Submitted:
23 January 2025
Posted:
24 January 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
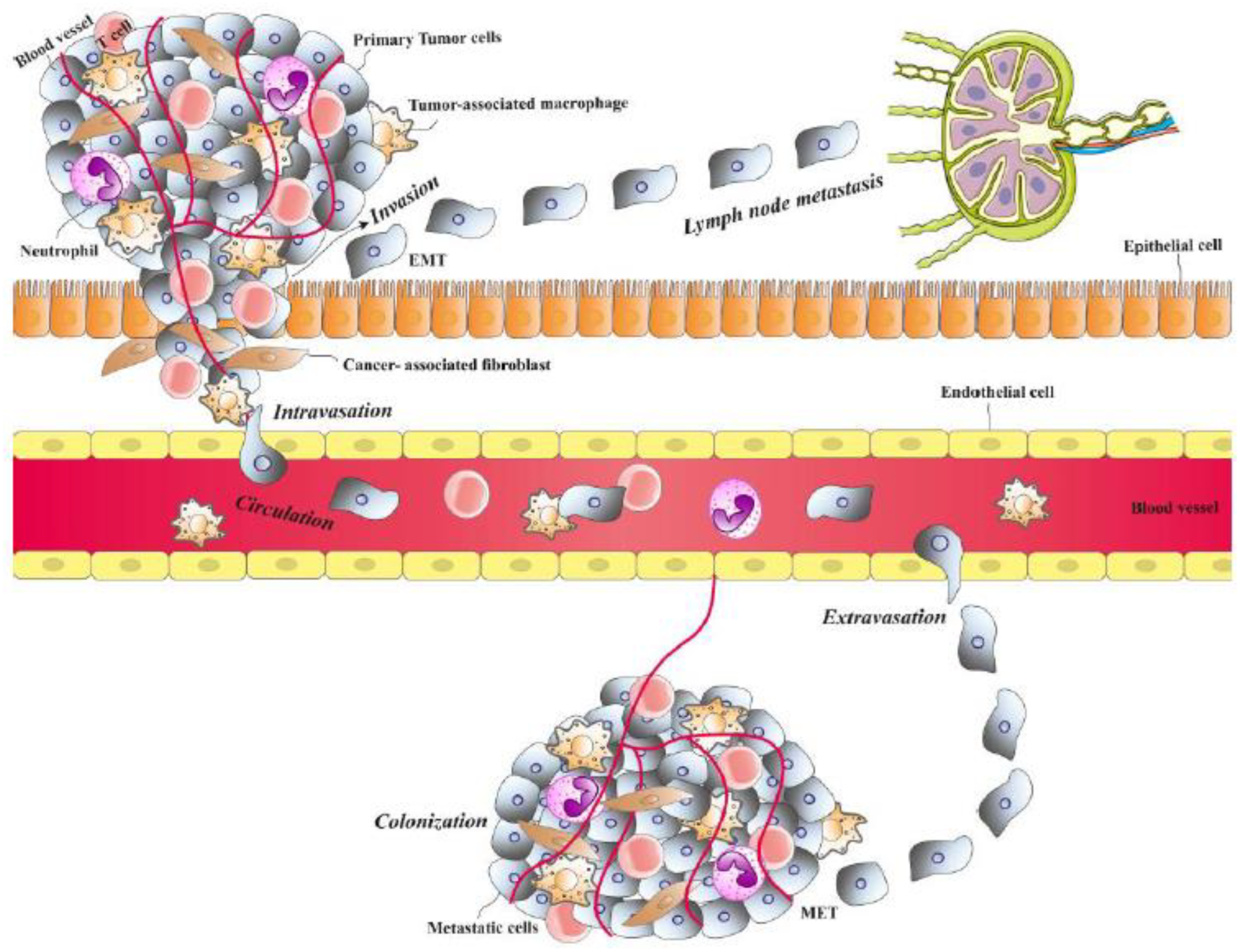
2. The Process of Metastasis
2.1. Invasion
2.2. Angiogenesis
2.3. Intravasation
2.4. Circulation and Extravasation
2.5. Colonization
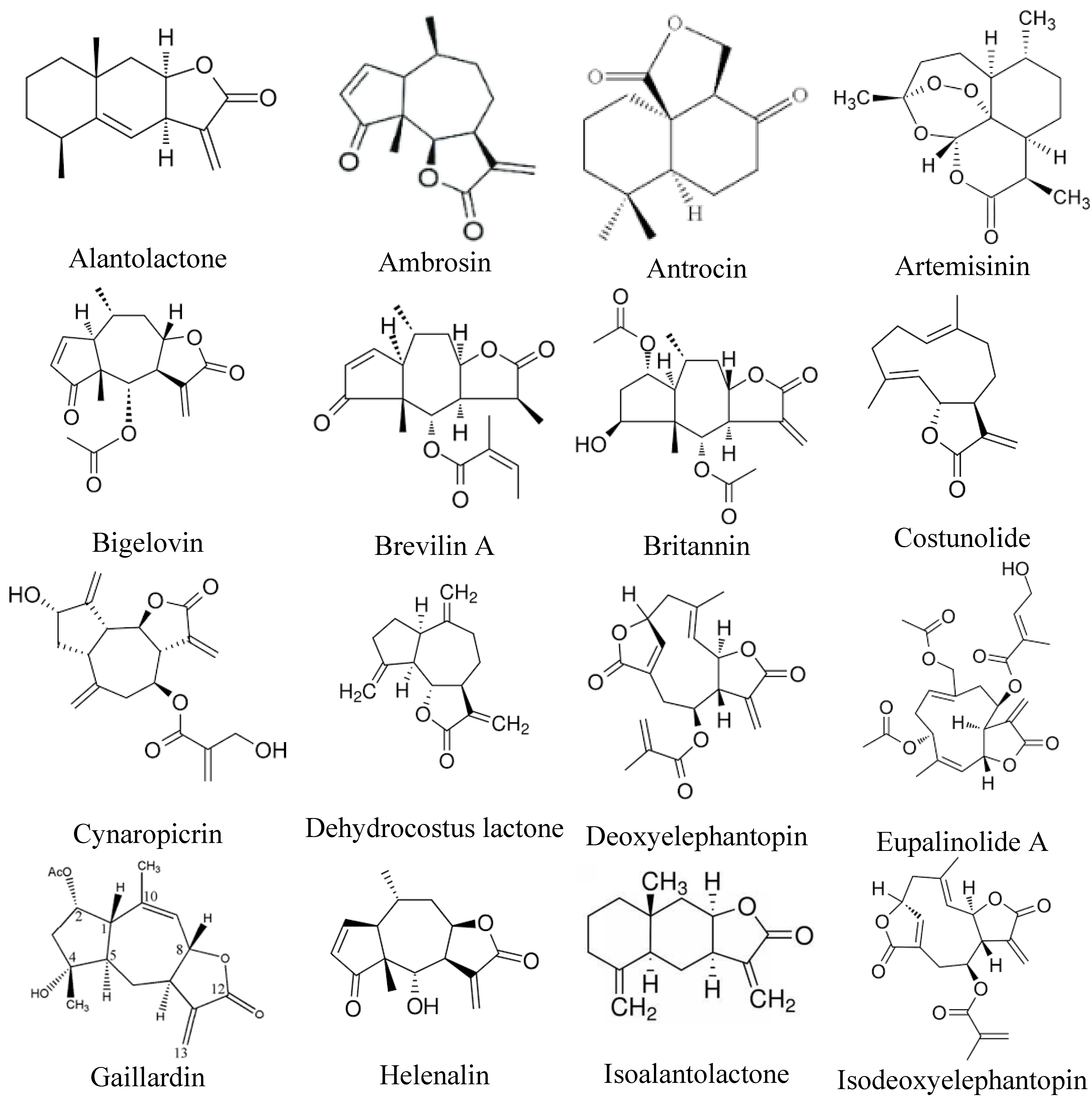
3. Sesquiterpene Lactones with the Ability to Inhibit Metastasis
3.1. Alantolactone
3.2. Ambrosin
3.3. Antrocin
3.4. Artemisinin
3.5. Brevilin A
3.6. Bigelovin
3.7. Britannin
3.8. Costunolide
3.9. Cynaropicrin
3.10. Dehydrocostus lactone
3.11. Deoxyelephantopin
3.12. Eupalinolide
3.13. Gaillardin
3.14. Helenalin
3.15. Isoalantolactone
3.16. Alantolactone
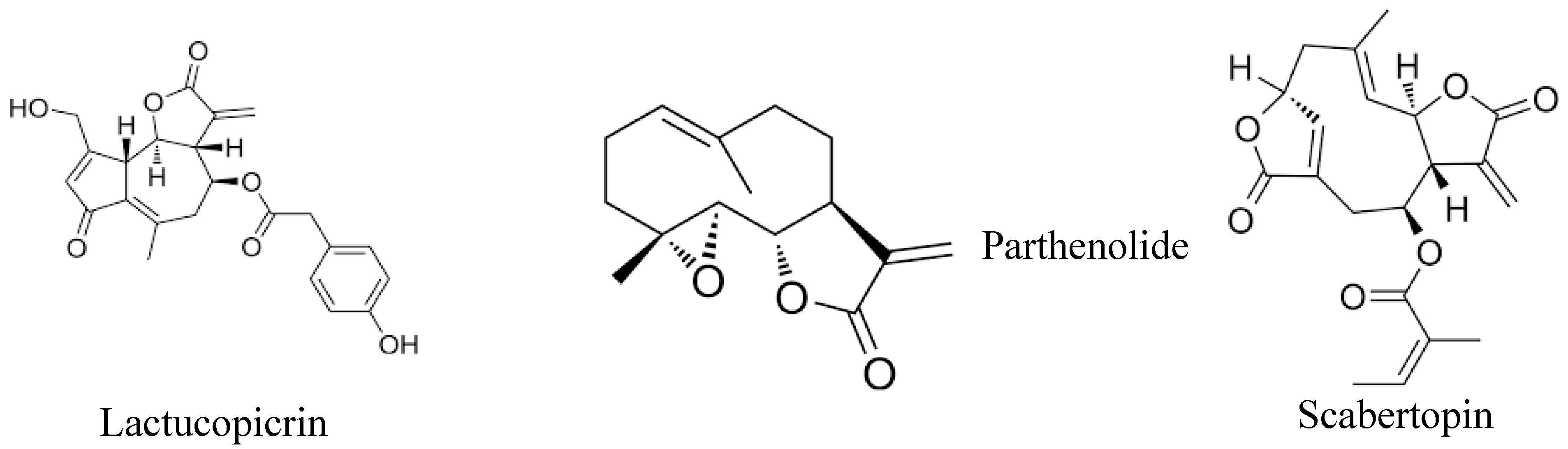
3.17. Lactucopicrin
3.18. Parthenolide
3.19. Scabertopin
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduc Target Ther, 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br J Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, J.; Li, G.; Chen, W.; Li, Z.; Lei, J. The role of extracellular vesicles in circulating tumor cell-mediated distant metastasis. Mol Cancer 2023, 22, 193. [Google Scholar] [CrossRef]
- Nolan, E.; Kang, Y.; Malanchi, I. Mechanisms of Organ-Specific Metastasis of Breast Cancer. Cold Spring Harb Perspect Med 2023, 13. [Google Scholar] [CrossRef]
- Cambria, E.; Coughlin, M.F.; Floryan, M.A.; Offeddu, G.S.; Shelton, S.E.; Kamm, R.D. Linking cell mechanical memory and cancer metastasis. Nat Rev Cancer 2024, 24, 216–228. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers (Basel) 2021, 13, 2053. [Google Scholar] [CrossRef]
- Rajabi, S.; Shakib, H.; Dastmalchi, R.; Danesh-Afrooz, A.; Karima, S.; Hedayati, M. Metastatic propagation of thyroid cancer; organ tropism and major modulators. Future Oncol 2020, 16, 1301–1319. [Google Scholar] [CrossRef]
- Shakib, H.; Rajabi, S.; Dehghan, M.H.; Mashayekhi, F.J.; Safari-Alighiarloo, N.; Hedayati, M. Epithelial-to-mesenchymal transition in thyroid cancer: a comprehensive review. Endocrine 2019, 66, 435–455. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Forsch Komplementmed 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front Pharmacol 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.I.; Verron, E.; Rohanizadeh, R. Molecular Mechanisms of Anti-metastatic Activity of Curcumin. Anticancer Res 2016, 36, 5639–5647. [Google Scholar] [CrossRef] [PubMed]
- Sokovic, M.; Ciric, A.; Glamoclija, J.; Skaltsa, H. Biological Activities of Sesquiterpene Lactones Isolated from the Genus Centaurea L. (Asteraceae). Curr Pharm Des 2017, 23, 2767–2786. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Nunes Dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the hallmarks of metastasis. Cancer Res 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef]
- Alečković, M.; McAllister, S.S.; Polyak, K. Metastasis as a systemic disease: molecular insights and clinical implications. Biochim Biophys Acta Rev Cancer 2019, 1872, 89–102. [Google Scholar] [CrossRef]
- Massague, J.; Ganesh, K. Metastasis-initiating cells and ecosystems. Cancer Discov 2021, 11, 971–994. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Maitra, A. Molecular envoys pave the way for pancreatic cancer to invade the liver. Nature 2019, 567, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J Clin Invest 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- van Zijl, F.; Krupitza, G.; Mikulits, W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 2011, 728, 23–34. [Google Scholar] [CrossRef]
- Harris, T.J.; Tepass, U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 2010, 11, 502–514. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat Rev Clin Oncol 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985, 315, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Geindreau, M.; Ghiringhelli, F.; Bruchard, M. Vascular Endothelial Growth Factor, a Key Modulator of the Anti-Tumor Immune Response. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Zavyalova, M.; Denisov, E.; Tashireva, L.; Savelieva, O.; Kaigorodova, E.; Krakhmal, N.; Perelmuter, V. Intravasation as a key step in cancer metastasis. Biochemistry (Moscow) 2019, 84, 762–772. [Google Scholar] [CrossRef]
- Dua, R.; Gui, G.; Isacke, C. Endothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systems. Eur J Surg Oncol 2005, 31, 824–832. [Google Scholar] [CrossRef]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am J Physiol Cell Physiol 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying collective cancer cell invasion. Nat Cell Biol 2012, 14, 777–783. [Google Scholar] [CrossRef]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov 2024, 14, 1375–1388. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018, 59, 455–467. [Google Scholar]
- Huang, R.; Kang, T.; Chen, S. The role of tumor-associated macrophages in tumor immune evasion. J Cancer Res Clin Oncol 2024, 150, 238. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Speicher, M.R. The biology of circulating tumor cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chakraborty, G.; Lee-Lim, A.P.; Mo, Q.; Decker, M.; Vonica, A.; Shen, R.; Brogi, E.; Brivanlou, A.H.; Giancotti, F.G. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 2012, 150, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Duda, D.G.; Duyverman, A.M.; Kohno, M.; Snuderl, M.; Steller, E.J.; Fukumura, D.; Jain, R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010, 107, 21677–21682. [Google Scholar] [CrossRef]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat Rev Cancer, 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Placke, T.; Örgel, M.; Schaller, M.; Jung, G.; Rammensee, H.-G.; Kopp, H.-G.; Salih, H.R. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Rese 2012, 72, 440–448. [Google Scholar] [CrossRef]
- Leach, J.; Morton, J.P.; Sansom, O.J. Neutrophils: homing in on the myeloid mechanisms of metastasis. Mol Immunol 2019, 110, 69–76. [Google Scholar] [CrossRef]
- Strilic, B.; Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef]
- Leong, H.S.; Robertson, A.E.; Stoletov, K.; Leith, S.J.; Chin, C.A.; Chien, A.E.; Hague, M.N.; Ablack, A.; Carmine-Simmen, K.; McPherson, V.A. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 2014, 8, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Stoletov, K.; Kato, H.; Zardouzian, E.; Kelber, J.; Yang, J.; Shattil, S.; Klemke, R. Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci 2010, 123, 2332–2341. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Salanti, A.; Gögenur, I. Premetastatic niches, exosomes and circulating tumor cells: Early mechanisms of tumor dissemination and the relation to surgery. Int J Cancer 2020, 146, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef]
- Rajabi, S.; Rajani, H.F.; Mohammadkhani, N.; Ramírez-Coronel, A.A.; Maleki, M.; Maresca, M.; Hajimehdipoor, H. Long non-coding RNAs as novel targets for phytochemicals to cease cancer metastasis. Molecules 2023, 28, 987. [Google Scholar] [CrossRef]
- Jamal, M.; Keywan, M. Steps in metastasis: an updated review. Med Oncol 2021, 38. [Google Scholar]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 2020, 5, 28. [Google Scholar] [CrossRef]
- Gau, D.M.; Chakraborty, S.; Boone, D.; Wells, A.; Roy, P. Abstract LB-043: MRTF| Profilin is an important signaling axis for metastatic outgrowth of triple negative breast cancer cells. Cancer Ress 2019, 79, LB-043–LB-043. [Google Scholar] [CrossRef]
- Rodrigues, G.; Hoshino, A.; Kenific, C.M.; Matei, I.R.; Steiner, L.; Freitas, D.; Kim, H.S.; Oxley, P.R.; Scandariato, I.; Casanova-Salas, I. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Bio 2019, 21, 1403–1412. [Google Scholar] [CrossRef]
- Rajabi, S.; Irani, M.; Moeinifard, M.; Hamzeloo-Moghadam, M. Britannin suppresses MCF-7 breast cancer cell growth by inducing apoptosis and inhibiting autophagy. Avicenna J Phytomed 2024, 14, 90–99. [Google Scholar]
- Hsu, C.Y.; Rajabi, S.; Hamzeloo-Moghadam, M.; Kumar, A.; Maresca, M.; Ghildiyal, P. Sesquiterpene lactones as emerging biomolecules to cease cancer by targeting apoptosis. Front Pharmacol 2024, 15, 1371002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Melotti, P.; Nicolis, E.; Tamanini, A.; Rolfini, R.; Pavirani, A.; Cabrini, G. Activation of NF-kB mediates ICAM-1 induction in respiratory cells exposed to an adenovirus-derived vector. Gene Ther 2001, 8, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Schnyder-Candrian, S.; Panski, A.; Bömmel, H.; Heim, M.; Duschl, A.; Moser, R. Phytochemical inhibition of interleukin-4-activated Stat6 and expression of VCAM-1. Biochem Biophys Res Commun 2002, 292, 841–847. [Google Scholar] [CrossRef]
- Chun, J.; Kim, Y. Alantolactone, a sesquiterpene lactone isolated from Inula Helenium L. selectively suppresses STAT3 activation and exhibits anticancer activity in MDA-MB-231 cells. Planta Medica 2015, 81, PM_11. [Google Scholar] [CrossRef]
- Liu, Y.R.; Cai, Q.Y.; Gao, Y.G.; Luan, X.; Guan, Y.Y.; Lu, Q.; Sun, P.; Zhao, M.; Fang, C. Alantolactone, a sesquiterpene lactone, inhibits breast cancer growth by antiangiogenic activity via blocking VEGFR2 signaling. Phytothera Res 2018, 32, 643–650. [Google Scholar] [CrossRef]
- Liu, J.; Liu, M.; Wang, S.; He, Y.; Huo, Y.; Yang, Z.; Cao, X. Alantolactone induces apoptosis and suppresses migration in MCF-7 human breast cancer cells via the p38 MAPK, NF-κB and Nrf2 signaling pathways. Int J Mol Med 2018, 42, 1847–1856. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Q.; Dai, X.; Wen, X.; Luo, X.; Duan, Y.; Yang, Z.; Dai, Q. Alantolactone enhances the sensitivity of melanoma to MAPK pathway inhibitors by targeting inhibition of STAT3 activation and down-regulating stem cell markers. Cancer Cell Int 2024, 24, 191. [Google Scholar] [CrossRef]
- Babaei, G.; Ansari, M.H.K.; Aziz, S.G.-G.; Bazl, M.R. Alantolactone inhibits stem-like cell phenotype, chemoresistance and metastasis in PC3 cells through STAT3 signaling pathway. Res Pharm Sci 2020, 15, 551–562. [Google Scholar]
- Zhang, Y.; Weng, Q.; Han, J.; Chen, J. Alantolactone suppresses human osteosarcoma through the PI3K/AKT signaling pathway. Mol Med Rep 2020, 21, 675–684. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Huang, H.; Yuan, X.; Zhang, P.; Ye, C.; Wei, M.; Huang, Y.; Luo, X.; Luo, J. Alantolactone inhibits proliferation, metastasis and promotes apoptosis of human osteosarcoma cells by suppressing Wnt/beta-catenin and MAPKs signaling pathways. Genes Dis 2022, 9, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Liu, J.; Zhang, C.; Jian, C.; Wang, L.; Zhang, Y.; Shi, C. Natural Product Alantolactone Targeting AKR1C1 Suppresses Cell Proliferation and Metastasis in Non-Small-Cell Lung Cancer. Front Pharmacol 2022, 13, 847906. [Google Scholar] [CrossRef]
- Abdelgaleil, S.; Badawy, M.; Suganuma, T.; Kitahara, K.; Abdelgaleil, S. Antifungal and biochemical effects of pseudoguaianolide sesquiterpenes isolated from Ambrosia maritima L. African J Microbiol Res 2011, 5, 3385–3392. [Google Scholar] [CrossRef]
- Villagomez, R.; Collado, J.A.; Muñoz, E.; Almanza, G.; Sterner, O. Natural and semi-synthetic pseudoguaianolides as inhibitors of NF-κB. J Biomed Sci Engin 2014, 2014. [Google Scholar] [CrossRef]
- Sotillo, W.S.; Villagomez, R.; Smiljanic, S.; Huang, X.; Malakpour, A.; Kempengren, S.; Rodrigo, G.; Almanza, G.; Sterner, O.; Oredsson, S. Anti-cancer stem cell activity of a sesquiterpene lactone isolated from Ambrosia arborescens and of a synthetic derivative. PloS one 2017, 12, e0184304. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shao, Z. Ambrosin exerts strong anticancer effects on human breast cancer cells via activation of caspase and inhibition of the Wnt/β-catenin pathway. Tropic J Pharmaceut Res 2021, 20, 809–814. [Google Scholar] [CrossRef]
- Fan, S.; Cui, Y.; Hu, Z.; Wang, W.; Jiang, W.; Xu, H. Ambrosin sesquiterpene lactone exerts selective and potent anticancer effects in drug-resistant human breast cancer cells (MDA-MB-231) through mitochondrial mediated apoptosis, ROS generation and targeting Akt/β-Catenin signaling pathway. J buon 2020, 25, 2221–2227. [Google Scholar]
- Rao, Y.K.; Wu, A.T.; Geethangili, M.; Huang, M.-T.; Chao, W.-J.; Wu, C.-H.; Deng, W.-P.; Yeh, C.-T.; Tzeng, Y.-M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem Res Toxicol 2011, 24, 238–245. [Google Scholar] [CrossRef]
- Chiu, K.-Y.; Wu, C.-C.; Chia, C.-H.; Hsu, S.-L.; Tzeng, Y.-M. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Let 2016, 373, 174–184. [Google Scholar] [CrossRef]
- Chen, J.-H.; Wu, A.T.; Tzeng, D.T.; Huang, C.-C.; Tzeng, Y.-M.; Chao, T.-Y. Antrocin, a bioactive component from Antrodia cinnamomea, suppresses breast carcinogenesis and stemness via downregulation of β-catenin/Notch1/Akt signaling. Phytomedicine 2019, 52, 70–78. [Google Scholar] [CrossRef]
- Chiu, K.-Y.; Chen, T.-H.; Wen, C.-L.; Lai, J.-M.; Cheng, C.-C.; Liu, H.-C.; Hsu, S.-L.; Tzeng, Y.-M. Antcin-H Isolated from Antrodia cinnamomea Inhibits Renal Cancer Cell Invasion Partly through Inactivation of FAK-ERK-C/EBP-β/c-Fos-MMP-7 Pathways. Evid Based Complemen Alternat Med 2017, 2017, 5052870. [Google Scholar] [CrossRef]
- Guo, Z. Artemisinin anti-malarial drugs in China. Acta Pharm Sin B 2016, 6, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol 2012, 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Liu, Y.; Zheng, H.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Li, F.; Hu, M. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 2016, 7, 31413. [Google Scholar] [CrossRef] [PubMed]
- Weifeng, T.; Feng, S.; Xiangji, L.; Changqing, S.; Zhiquan, Q.; Huazhong, Z.; Peining, Y.; Yong, Y.; Mengchao, W.; Xiaoqing, J. Artemisinin inhibits in vitro and in vivo invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine 2011, 18, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.A.K.; Efferth, T.; Asangani, I.A.; Allgayer, H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Inte J Cancer 2010, 127, 1475–1485. [Google Scholar] [CrossRef]
- Ma, Y.; Kim, B.H.; Yun, S.K.; Roh, Y.S. Centipeda minima Extract Inhibits Inflammation and Cell Proliferation by Regulating JAK/STAT Signaling in Macrophages and Keratinocytes. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Cui, X.; Lv, D.; Jin, L.; Khan, M.; Ma, T. Brevilin A promotes oxidative stress and induces mitochondrial apoptosis in U87 glioblastoma cells. Onco Targets Ther 2018, 11, 7031–7040. [Google Scholar] [CrossRef]
- Su, T.; Wang, Y.P.; Wang, X.N.; Li, C.Y.; Zhu, P.L.; Huang, Y.M.; Yang, Z.Y.; Chen, S.B.; Yu, Z.L. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of brevilin A. Life Sci 2020, 241, 117169. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, H. In vitro evaluation of anti-hepatoma activity of brevilin A: involvement of Stat3/Snail and Wnt/beta-catenin pathways. RSC Adv 2019, 9, 4390–4396. [Google Scholar] [CrossRef]
- Qu, Z.; Lin, Y.; Mok, D.K.; Bian, Q.; Tai, W.C.; Chen, S. Brevilin A, a Natural Sesquiterpene Lactone Inhibited the Growth of Triple-Negative Breast Cancer Cells via Akt/mTOR and STAT3 Signaling Pathways. Onco Targets Ther 2020, 13, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Tan, J.; Chen, H.; Shi, Z.; Kwan, H.Y.; Su, T. Brevilin A exerts anti-colorectal cancer effects and potently inhibits STAT3 signaling invitro. Heliyon 2023, 9, e18488. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Meng, M.; Li, B.; Chen, H.; Tan, J.; Xu, K.; Xiao, S.; Kwan, H.Y.; Liu, Z.; Su, T. Brevilin A is a potent anti-metastatic CRC agent that targets the VEGF-IL6-STAT3 axis in the HSCs-CRC interplay. J Transl Med 2023, 21, 260. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Z.; Tan, N.H.; Ji, C.J.; Fan, J.T.; Huang, H.Q.; Han, H.J.; Zhou, G.B. Apoptosis inducement of bigelovin from Inula helianthus-aquatica on human Leukemia U937 cells. Phytother Res 2009, 23, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.; Chan, B.C.; Kwok, H.F.; Wong, Y.L.; Leung, H.W.; Ji, C.J.; Fung, K.P.; Leung, P.C.; Tan, N.H.; Lau, C.B. Anti-angiogenesis and immunomodulatory activities of an anti-tumor sesquiterpene bigelovin isolated from Inula helianthus-aquatica. Eur J Med Chem 2013, 59, 243–252. [Google Scholar] [CrossRef]
- Li, M.; Yue, G.G.; Song, L.H.; Huang, M.B.; Lee, J.K.; Tsui, S.K.; Fung, K.P.; Tan, N.H.; Lau, C.B. Natural small molecule bigelovin suppresses orthotopic colorectal tumor growth and inhibits colorectal cancer metastasis via IL6/STAT3 pathway. Biochem Pharmacol 2018, 150, 191–201. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer Targets and Signaling Pathways Activated by Britannin and Related Pseudoguaianolide Sesquiterpene Lactones. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Abdolmohammadi, M.H.; Roozbehani, M.; Hamzeloo-Moghadam, M.; Heidari, F.; Fallahian, F. Peroxisome proliferator-activated receptor gamma (PPARγ) pathway mediates anticancer activity of Britannin, isolated from Inula aucheriana DC., in human gastric cancer cells. Res Square 2022. [Google Scholar] [CrossRef]
- Li, H.; Du, G.; Yang, L.; Pang, L.; Zhan, Y. The Antitumor Effects of Britanin on Hepatocellular Carcinoma Cells and its Real-Time Evaluation by In Vivo Bioluminescence Imaging. Anticancer Agents Med Chem 2020, 20, 1147–1156. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhang, Z.H.; Li, M.Y.; Wang, J.Y.; Xing, Y.; Ri, M.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Zuo, H.X. Britannin stabilizes T cell activity and inhibits proliferation and angiogenesis by targeting PD-L1 via abrogation of the crosstalk between Myc and HIF-1α in cancer. Phytomedicine 2021, 81, 153425. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, B.; Qu, M.; Liu, F.; Wu, X. Britannin inhibits cell proliferation, migration and glycolysis by downregulating KLF5 in lung cancer. Exp Ther Med 2024, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Inte J Mol Sci 2015, 16, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, S.G.; Woo, S.M.; Yun, Y.J.; Jo, J.; Kim, W.; Shin, Y.C.; Ko, S.G. Saussurea lappa Clarke-Derived Costunolide Prevents TNF alpha -Induced Breast Cancer Cell Migration and Invasion by Inhibiting NF- kappa B Activity. Evid Based Complement Alternat Med 2013, 2013, 936257. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Park, S.; Zhang, H.; Park, S.; Kwon, W.; Kim, E.; Zhang, X.; Jang, S.; Yoon, D.; Choi, S.K.; et al. Targeting AKT with costunolide suppresses the growth of colorectal cancer cells and induces apoptosis in vitro and in vivo. J Exp Clin Cancer Res 2021, 40, 114. [Google Scholar] [CrossRef]
- Jeong, S.J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett 2002, 187, 129–133. [Google Scholar] [CrossRef]
- Wei, M.; Li, J.; Qiu, J.; Yan, Y.; Wang, H.; Wu, Z.; Liu, Y.; Shen, X.; Su, C.; Guo, Q.; et al. Costunolide induces apoptosis and inhibits migration and invasion in H1299 lung cancer cells. Oncol Rep 2020, 43, 1986–1994. [Google Scholar] [CrossRef]
- Tabata, K.; Nishimura, Y.; Takeda, T.; Kurita, M.; Uchiyama, T.; Suzuki, T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J Pharmacol Sci 2015, 127, 397–403. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Comino, C.; Moglia, A.; Cankar, K.; Genre, A.; Hehn, A.; Bourgaud, F.; Beekwilder, J.; Lanteri, S. Accumulation of cynaropicrin in globe artichoke and localization of enzymes involved in its biosynthesis. Plant Sci 2015, 239, 128–136. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, Y.; Shen, Y.; Xiao, S.; Yang, L.; Xiang, Y.; Dai, X.; Hu, W.; Zhou, B.; Liu, Z.; et al. Cynaropicrin Shows Antitumor Progression Potential in Colorectal Cancer Through Mediation of the LIFR/STATs Axis. Front Cell Dev Biol 2020, 8, 605184. [Google Scholar] [CrossRef]
- De Cicco, P.; Busa, R.; Ercolano, G.; Formisano, C.; Allegra, M.; Taglialatela-Scafati, O.; Ianaro, A. Inhibitory effects of cynaropicrin on human melanoma progression by targeting MAPK, NF-kappaB, and Nrf-2 signaling pathways in vitro. Phytother Res 2021, 35, 1432–1442. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int J Mol Sci 2015, 16, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Wang, H.; Pan, Y.-R.; Zhang, L.; Guo, Q.; Yan, Y.; Li, J.; Fu, J.; Fan, X.; Wang, Y. Dehydrocostus lactone exerts the antitumor effect in non-small cell lung cancer H1299 cells. TMR Cancer 2020, 3, 101–111. [Google Scholar] [CrossRef]
- Zhang, R.; Hao, J.; Wu, Q.; Guo, K.; Wang, C.; Zhang, W.K.; Liu, W.; Wang, Q.; Yang, X. Dehydrocostus lactone inhibits cell proliferation and induces apoptosis by PI3K/Akt/Bad and ERS signalling pathway in human laryngeal carcinoma. J Cell Mol Med 2020, 24, 6028–6042. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liang, Q.; Gong, Z.; Yu, W.; He, S.; Xi, L. Antitumor activities of the four sesquiterpene lactones from Elephantopus scaber L. Exp Oncol 2006, 28, 106–109. [Google Scholar]
- Kabeer, F.A.; Rajalekshmi, D.S.; Nair, M.S.; Prathapan, R. Molecular mechanisms of anticancer activity of deoxyelephantopin in cancer cells. Integr Med Res 2017, 6, 190–206. [Google Scholar] [CrossRef]
- Farha, A.K.; Dhanya, S.R.; Mangalam, S.N.; Remani, P. Anti-metastatic effect of deoxyelephantopin from Elephantopus scaber in A549 lung cancer cells in vitro. Nat Prod Res 2015, 29, 2341–2345. [Google Scholar] [CrossRef]
- Cvetanova, B.; Li, M.Y.; Yang, C.C.; Hsiao, P.W.; Yang, Y.C.; Feng, J.H.; Shen, Y.C.; Nakagawa-Goto, K.; Lee, K.H.; Shyur, L.F. Sesquiterpene Lactone Deoxyelephantopin Isolated from Elephantopus scaber and Its Derivative DETD-35 Suppress BRAF(V600E) Mutant Melanoma Lung Metastasis in Mice. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Lee, W.L.; Shyur, L.F. Deoxyelephantopin impedes mammary adenocarcinoma cell motility by inhibiting calpain-mediated adhesion dynamics and inducing reactive oxygen species and aggresome formation. Free Radic Biol Med 2012, 52, 1423–1436. [Google Scholar] [CrossRef]
- Chao, W.W.; Cheng, Y.W.; Chen, Y.R.; Lee, S.H.; Chiou, C.Y.; Shyur, L.F. Phyto-sesquiterpene lactone deoxyelephantopin and cisplatin synergistically suppress lung metastasis of B16 melanoma in mice with reduced nephrotoxicity. Phytomedicine 2019, 56, 194–206. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Tao, Y.; Fan, X.; Shen, X.; Tian, S. Eupatorium lindleyanum DC. sesquiterpene fraction F1012-2 regulates p53/NF-κB signaling pathways in human breast cancer. Archive Biol Sci 2022, 74, 291–299. [Google Scholar] [CrossRef]
- Lou, C.; Chen, Y.; Zhang, J.; Yang, B.; Zhao, H. Eupalinolide J Suppresses the Growth of Triple-Negative Breast Cancer Cells via Targeting STAT3 Signaling Pathway. Front Pharmacol 2019, 10, 1071. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shen, J.W.; Zhou, D.H.; Zhao, Y.P.; Wang, W.Q.; Zhu, Y.; Zhao, H.J. Precise discovery of a STAT3 inhibitor from Eupatorium lindleyanum and evaluation of its activity of anti-triple-negative breast cancer. Nat Prod Res 2019, 33, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, J.; Zhang, J.; Yao, L.; Jiang, B.; Du, S.; Li, F.; Peng, Q.; Qin, L.; Wang, Y.; et al. Eupalinolide B suppresses pancreatic cancer by ROS generation and potential cuproptosis. iScience 2024, 27, 110496. [Google Scholar] [CrossRef]
- Hu, H.; Bai, H.; Huang, L.; Yang, B.; Zhao, H. Eupalinolide J Inhibits Cancer Metastasis by Promoting STAT3 Ubiquitin-Dependent Degradation. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, F.; Cao, Z.; Wang, T.; Pan, L.; Luo, W.; Ding, W.; Li, J.; Jin, L.; Liu, H. Eupalinolide A induces autophagy via the ROS/ERK signaling pathway in hepatocellular carcinoma cells in vitro and in vivo. Inte J Oncol 2022, 61, 1–16. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, L.; Zhang, X. Eupalinilide B as a novel anti-cancer agent that inhibits proliferation and epithelial-mesenchymal transition in laryngeal cancer cells. J Int Med Res 2022, 50, 3000605211067921. [Google Scholar] [CrossRef]
- Fallahian, F.; Aghaei, M.; Abdolmohammadi, M.H.; Hamzeloo-Moghadam, M. Molecular mechanism of apoptosis induction by Gaillardin, a sesquiterpene lactone, in breast cancer cell lines : Gaillardin-induced apoptosis in breast cancer cell lines. Cell Biol Toxicol 2015, 31, 295–305. [Google Scholar] [CrossRef]
- Roozbehani, M.; Abdolmohammadi, M.H.; Hamzeloo-Moghadam, M.; Irani, S.; Fallahian, F. Gaillardin, a potent sesquiterpene lactone induces apoptosis via down-regulation of NF-kappabeta in gastric cancer cells, AGS and MKN45. J Ethnopharmacol 2021, 281, 114529. [Google Scholar] [CrossRef]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB. Biol Chem 1997, 378, 951–961. [Google Scholar] [CrossRef]
- Mun, H.; Townley, H.E. Mechanism of Action of the Sesquiterpene Compound Helenalin in Rhabdomyosarcoma Cells. Pharmaceuticals (Basel) 2021, 14. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Zhang, Q.; Zhang, B.; Yang, B.; Lin, N.M. Active ingredients of Inula helenium L. exhibits similar anti-cancer effects as isoalantolactone in pancreatic cancer cells. Nat Prod Res 2020, 34, 2539–2544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Hui, X.G.; Huo, L.; Sun, D.X.; Peng, W.; Zhang, Y.; Li, X.B.; Ma, T.; Li, W.H.; Liang, J.; et al. Antiproliferative effects of isoalantolactone in human liver cancer cells are mediated through caspase-dependent apoptosis, ROS generation, suppression of cell migration and invasion and targeting Ras/Raf/MEK signalling pathway. Acta Biochim Pol 2022, 69, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, L.; Feng, L.; Zhang, Z.; Song, J.; Liu, D.; Jia, X. Isoalantolactone inhibits the migration and invasion of human breast cancer MDA-MB-231 cells via suppression of the p38 MAPK/NF-kappaB signaling pathway. Oncol Rep 2016, 36, 1269–1276. [Google Scholar] [CrossRef]
- Li, J.; Zhu, P.; Chen, Y.; Zhang, S.; Zhang, Z.; Zhang, Z.; Wang, Y.; Jiang, X.; Lin, K.; Wu, W.; et al. Isoalantolactone Induces Cell Cycle Arrest, Apoptosis and Autophagy in Colorectal Cancer Cells. Front Pharmacol 2022, 13, 903599. [Google Scholar] [CrossRef]
- Hu, F.; Yang, P. Isoalantolactone exerts anticancer effects on human HEC-1-B endometrial cancer cells via induction of ROS mediated apoptosis and inhibition of MEK/ERK signalling pathway. Acta Biochim Pol 2022, 69, 453–458. [Google Scholar] [CrossRef]
- Peng, W.; Hui, X.-g.; Huo, L.; Sun, D.-x.; Wu, Z.-c.; Zhang, Y.; Li, X.-b.; Ma, T.; Li, W.-h.; Liang, J. Isoalantolactone inhibits the proliferation of human liver cancer cells by inducing intrinsic apoptosis. Tropi J Pharmaceu Res 2024, 23, 273–278. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Xiong, J.; Xie, L.; Ying, S.; Jia, Y.; Yao, Y.; Song, X.; Zeng, Z.; Yuan, J. Isoalantolactone inhibits pancreatic cancer proliferation by regulation of PI3K and Wnt signal pathway. PloS one 2021, 16, e0247752. [Google Scholar] [CrossRef]
- Yan, G.R.; Tan, Z.; Wang, Y.; Xu, M.L.; Yu, G.; Li, Y.; He, Q.Y. Quantitative proteomics characterization on the antitumor effects of isodeoxyelephantopin against nasopharyngeal carcinoma. Proteomics 2013, 13, 3222–3232. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Huang, Z.H.; Huang, X.H.; Zheng, W.B.; Yin, X.F.; Li, Y.L.; Li, B.; He, Q.Y. Isodeoxyelephantopin induces protective autophagy in lung cancer cells via Nrf2-p62-keap1 feedback loop. Cell Death Dis 2017, 8, e2876. [Google Scholar] [CrossRef]
- Babykutty, S.; George, A.; Vijayan, S.J.; Sidheek, S.P.; Sulfath, S.; Moly, A.G.; Thomas, J.A.; Xavier, H.; Chellama, P.; Rajalekshmi, D.S. Isodeoxyelephantopin (IDOE) Retards Tumor Cell Migration by Downregulating MMP-2/9 Expression in Triple Negative Breast Cancer. J Applie Pharmaceut Sci Res 2021, 4, 21–28. [Google Scholar] [CrossRef]
- Ichikawa, H.; Nair, M.S.; Takada, Y.; Sheeja, D.B.; Kumar, M.A.; Oommen, O.V.; Aggarwal, B.B. Isodeoxyelephantopin, a novel sesquiterpene lactone, potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis through suppression of nuclear factor-kappaB (nf-kappaB) activation and nf-kappaB-regulated gene expression. Clin Cancer Res 2006, 12, 5910–5918. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; You, K.R.; Lee, S.Y.; Song, C.H.; Kim, D.G. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 2002, 277, 38954–38964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lan, D.; Ning, S.; Ruan, L. Anticancer action of lactucopicrin in SKMEL-5 human skin cancer cells is mediated via apoptosis induction, G2/M cell cycle arrest and downregulation of m=TOR/PI3K/AKT signalling pathway. J BUON 2018, 23, 224–228. [Google Scholar]
- Meng, Q.; Tang, B.; Qiu, B. Growth inhibition of Saos-2 osteosarcoma cells by lactucopicrin is mediated via inhibition of cell migration and invasion, sub-G1 cell cycle disruption, apoptosis induction and Raf signalling pathway. J buon 2019, 24, 2136–2140. [Google Scholar]
- Rotondo, R.; Oliva, M.A.; Staffieri, S.; Castaldo, S.; Giangaspero, F.; Arcella, A. Implication of Lactucopicrin in Autophagy, Cell Cycle Arrest and Oxidative Stress to Inhibit U87Mg Glioblastoma Cell Growth. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Liu, D.; Han, Y.; Liu, L.; Ren, X.; Zhang, H.; Fan, S.; Qin, T.; Li, L. Parthenolide inhibits the tumor characteristics of renal cell carcinoma. Int J Oncol 2021, 58, 100–110. [Google Scholar] [CrossRef]
- Bork, P.M.; Schmitz, M.L.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett 1997, 402, 85–90. [Google Scholar] [CrossRef]
- Jafari, N.; Nazeri, S.; Enferadi, S.T. Parthenolide reduces metastasis by inhibition of vimentin expression and induces apoptosis by suppression elongation factor alpha - 1 expression. Phytomedicine 2018, 41, 67–73. [Google Scholar] [CrossRef]
- Kim, S.L.; Park, Y.R.; Lee, S.T.; Kim, S.W. Parthenolide suppresses hypoxia-inducible factor-1alpha signaling and hypoxia induced epithelial-mesenchymal transition in colorectal cancer. Int J Oncol 2017, 51, 1809–1820. [Google Scholar] [CrossRef]
- Liu, Y.C.; Kim, S.L.; Park, Y.R.; Lee, S.T.; Kim, S.W. Parthenolide promotes apoptotic cell death and inhibits the migration and invasion of SW620 cells. Intest Res 2017, 15, 174–181. [Google Scholar] [CrossRef]
- Kishida, Y.; Yoshikawa, H.; Myoui, A. Parthenolide, a natural inhibitor of Nuclear Factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res 2007, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- D'Anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis 2013, 4, e891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.M.; Park, Y.R.; Seo, S.Y.; Kim, I.H.; Lee, S.T.; Kim, S.W. Parthenolide inhibits transforming growth factor beta1-induced epithelial-mesenchymal transition in colorectal cancer cells. Intest Res 2019, 17, 527–536. [Google Scholar] [CrossRef]
- Christina, Y.I.; Rifa'i, M.; Widodo, N.; Djati, M.S. The combination of Elephantopus scaber and Phaleria macrocarpa leaves extract promotes anticancer activity via downregulation of ER-alpha, Nrf2 and PI3K/AKT/mTOR pathway. J Ayurveda Integr Med 2022, 13, 100674. [Google Scholar] [CrossRef]
- Gao, Y.; Nie, Z.; Cao, H.; Huang, D.; Chen, M.; Xiang, Y.; Yu, X.; Zhang, S. Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro. Cancers (Basel) 2022, 14, 5976. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014, 6, 114–118. [Google Scholar]
| Compound | Plant source | Concentration | Cancer Type | Cancer Model | Altered Factors | Ref. | |
|---|---|---|---|---|---|---|---|
| Alantolactone | Inula helenium | 1–100 μM for cell lines | Breast | HUVEC cells for CAM assay | Reduced cell mobility and migration, Suppressed new blood vessel formation | [66] | |
| 5 mg/kg/day over 15 days for animal model |
BABL/c nude mice with MDA-MB-231 xenografts | ||||||
| 10, 20 and 30 μM | Breast | MCF-7 cells | Suppressed colony formation and migration, Inhibited invasion and cell migration by downregulating MMP-2, MMP-7, and MMP-9 | [67] | |||
| 2.5, 5 and 10 μM for cell lines | Melanoma | A375, A2058, A375R (vemurafenib-resistant variant of A375), HK-2 cell line, |
Inhibited STAT3 signaling | [68] | |||
| 10 mg/kg over 12 days for animalmodel | BALB/c nude mice | ||||||
| 0.01 and 0.1 μM | Prostate | PC3 cells | antagonized the STAT3 signaling pathway, leading to the upregulation of p53 and the downregulation of Oct-4, CD44, CD133, and Nanog expression, reduced stemness traits and inhibited migration in metastatic prostate cancer cells | [69] | |||
| U2OS, 0, 5, 10 or 20 μM HOS, 0, 15, 30 or 60 μM |
Osteosarcoma | U2OS and HOS cells | Reduced cell migration, invasion, and aggressiveness | [70] | |||
| 0, 4, 8, and 10 μM for cell lines |
Osteosarcoma | 143B, MG63, U2OS, and SaoS2, |
Decreased EMT-related markers, including Vimentin, Snail, and N-cadherin, Increased the epithelial marker E-cadherin, Reduced cell invasion, migration, and proliferation, Decreased in the expression of MMP-9, MMP-2, and MMP-7 | [71] | |||
| 5, 15 and 25 mg/kg over 21 days for animal model |
Athymic mice |
||||||
| 3, 10, and 30 μM for cell lines |
Lung | NCI-H460 cell lines |
Inhibited AKR1C1, resulting in a reduction in AKR1C1 expression, Decreased metastasis and cell growth | [72] | |||
| 10 and 20 mg/kg Over 21 days for animal model |
BALB/c nude mice | ||||||
| Ambrosin |
Hymenoclea salsola and Ambrosia maritima |
1, 2.5, or 5 μM. |
Breast | MCF-7, JIMT-1, and HCC1937, MCF-10A |
Reduced the populations of breast cancer stem cells, Inhibited cell migration | [75] | |
| 8, 32 and 64 μM | Breast | MDA-MB 231 cell | Inhibited cell proliferation, Downregulation of the Wnt/β-catenin pathway | [76] | |||
| 12.5, 25 and 50 μM |
Breast | MDA-MB 231 | Inhibited colony formation, Reduced MMP expression, Decreased the levels of phosphorylated GSK-3β and Akt, thereby inhibiting the Akt/β-catenin signaling pathway | [77] | |||
| Antrocin | Antrodia camphorata | 25, 50, and 75 μg/ml | Bladder | 5637 and T24 | Inhibited invasion, migration, and cell proliferation, Reduced the phosphorylation of FAK and paxillin, leading to the disruption of filopodia and lamellipodia formation, Increased E-cadherin levels, Decreased vimentin expression, Reduced MMP-2 activity | [79] | |
| 50,100,200 μM for cell lines |
Breast | MCF7 and MDA-MB-231 |
Downregulating the expression of oncogenes and stemness-related markers such as β-catenin, Akt, and Notch1, Reduction in migration, tumorigenesis, and proliferation | [80] | |||
| 30 mg/kg over two weeks for animal model |
NOD/SCID mice |
||||||
| 20, 50, 100, 200, and 300 μM |
Kidney | RCC 786-0 cells | Inhibited the Src, FAK, and ERK1/2 signaling pathways, leading to a reduction in the phosphorylation of paxillin, C/EBP-β, and total c-Fos levels, Decreased the expression of MMP-7 and vimentin, Disrupted cell migration, invasion, and the formation of lamellipodia | [81] | |||
| Artemisinin | Artemisia annua | 7.5, 15, or 30 μM | Lung | A549 and H1299 cell lines | Inhibited the migration and invasion, Suppressed the activity of MMPs, Suppressed the expression of EMT-related proteins, including N-cadherin and vimentin, and cancer stem cell (CSC) markers like Nanog, Sox2, and Oct3/4, increased E-cadherin expression, Disrupted the Wnt/β-catenin signaling pathway | [84] | |
| 12.5, 25, 50, or 75 μM | Liver | HepG2 and SMMC-7721 cells | Decreased the expression of MMP2 and upregulation of TIMP2, Inhibited the activation of p38 and ERK1/2, Enhanced cell adhesion by increasing Cdc42 activity, which activated E-cadherin, Inhibited cell motility, migration, and metastasis | [85] | |||
| 2.5 μM | Lung | H1395, A549, LXF289 cells, Calu3 and H1299, H460 | Inhibited the expression of MMP-2, MMP-7, and u-PA, leading to in the inhibition of metastasis and invasion | [86] | |||
| Brevilin A | Centipeda minima | 0.25 and 0.5 μM for cell lines |
Melanoma | A375 and A2058 |
Inhibited the JAK2/STAT3 pathway by reducing the phosphorylation of JAK2 and STAT3, Suppressed cell invasion and migration | [89] | |
| 4.5 mg/kg and 9 mg/kg Over 21 for animal model |
nu/nu BALB/c mice | ||||||
| 5 , 10 , and 15 μM | Liver | HepG2 and SMMC-7221 | The downregulation of MMP-2 and MMP-9, inhibited the Wnt/β-catenin and STAT3/Snail signaling pathways, resulting in decreased cell invasion | [90] | |||
| 1.25, 2.5, 5, 10, 20 μM for cell lines |
Breast | MDA-MB 231 and MDA-MB 468 |
Inhibited cell migration and reduced the phosphorylation and expression of Akt, mTOR, and STAT3, thereby suppressing the Akt-mTOR and STAT3 signaling pathways | [91] | |||
|
25 and 50 mg/kg/day over 22 days for animal model |
BALB/c nude mice | ||||||
| 2.5, 5, 10 μM | Colorectal | HCT-116 and CT26 | Suppressed the expression of MMP-2 and VEGF, Inhibited STAT3, Suppressed angiogenesis, Reduced cell migration and invasion | [92] | |||
| 2.5, 5 and 10 μM for cell lines |
Colorectal | LOVO, HCT-116, HT29 and CT26, NCM460, Human hepatic stellate cell line LX-2 and the mouse hepatic stellate cell line JS1 |
Inhibited colorectal liver metastasis and tumor growth by targeting the VEGF-IL6-STAT3 axis | [93] | |||
| 4 and 8 mg/kg Over 2 weeks for animal model |
|||||||
| BALB/c mice |
|||||||
| Bigelovin | Inula helianthus-aquatica C. Y. Wu | Zebrafish Embryos: 25, 50, and 100 μM Endothelial Cells (HMEC-1): 400–1600 nM Human PBMCs: 62.5–250 nM Monocyte Adhesion (THP-1 to HMEC-1): 500–4000 nM |
Non-cancerous cell lines | Zebrafish embryos, Endothelial Cells (HMEC-1), Human PBMCs and THP-1 Monocytes |
Suppressed the formation of subintestinal vessels in zebrafish embryos, Induced anti-angiogenic effects by downregulating angiogenesis-related genes (Ang-1, Ang-2, Tie-1, and Tie-2), reducing Th1 cytokine production (IFN-γ, IL-2, and IL-12), and inhibiting CAM gene expression (ICAM-1, VCAM-1, and E-selectin) | [95] | |
| 0.75, 1.5 or 3 μM for cell lines | Colon | colon 26-M01, and HCT116 |
Significant changes in key molecules, including p-STAT3, STAT3, Rock, β-catenin, N-cadherin, Rac1/2/3, and RhoA, resulting in the disruption of the IL6-STAT3 and cofilin pathways, Inhibited cell motility, migration, EMT, angiogenesis, and cell growth, Suppressed liver and lung metastasis | [96] | |||
| 0.3, 1 and 3 mg/kg over 18 days for animal model | BALB/c mice | ||||||
| Britannin | Inula britannica L | 20, 40, 80 μM | Gastric | AGS and MKN-45 | Reduction in the expression of MMP-9, TWIST-1, and COX-2 | [98] | |
| 2.7 and 6 μM | Liver | BEL-7402 and HepG2 | Suppressed tumor cell migration | [99] | |||
| 1, 3, 10 μM for cell lines | Colorectal, lung, cervical, and liver | HCT116, A549, HeLa, Hep3B, HUVECs |
Inhibited invasion, migration, and angiogenesis by reducing PD-L1 levels, Suppressed the expression of VEGF and MMP-9 | [100] | |||
| BALB/c nude mice | |||||||
| 5 mg/kg or 15 mg/kg Over 30 days for animal model |
|||||||
| 5, 10, 20 μM | Lung | A549 | reduced KLF5 expression, Inhibited cell migration | [101] | |||
| Costunolide | Saussurea lappa | 20, 50 μM for cell lines |
Breast | MDA-MB 231 |
Inhibited NF-κB, which led to the suppression of TNFα-induced migration and invasion of cancer cells, Downregulation of MMP-9, Suppressed metastasis | [103] | |
| 20 μM over 30 days for animal model | nude (Nu/Nu) mice | ||||||
| 2.5, 5, and 10 μM | Colorectal | HCT-15, HCT-116, and DLD1 | Reduction in cell migration and invasion, Decreased vimentin and N-cadherin levels, Increased E-cadherin expression | [104] | |||
| 1, 5, 25 μM for cell lines |
Skin | HUVECs, Human epidermoid carcinoma KB3-1 Cells |
Interfered with the VEGFR KDR/Flk-1 signaling pathways related to angiogenic factors, leading to the suppression of pro-angiogenic activity | [105] | |||
| 100 mg/kg over 7 days for animal model | BALB/c mice | ||||||
| 12, 24, and 48 μM | Lung | H1299 | Inhibited cell migration and invasion, suppressed the EMT process by upregulation of E-cadherin and a downregulation of N-cadherin, Reduction in mRNA expression levels of integrins α2 and β1, as well as MMP2 |
[106] | |||
| 0.1-10 μM | Neuroblastoma | NB-39 | inhibition of cell migration and invasion, the downregulation of MMP-2 | [107] | |||
| Cynaropicrin | Cynara scolymus L | 5, 7.5, 10 μM | Colorectal | HCT116, RKO, and DLD-1, | Reduction in cell migration | [109] | |
| 3, 10, 30 μM | Melanoma | A375 | Reduction in the MAPK/ERK and NF-κB pathways, Inhibited cell motility and invasion | [110] | |||
| Dehydrocostus lactone | Aucklandiae Radix | 2.0, 4.0, 8.0 and 16.0 μM | Lung | H1299 cells | Inhibited cell migration and invasion, Upregulation of the expression of E-cadherin, Downregulation of N-cadherin, Snail, integrin α2, and MMP-2 | [112] | |
| 3 μg/mL | Larynx | TU212 and HBE | Downregulation of MMP-2 and MMP-9, Inhibited cell migration and invasion | [113] | |||
| Deoxyelephantopin | Elephantopus scaber | HCT 116 (3.73), K562 (0.5), KB (0.41), and T47D (0.91) μg/mL | Colorectal, chronic myeloid leukemia, oral, and breast | HCT 116, K562, KB, and T47D cell | Suppressed cell migration and invasion, Decreased the expression of uPA, uPAR, MMP-2, and MMP-9, Upregulation of TIMP-1 and TIMP-2 | [115] | |
| 12.28 µg/ml | Lung | A549 cells | Decreased migration and invasion, Decreased the expression levels of NF-kB, IkBa, MMP-2, MMP-9, uPA, and uPAR , Increased TIMP-2 levels , Reduced protein levels of p-ERK 1/2 and p-Akt, along with increased levels of p-p38 and p-JNK, contributing to metastasis suppression | [116] | |||
| 1.5, 3, 6 μM for cell lines |
Melanoma | A375LM5IF4g/Luc, |
Inhibited N-cadherin, MMP2, vimentin, and integrin-4, Suppressed pulmonary vascular permeability, VEGF+ , neovascularization marker CD31, and N-cadherin | [117] | |||
| 20 mg/kg over 27 days for animal model | NOD/SCID mice | ||||||
| 1.0, 2.5, 5.0 and 150 μM | Adenocarcinoma | TS/A cells | Inhibited cell motility. Disrupted adhesion formation by inhibiting m-calpain's enzymatic activity. Inhibited lamellipodia formation and actin filament organization | [118] | |||
| 0.5 to 3 μM for cell lines |
Melanoma | B16 murine melanoma cell line | Inhibited cell migration, suppressed lung metastasis | [119] | |||
| 10 mg/kg over 21 days for animal model | C57BL/6J mice | ||||||
| Eupalinolide | Eupatorium lindleyanum DC. | 10 μM | Breast | MDA-MB-468, | STAT3 inhibitory effects | [122] | |
| 2.5, 5, 10 μM | Pancreas | MiaPaCa-2, PANC-1, and PL-45 | Inhibition of cell migration and invasion | [123] | |||
| 4, 8 μM | Breast | MDA-MB-231, MDA-MB-468, and MCF-7 |
Downregulation of STAT3 and p-STAT3 | [121] | |||
| 1.25, 2.5 μM for cell lines |
Breast | U251 and MDA-MB-231 cells |
Decreased in STAT3, p-STAT3, MMP-9, and MMP-2, Suppressed cancer cell metastasis |
[124] | |||
| 30 mg/kg over 18 days for animal model |
BALB/c nu/nu mice | ||||||
| 7, 14 or 28 μM | Liver | MHCC97-L and HCCLM3 | Suppressed cell motility and migration through the downregulation of vimentin and the upregulation of ZEB1, N-cadherin, and fibronectin | [125] | |||
| 1, 2 μM | Larynx | TU212 cells | Inhibited EMT markers by a decrease in N-cadherin expression and an increase in E-cadherin, Reduction in cell motility, Suppressed LSD1 | [126] | |||
| Gaillardin | Inula oculus-christi | 20, 40, and 80 μM | Gastric | MKN45 and AGS | Suppressed NF-κB, which subsequently downregulated its target genes, including COX-2, TWIST-1, and MMP-9 | [128] | |
| Helenalin | Arnica montana and Arnica chamissonis ssp. Foliosa | 2.5, 5 μM | Rhabdomyosarcoma | RH30 and RD cells | Inhibited cell migration | [130] | |
| Isoalantolactone | Inula helenium L. | 25, 75, 150 μM | Liver | Hep-G2 | Reduced cell invasion and migration | [132] | |
| 1, 2, 4 μM | Breast | MDA-MB-231 | Inhibited the p38 and MAPK/NF-κB signaling pathways, leading to the suppression of cell migration and invasive activities | [133] | |||
| 2 or 4 μg/ml | Pancreas | PANC-1 and SW1990 | Suppressed colony formation and cell migration | [131] | |||
| 5, 10, 20 μM | Colorectal | HCT116 and SW620 | Suppressed colony formation | [134] | |||
| 5, 10, 20 μM | Endometrium | HEC-1-B | Inhibited both migratory properties and invasiveness | [135] | |||
| 4.5, 9.0, or 18 μM | Liver | HuH7 | Inhibition of cell invasion | [136] | |||
| 20 μM | Pancreas | PANC-1, AsPC-1, and BxPC-3 | Suppressed cell migration and invasion | [137] | |||
| Isodeoxyelephantopin | Elephantopus scaber L. | 0.4, 0.8, 1.6, 3.2, 6.4 , 12.8 μM | Lung | H1299 and A549 | Suppressed colony formation | [139] | |
| 50 μM | Breast | MDA-MB-231 | Suppressed cell migration, Inhibited the expression of MMP-2 and MMP-9 | [140] | |||
| 2 μM | Lung | H1299 | Inhibited cell invasion, Suppressed the expression of MMP-9 and ICAM-1 |
[141] | |||
| Lactucopicrin | Lactuca virosa | 7.5, 15, 30 μM | Skin | SKMEL-5 | Reduction in p-PI3K, p-Akt, and p-mTOR levels | [143] | |
| 12.5, 25, 50 μM | Osteosarcoma | Saos-2 cells | Inhibited cell migration and invasion | [144] | |||
| 7.5 and 10 μM | Glioblastoma | U87MG cells | Reduction in colony formation, Decreased cell motility, Reduction in Akt phosphorylation levels | [145] | |||
| Parthenolide | Tanacetum parthenium | 4 and 8 μM | Kidney | 786-O and ACHN cells | Decreased cell proliferation, Inhibited both cell migration and invasion, Suppressed MMP-2 and MMP-9 expression levels, Increased E-cadherin levels, Decreased N-cadherin, vimentin, and Snail levels, Inhibited ALDH1, CD133, Oct4, and Sox2 , Reduction in the number of spheres, Decline in p-PI3K and p-AKT expression | [146] | |
| 2 μM |
MCF-7 | Downregulated vimentin expression | [148] | ||||
| 2.5, 5, 10, 20, 40 μM for cell lines |
Colorectal | HT-29, DLD-1, and HCT116 cells | Decreased HIF-1α expression, suppressed migration and invasion, Reduced the expression of MMP-2 and MMP-9, Increased E-cadherin levels, Downregulated EMT markers such as β-catenin, vimentin, Slug, Snail, and Twist, Reduced levels of CA IX, a marker of hypoxia, Decreased the number of NF-κB subunit p65-positive cells, Reduced angiogenesis marker von Willebrand factor (VWF) and EMT marker vimentin | [149] | |||
| 4 mg/kg over 27 days for animal model |
|||||||
| Female athymic nude mice | |||||||
| 5, 10, 20 μM | Colorectal | SW620 cells | Inhibited cell migration and invasion, Upregulation of E-cadherin, Downregulation of β-catenin, Snail, vimentin, MMP-2, MMP-9, and COX-2 expression | [150] | |||
| 0.2, 2, 10, 20, 200 μg/mL for cell lines |
Osteosarcoma | LM8 cells |
Suppression of NF-κB DNA binding and transcriptional activity, Reduction in VEGF expression, Inhibited tumor invasion, Reduced pulmonary metastasis, Decreased p65 expression, Suppressed VEGF expression in metastatic lung tumors and surrounding lung tissue | [151] | |||
| 0.01, 0.1, 1, or 100 μg/kg or 1 mg/kg daily over 25 days for animal model | C3H male mice | ||||||
| 15, 25, 50 μM for cell lines |
MDA-MB 231 |
Downregulation of NF-κB activity, Inhibited cell migration, Reduced vimentin expression, Reduction in the levels of VEGF, MMP2, MMP9, and p65 | [152] | ||||
| 50 mg/Kg daily over 16 days for animal model | Nude athymic mice | ||||||
| 5 μM | Colorectal | SW480 and HT-29 cells | Inhibited TGF-β1-induced EMT, Reducing cell migration and invasion, decreased vimentin, β-catenin, Snail, and Slug levels, Increased E-cadherin expression | [153] | |||
| Scabertopin | Elephantopus scaber L. | 10, 15 μM | Bladder | J82 cells | Suppressed cell migration and invasion, Decreased the expression levels of MMP-9, phospho-FAK (Tyr397), phospho-AKT (Ser472, Ser473, Ser474), and phospho-PI3K (Tyr607) | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





