lead-free materials for diagnostic X-ray shielding: Recent advances, challenges and future perspectives," Hybrid Advances, vol. 4, p. 100100, 2023/12/01/ 2023. [CrossRef]
- H. Alsaab and S. Zeghib, "Analysis of X-ray and gamma ray shielding performance of prepared polymer micro-composites," Journal of Radiation Research and Applied Sciences, vol. 16, no. 4, p. 100708, 2023/12/01/ 2023. [CrossRef]
- S.-C. Kim, "Construction of a Medical Radiation-Shielding Environment by Analyzing the Weaving Characteristics and Shielding Performance of Shielding Fibers Using X-ray-Impermeable Materials," Applied Sciences, vol. 11, no. 4. [CrossRef]
- J. Wang et al., "Preparation of eGaIn NDs/TPU Composites for X-ray Radiation Shielding Based on Electrostatic Spinning Technology," Materials, vol. 17, no. 2. [CrossRef]
- Bawazeer et al., "Evaluation of X-ray radiation shielding performance of Bi2O3 and BaTiO3 embedded in PVP and PEG polymer nanocomposite," Radiation Effects and Defects in Solids, 07/22 2024. [CrossRef]
- V. More, Z. Alsayed, M. S. Badawi, A. A. Thabet, and P. P. Pawar, "Polymeric composite materials for radiation shielding: a review," Environmental Chemistry Letters, vol. 19, no. 3, pp. 2057-2090, 2021/06/01 2021. [CrossRef]
- Yao, X. Li, K. G. Neoh, Z. Shi, and E. T. Kang, "Antibacterial activities of surface modified electrospun poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) fibrous membranes," Applied Surface Science, vol. 255, no. 6, pp. 3854-3858, 2009/01/01/ 2009. [CrossRef]
- Kazemi and M. R. Yaftian, "PVDF-HFP-based polymer inclusion membrane functionalized with D2EHPA for the selective extraction of bismuth(III) from sulfate media," Scientific Reports, vol. 14, no. 1, p. 11622, 2024/05/21 2024. [CrossRef]
- L. Shi, R. Wang, Y. Cao, D. Liang, and J.-H. Tay, "Effect of additives on the fabrication of poly(vinylidene fluoride- co-hexafluropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes," Journal of Membrane Science - J MEMBRANE SCI, vol. 315, pp. 195-204, 05/01 2008. [CrossRef]
- M. J. Toh, P. C. Oh, and M. I. S. Mohd Shaufi, "Preparation of Highly Hydrophobic PVDF-HFP Membrane with Anti-Wettability Characteristic," IOP Conference Series: Materials Science and Engineering, vol. 778, no. 1, p. 012176, 2020/04/01 2020. [CrossRef]
- M. S. Gharissah et al., "Composites cement/BaSO4/Fe3O4/CuO for improving X-ray absorption characteristics and structural properties," Scientific Reports, vol. 12, no. 1, p. 19169, 2022/11/10 2022. [CrossRef]
- X. Chen, L. Wang, J. Shi, H. Shi, and Y. Liu, "Effect of Barium Sulfate Nanoparticles on Mechanical Properties and Crystallization Behaviour of HDPE," Polymers and Polymer Composites, vol. 18, no. 3, pp. 145-152, 2010/03/01 2010. [CrossRef]
- H. A. Maghrabi, A. Vijayan, F. Mohaddes, P. Deb, and L. Wang, "Evaluation of X-ray radiation shielding performance of barium sulphate-coated fabrics," Fibers and Polymers, vol. 17, no. 12, pp. 2047-2054, 2016/12/01 2016. [CrossRef]
- H. Agarwal, S. Yadav, and G. Jaiswar, "Effect of nanoclay and barium sulfate nanoparticles on the thermal and morphological properties of polyvinylidene fluoride nanocomposites," Journal of Thermal Analysis and Calorimetry, vol. 129, no. 3, pp. 1471-1479, 2017/09/01 2017. [CrossRef]
- L. A. Silva, A. M. S. Batista, T. Serodre, A. T. B. Neto, C. A. Furtado, and L. O. Faria, "Enhancement of X-ray Shielding Properties of PVDF/BaSO4 Nanocomposites Filled with Graphene Oxide," MRS Advances, vol. 4, no. 3, pp. 169-175, 2019/01/01 2019. [CrossRef]
- S. Banerjee, "Simple derivation of Young, Wenzel and Cassie-Baxter equations and its interpretations," 09/11 2008. [CrossRef]
- D. K. Owens and R. C. Wendt, "Estimation of the surface free energy of polymers," Journal of Applied Polymer Science, vol. 13, no. 8, pp. 1741-1747, 1969/08/01 1969. [CrossRef]
- J. Yuennan, N. Tohluebaji, C. Putson, N. Muensit, and P. Channuie, "Enhanced electroactive β-phase and dielectric properties in P(VDF-HFP) composite flexible films through doping with three calcium chloride salts: CaCl, CaCl·2HO, and CaCl·6HO," Polymers for Advanced Technologies, vol. 35, no. 6, p. e6437, 2024. [CrossRef]
- K. Selvakumar and R. Manimuthu, "Investigation on meta-polybenzimidazole blend with sulfonated PVdF-HFP proton conducting polymer electrolytes for HT-PEM fuel cell application," Journal of Materials Science: Materials in Electronics, vol. 29, 09/01 2018. [CrossRef]
- H. Li and S. Lim, "Boosting Performance of Self-Polarized Fully Printed Piezoelectric Nanogenerators via Modulated Strength of Hydrogen Bonding Interactions," (in eng), Nanomaterials (Basel), vol. 11, no. 8, Jul 25 2021. [CrossRef]
- T. Mälzer, L. Mathies, T. Band, R. Gorgas, and H. S. Leipner, "Influence of Different Solvents and High-Electric-Field Cycling on Morphology and Ferroelectric Behavior of Poly(Vinylidene Fluoride-Hexafluoropropylene) Films," Materials, vol. 14, no. 14. [CrossRef]
- Y. Guo and H. Zhao, "Femtosecond laser processed superhydrophobic surface," Journal of Manufacturing Processes, vol. 109, pp. 250-287, 2024/01/17/ 2024. [CrossRef]
- P. S. Souza, A. J. Santos, M. A. P. Cotrim, A. M. Abrão, and M. A. Câmara, "Analysis of the surface energy interactions in the tribological behavior of ALCrN and TIAlN coatings," Tribology International, vol. 146, p. 106206, 2020/06/01/ 2020. [CrossRef]
- H. Bala et al., "In situ preparation and surface modification of barium sulfate nanoparticles," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 274, no. 1, pp. 71-76, 2006/02/15/ 2006. [CrossRef]
- Wang, Y. Zhang, L. Shi, J. Li, and Z. Guo, "Advances in the theory of superhydrophobic surfaces," Journal of Materials Chemistry, 10.1039/C2JM32780E vol. 22, no. 38, pp. 20112-20127, 2012. [CrossRef]
- Li et al., "A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines," Coatings, vol. 13, p. 301, 01/28 2023. [CrossRef]
- Y. Bormashenko, R. Pogreb, O. Stanevsky, and E. Bormashenko, "Vibrational spectrum of PVDF and its interpretation," Polymer Testing - POLYM TEST, vol. 23, pp. 791-796, 10/01 2004. [CrossRef]
- Ramesh, "One-step fabrication of biomimetic PVDF-BaTiO3 nanofibrous composite using DoE," Materials Research Express, vol. 5, p. 085308, 07/05 2018. [CrossRef]
- Siva, T. Shakthi, and J. Hemalatha, "Synthesis and ferroelectric investigations of poly(vinylidene fluoride- co -hexafluoropropylene)-Mg(NO 3 ) 2 films: ARTICLE," Journal of Applied Polymer Science, vol. 133, 06/01 2016. [CrossRef]
- Á. B. Sifontes et al., "Obtaining Highly Crystalline Barium Sulphate Nanoparticles via Chemical Precipitation and Quenching in Absence of Polymer Stabilizers," Journal of Nanomaterials, vol. 2015, no. 1, p. 510376, 2015/01/01 2015. [CrossRef]
- L. Staicu, T. Bajda, L. Drewniak, and L. Charlet, "Power Generation: Feedstock for High-Value Sulfate Minerals," Minerals, vol. 10, p. 188, 02/19 2020. [CrossRef]
- D. Li and M. Liao, "Study on the dehydrofluorination of vinylidene fluoride (VDF) and hexafluoropropylene (HFP) copolymer," Polymer Degradation and Stability, vol. 152, pp. 116-125, 2018/06/01/ 2018. [CrossRef]
- Mohammed, S. Salman, and F. M.Noori, "Preparation and Characterizations of Poly (vinylidene fluoride)(PVDF)/Ba0. 6Sr0. 4TiO3 (BST) Nanocomposites," International Journal of Applied Engineering Research, vol. 13, pp. 5008-5013, 01/01 2018.
- Q. Chang, S. Guo, and X. Zhang, "Radiation shielding polymer composites: Ray-interaction mechanism, structural design, manufacture and biomedical applications," Materials & Design, vol. 233, p. 112253, 08/01 2023. [CrossRef]
- Baeyens et al., "Basic Concepts of Radiation Biology," in Radiobiology Textbook, S. Baatout Ed. Cham: Springer International Publishing, 2023, pp. 25-81.
| Sample | Porosity (%) |
Root means square roughness (Rq) (nm) |
Crystallinity (Xc) (%) |
|---|---|---|---|
| Pure | 0.30 | 66.57 | 60.10 |
| 5% BaSO4 | 1.60 | 138.93 | 71.24 |
| 10% BaSO4 | 4.67 | 139.96 | 73.81 |
| 20% BaSO4 | 15.05 | 180.66 | 79.00 |
| 30% BaSO4 | 20.40 | 237.45 | 80.44 |
| 40% BaSO4 | 25.21 | 590.32 | 83.53 |
| Sample | TGA Analysis Results | DTG Analysis Results | ||||
|---|---|---|---|---|---|---|
| Decomposition Temperature (°C) | Weight Loss Rate (%/°C) |
Residual Mass (%) |
Peak Decomposition Temperature (°C) | Maximum Weight Loss Rate (%/min) |
Residual Mass (%) |
|
| pure | 468.87 | 0.55 | 19.58 | 499.09 | 27.55 | -0.06 |
| 5% BaSO4 | 478.78 | 0.46 | 35.50 | 503.72 | 21.36 | -0.07 |
| 10% BaSO4 | 483.38 | 0.34 | 51.34 | 505.7 | 14.03 | -0.28 |
| 20% BaSO4 | 486.69 | 0.27 | 60.60 | 506.86 | 12.10 | -0.16 |
| 30% BaSO4 | 495.29 | 0.24 | 66.15 | 512.65 | 9.93 | -0.19 |
| 40% BaSO4 | 498.26 | 0.18 | 74.11 | 513.15 | 7.73 | -0.23 |
| Thickness (cm) | Attenuation (%) | ||||
|---|---|---|---|---|---|
| 5%BaSO4 | 10%BaSO4 | 20%BaSO4 | 30%BaSO4 | 40%BaSO4 | |
| 0.02 | 1.48 | 4.95 | 10.99 | 19.48 | 22.71 |
| 0.04 | 4.92 | 8.96 | 19.05 | 36.65 | 39.81 |
| 0.06 | 7.65 | 14.65 | 26.11 | 47.27 | 49.77 |
| 0.08 | 10.47 | 19.26 | 31.68 | 54.54 | 58.49 |
| 0.1 | 13.87 | 24.02 | 39.32 | 61.39 | 65.64 |
| 0.12 | 15.50 | 27.41 | 44.95 | 66.25 | 71.52 |
| X-ray energy (keV) | Attenuation (%) of the 0.2 mm thickness | ||||
|---|---|---|---|---|---|
| 5%BaSO4 | 10%BaSO4 | 20%BaSO4 | 30%BaSO4 | 40%BaSO4 | |
| 60 | 1.48 | 4.95 | 10.99 | 19.48 | 22.71 |
| 80 | 2.28 | 2.12 | 7.33 | 10.96 | 13.95 |
| 100 | 0.04 | 1.20 | 3.74 | 6.29 | 7.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




