1. Introduction
The sympathetic nervous system (SNS) together with the parasympathetic nervous system (PSNS) constitute the autonomic nervous system (ANS) also called involuntary nervous system because it functions without conscious control . The physiology of most organs in the body is influenced by the both the SNS and PSNS that play a major homeostatic role. The efferent nervous signaling of the SNS originates in preganglionic neurons located in the lateral horn of the gray matter of the spinal cord or in the brainstem. The cholinergic axons of these neurons synapse with post ganglionic neurons located in paravertebral ganglia outside the spinal cord. In turn, post ganglionic neurons send adrenergic axons to peripheral tissues. The post ganglionic fibers of the SNS do not synapse directly with target cells but rather release their neurotransmitters along a significant portion of the axon and, by doing this, affect a large area of the target tissue. This happens through multiple swelling on the nerve terminals, called varicosities (Mc Corry 2009)Therefore, the nervous signaling may activate adrenergic receptors on many cells simultaneously. In steady state situation, in mammals, the SNS is activated during the active phase of the circadian rest-activity rhythm by the suprachiasmatic nucleus (SCN), the master biological clock located in the hypothalamus (Buijs et al. 2006). As the overall main effect of the SNS is to prepare the organism for physical activity by modulating the blood flow in peripheral organs, its daily activation is essential for the homeostatic response to the environment. Furthermore, the SNS may be fully activated by psychogenic stimuli such as stress, determining the fight-or flight response (Scott-Solomon et al. 2021) . The peripheral sympathetic neurotransmitters are catecholamines. The biosynthetic pathway forming catecholamines is the following: the non essential amino acid tyrosine is hydroxylated to the meta position to form 3,4-dihydroxy-L-phenylalanine (L-DOPA) by the enzyme tyrosine hydroxylase. Then, under the action of DOPA decarboxylase , L-DOPA becomes dopamine (DA) that in turn is converted to norepinephrine (NE) by dopamine b-hydroxylase. Finally, epinephrine (E) is formed by the action of phenylethanolamine N-methyltranferase on NE. NE and E bind and activate G-coupled receptors called adrenergic receptors (ARs), a term derived by adrenaline and noradrenaline that stands for E and NE . Based on pharmacological studies and molecular cloning, we can recognize nine types of ARs, that is a1, a2 and b, each of which is present in three subtypes, namely alpha1A, alpha1B, alpha1D; alpha2A, alpha2B, alpha2C and beta1, beta2, beta3 (Graham 1990). Relevant to the present review, it has been recently demonstrated that some sympathetic fibers may acquire postnatally the ability to conveys cholinergic signals in the bone marrow (BM) acting on alpha7 nicotinic receptors (Fielding et al. 2022).
The hematopoietic system is shielded in the hardest organs of the body, i.e. the bones and this location may possibly reflect its homeostatic relevance. In fact, hematopoiesis takes place in the BM and produces an astonishing 500 billions of cells every day (Kaushansky 2018). These highly specialized cells constitute the blood and the immune system and have functions as distinct as transporting oxygen and building the immune response (Fielding et al. 2022). A miniscule and rare population of hematopoietic stem cells (HSCs) endowed with self-renewal as well as differentiation capabilities is responsible for such an amazing performance (Wang et al. 1997). Nevertheless, in steady state condition HSCs are very quiescent cells and enter the cell cycle quite rarely (Cheshier 1999). In fact, homeostatic hematopoiesis is essentially dependent on the proliferation of lineage specific progenitor cells (Höfer and Rodewald 2018). On the contrary, in emergency situations such as serious bleeding or infection, HSCs are mobilized to produce progenitor and effector cells (Cora et al. 2018; Kandarakov et al. 2022).
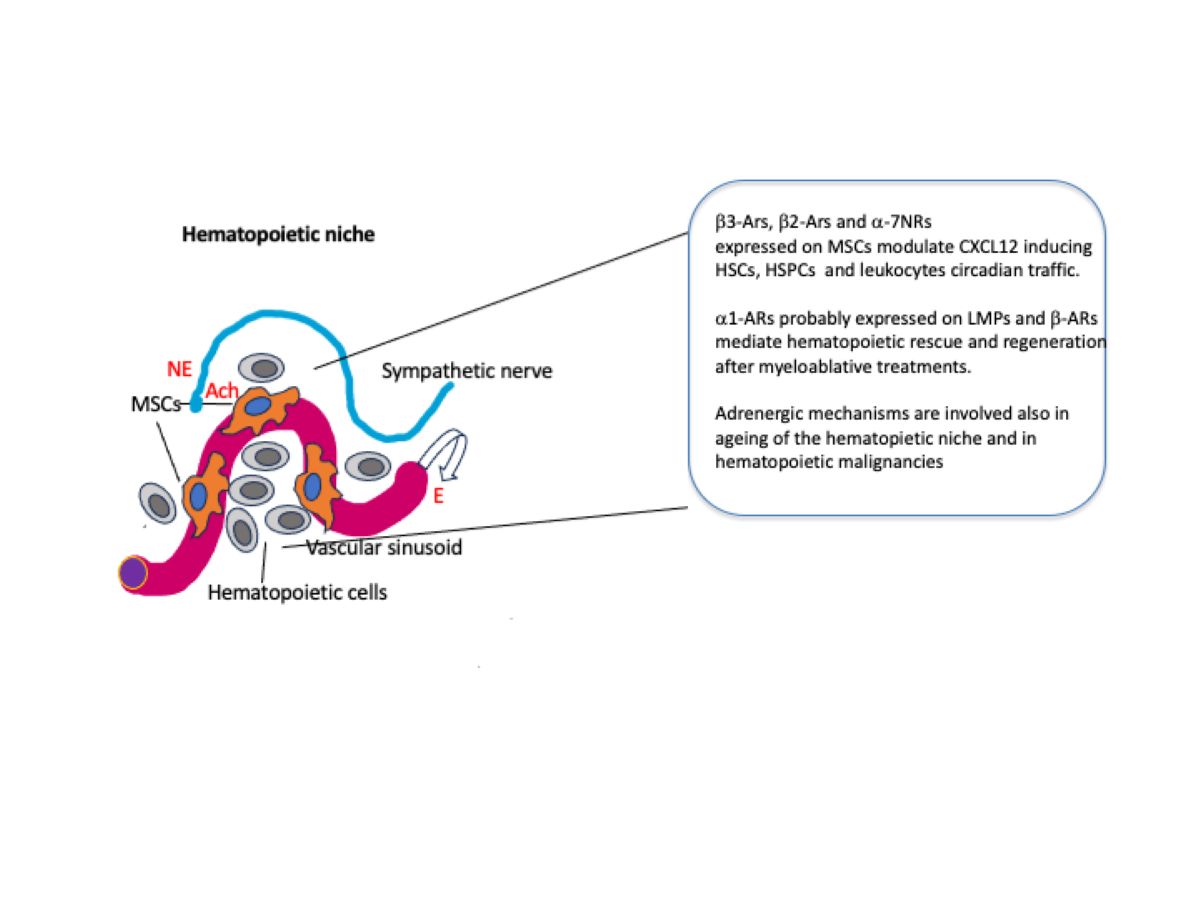
HSCs reside in the BM, a loose tissue which permeates the bone trabeculae and is constituted essentially by stromal cells, vessels and hematopoietic cells. The arteries supplying the BM enter through the nutrient foramen and branch into smaller arterioles which then connect to the vascular sinusoids thru transition zone vessels and finally, by a central venous sinus, into the veins leaving the bone via the nutrient channel. The arterioles are wrapped in sympathetic nerve fibers, perivascular mesenchymal cells and non-myelinating Schwann cells (Cora et al. 2018; Fielding and Méndez-Ferrer 2020).
The evidence that outside the BM, HSCs loose their function led to the concept of hematopoietic niche ( Morrison and Scadden 2014). This structure supports hematopoiesis through a complex ad close interaction of many cell types supplying growth factors and adhesion molecules. The exact location of the niches is still not clear, some studies place them in the proximity of the endosteal region, others close to the vascular sinusoids ( Wei and Frenette 2018). At variance, it has been recently proposed that hematopoietic niches are distributed at random in the BM ( Kokkaliarisetal. 2020). More recent studies showed that myeloid-biased HSCs are located near sinusoids while lymphoid-biased HSCs are in proximity of arterioles ( Pinho etal al. 2018). It remains to elucidate where are located the niches supporting unbiased HSCs. In any case, endothelial cells and perivascular cells that include mesenchymal stromal cells (MSCs) are crucial for production of CXCL12 and stem cell factor (SCF) that are fundamental for the maintenance of both HSCs and their immediate progeny, i.e. the hematopoietic stem and progenitor cells (HSPCs) (Wei and Frenette 2018; Kandarakov et al. 2022). Besides endothelial cells and MSCs, that comprehend different cell subpopulations (Kandarakov et al. 2022), a number of other cell types contribute to the hematopoietic function of the BM niche. Osteoblasts, megakaryocytes, macrophages, neutrophils, adipocytes and regulatory T cells have been reported to play a role in the hematopoietic niche (Cossio et al. 2019;Kandarakov et al. 2022).
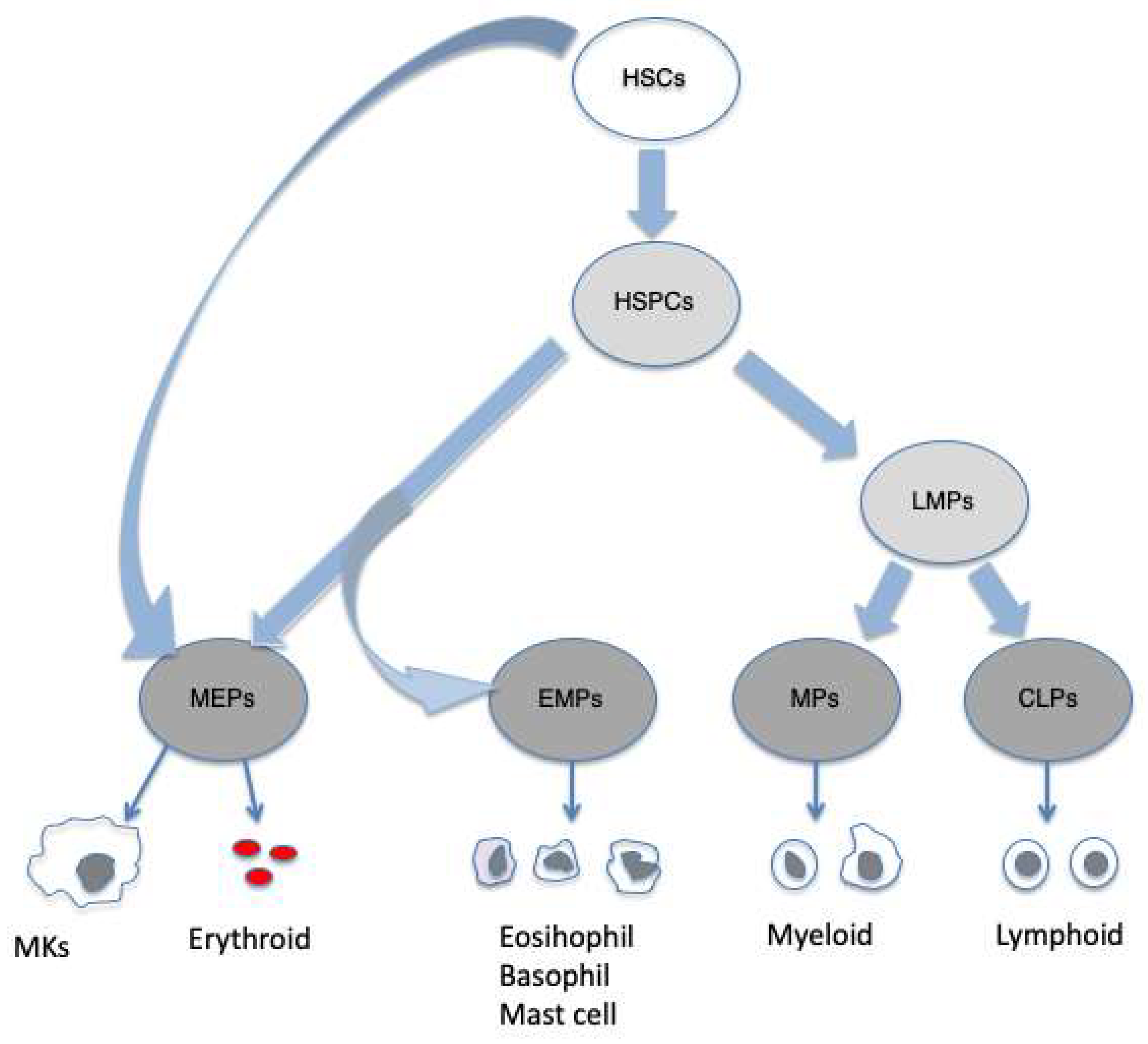
Hematopoiesis is a very complex and tightly regulated mechanisms. The classical hierarchical model describes the first step of HSCs differention as leading to the formation of multipotent progenitor cells (MMPs). The differentiation of MMPs
into common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) constitutes the second step. The progeny of CMPs are granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythroid progenitors (MEPs). GMPs give rise to monocytes, macrophages, mast cells and granulocytes and MEPs differentiate into erythrocytes and
megakaryocytes. The lymphoid cells derived from CLPs include T an B lymphocytes, natural killer (NK) cells, dendritic cells (DCs) and the innate lymphoid cells (ILCs) (Akashi et al.2000; Nakorn et al. 2003; Pronk et al. 2007;Karsunky etal. 2008; Vivier et al. 2018). However , more recently a different model has been proposed in which HSCs are described as an heterogeneous cell populations with lineage- priming including multipotent and megakaryocyte/platelet-biased HSCs. In this model , hematopoiesis is seen as a gradual transition with cells acquiring increasing lineage priming while losing pluripotency. Early stage MEPs are produced with a shared pathway with mast cells, eosinophils and basophils, but separate from other myeloid and lymphoid lineages. In addition, megakaryocytes may be directly produced by HSCs , a pathway that could be important in emergency situations or in malignancies (Psaila and Mead 2019),
Figure 1.
Hematopoiesis occurs by a continuous flow of differentiation steps in which multipotency may coexists with various degrees of lineage priming. Lymphoid-primed multipotent progenitor cells (LMPs); eosinophil, basophil-mast cells progenitors (EMPs); myeloid progenitors (EMPs); megacaryocytes (MK).
2. Sympathetic Modulation of Hematopoiesis
The notion that BM is innervated by myelinated and non-myelinated nerve fibers dates back to 1916n (Drinker and Drinker, 1916). However, the evidence that such an innervation enter the BM parenchyma and belongs largely to the ANS is much more recent (Calvon1968). Similarly, the first evidence that adrenergic agents could influence regenerative hematopoiesis in a model of syngeneic BM transplantation was published only in 1992 (Maestroni et al. 1992). Later it was also shown that NE concentration in the BM shows a circadian rhythm that could be abolished by chemical sympathectomy suggesting that the NE oscillation was dependent on the rhythmic oscillation of the SNS activity. Remarkably, the NE rhythm correlated positively with the proliferative activity of BM cells suggesting a role of NE in steady state hematopoiesis (Maestroni et al. 1998). The intriguing evidence that NE may affect proliferation of BM cells came also from another study showing that the administration of exogenous NE could protect mice inoculated with lethal doses of the cytotoxic drug carboplatin. The hematopoietic rescue was apparently exerted at the level of myeloid progenitor cells ( Maestroni et al. 1997). This finding was then confirmed in both mice and humans even at the level of HSCs (Spiege et al. 2007; Lucas et al. 2013). The above reported studies (Maestroni et al. 1992; Maestroni et val.1997; Maestroni et al. 1998), claimed that the adrenergic effects observed involved alpha1-ARs apparently expressed on lymphoid stem/progenitor cells (Togni and Maestroni, 1996) and that their activation by NE promoted lymphoid differentiation while inhibiting myeloid differentiation, a finding that has not yet been confirmed by other groups.
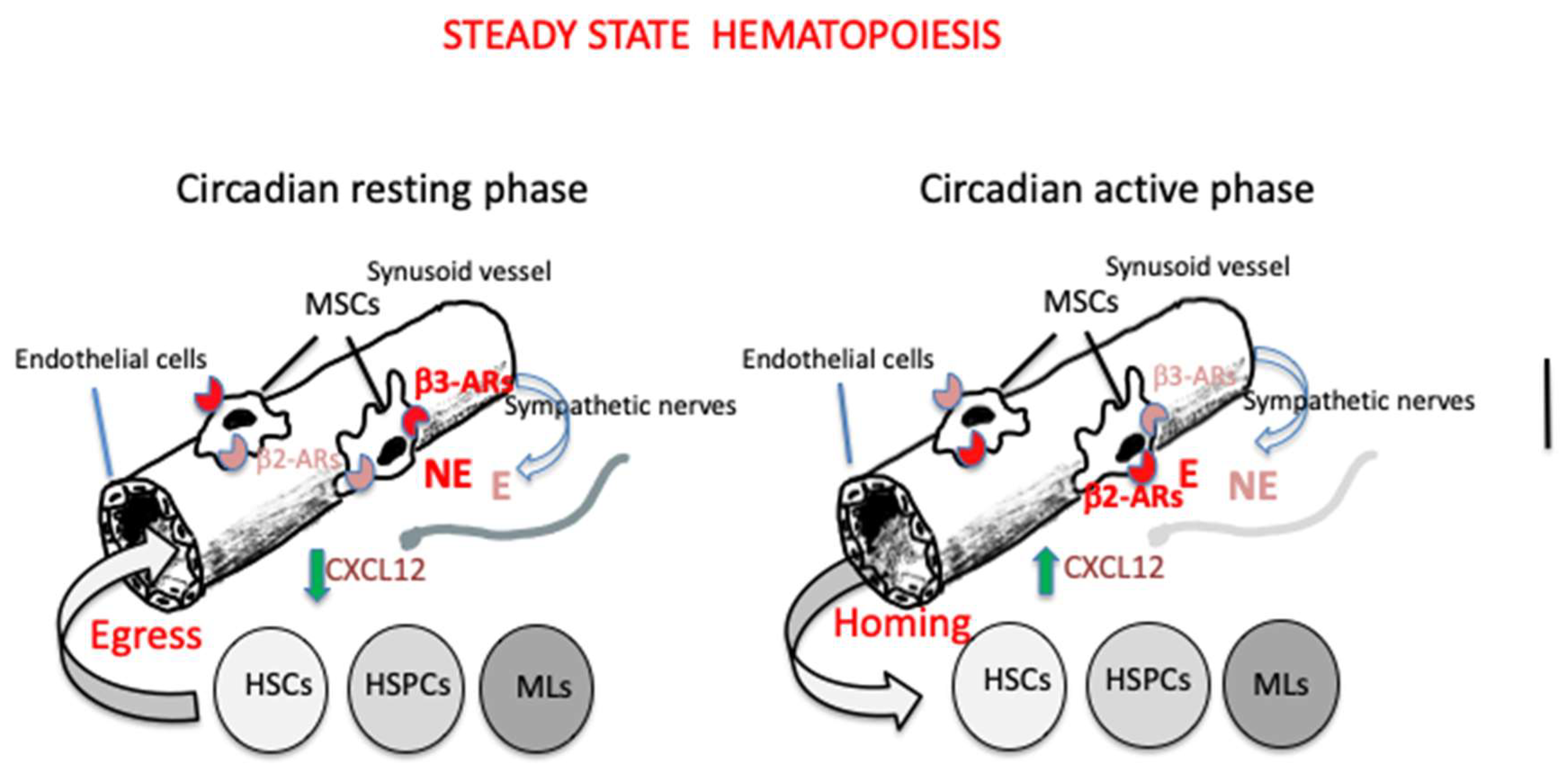
2.1. Steady State Hematopoiesis
Every day, HSCs, HSPCs and mature leukocytes egress from the BM, enter the blood circulation and then return back home and, in the case of mature leukocytes, may infiltrate peripheral extramedullary tissues (Méndez-Ferrer et al. 2008). This circadian rhythm is mainly regulated by photic signals provided by the light/dark cycle of the day. The photic information activates retinal ganglion cells expressing the photopigment melanopsin and reach the SCN via the retino-hypothalamic tract. In turn, the SCN synchronized with the external environment, signals to peripheral organs, including the BM, via the SNS (Méndez-Ferrer et al. 2008; Maestroni 2023). One of the key factors governing the cellular traffic in and out the BM is the expression of the chemokine CXCL12 by a particular cell population surrounding sinusoidal endothelial cells or located in the proximity of the endosteum. These CXCL12-abundant reticular cells (CAR cells) largely overlap with mesenchymal cells expressing the green fluorescent protein (GFP) and containing the intermediate filament protein nestin (Nes-GFP+ cells) and with leptin receptor positive (LepR+ )cells ( Fielding and Méndez-Ferrer 2020). CXCL12 binds to its receptor CXCR4 expressed on most cells including hematopoietic and endothelial cells and organizes cell migration, cell homing and cell retention in the BM (Bianchi and Mezzapelle 2020). Nes-GFP+ cells co-localize with HSCs in the niche and are associated with adrenergic nerve fibers (Méndez-Ferrer 2010) that are maintained in the BM by nerve growth factor (NGF) produced by LepR+ cells (Méndez-Ferrer 2010). Nes-GFP+ cells express beta3-ARs whose activation downregulates CXCL12 expression promoting HSCs, HSPCs and leukocytes migration. This happens in the resting phase of the circadian rhythm in both mice and humans Méndez-Ferrer et al. 2008; Méndez-Ferrer et al.2017). On the contrary, during the activity phase (day in humans and night in mice) the opposite occurs, the expression of CXCL12 increases and cells return to the BM (Méndez-Ferrer et al. 2008). The action of beta3-ARs, that would induce cell egress, is contrasted by beta2-ARs that favor BM homing. beta2-ARs are involved also in the leukocytes adhesion to endothelial cells and subsequent recruitment in peripheral tissues (Méndez-Ferrer et al. 2010).Thus, rather surprisingly, similar noradrenergic mechanisms seem responsible for two opposite effects, i.e. BM egress during the resting phase of the circadian cycle and BM homing during the active phase. A recent study addressed this problem in mice and found that during the light phase of the photoperiod (resting phase), sympathetic fibers, that acquired cholinergic properties postnatally (Fielding et al. 2022), reduce vascular adhesion through acetylcholine collaborating with beta3-ARs to allow HSCs and leukocytes to enter the blood circulation (García-García et al.2020). Moreover, the expression of beta3-ARs and beta2-ARs are in phase with the peak of their high affinity adrenergic ligands , that is NE for b3-ARs during day time and E for beta2-ARs during the night. In addition, during the night, the central parasympathetic inhibition of the sympathetic tone decreases beta3-ARs activity while allowing the activation of beta2-ARs by E secreted upon activation of the adrenal glands by the hypothalamo-pituitary axis. This combination finally results in increased CXCL12 expression and BM homing (Méndez-Ferre et al. 2008; García-García et al.2020). Thus, it is suggested that the circadian traffic of HSCs and leukocytes is directed by both the SNS and the PSNS.
The current opinion about the physiological relevance of the circadian regulation of HSCs, HSPCs and mature leukocytes circulation as well as of their homing in the BM or peripheral tissues, claims that such a traffic is necessary to maintain an efficient hematopoietic niche as well as to patrol the organism to ensure the appropriate response in case of infection [Méendez-Ferre et al. 2008; Maestroni 2023).
A scheme illustrating this cellular traffic in steady state hematopoiesis is reported in
Figure 2.
2.2. HSCs-Based Therapeutics and Regenerative Hematopoiesis
HSCs transplantation is widely used to treat hematological malignancies, bone marrow failure and autoimmune diseases (Bozdağ et al.2018). Critical issues in this procedure are the number of HSCs that can be harvested and their successful engraftment in the host. The evidence that HSCs do circulate in the blood and undergo self renewal in the BM niche at different times of the circadian cycle provides the possibility to improve the efficacy of both procedures. Therefore, apheresis to collect HSCs should be performed at the beginning of the resting phase rather than in the morning and the opposite schedule should be applied for HSCs injection. In addition, as both phenomena are regulated by adrenergic signals activating beta3-ARs for HSCs egress beta2-ARs for HSCs homing, and one could envisage a pharmacological approach using the appropriate adrenergic agonists/antagonists to further improve the success of these therapeutic trials. This would stand also for the clinical use of granulocyte colony stimulating factor (G-CSF), used to enforce HSCs mobilization, because it has been shown that even its action depends on the SNS activity. In fact, inhibition of the adrenergic signaling prevents the downregulation of CXCL12 and hence the mobilization of HSCs, while administration of beta2-ARs agonists had the opposite effect (Katayama et al. 2006). At variance, a recent study in mice shows that a CXCR4 antagonist synergizes with the beta-ARs blocker propranolol in inducing HSCs mobilization (Sukhtankar et al. 2023).
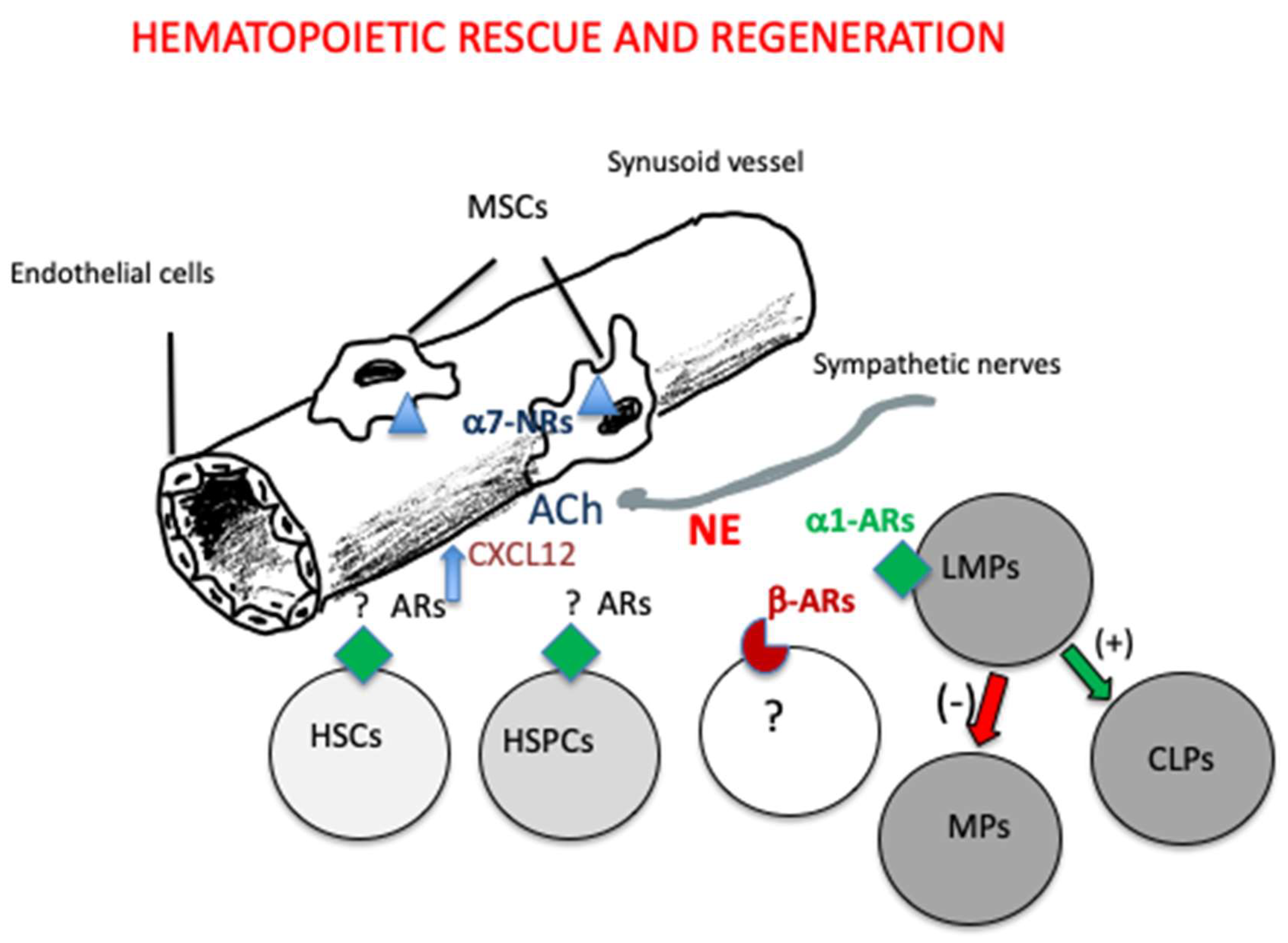
The use of adrenergic agents might be considered also to enhance regeneration of the hematopoietic system after irradiation or chemotherapy in cancer patients. In fact, it has been established that noradrenergic signals to beta-ARs are required to regenerate hematopoiesis after myeloablative treatment (Maestroni et a. 1997; Lucas et al. 2013). In addition, due to the ability of alpha7-nicotinic receptors on nes-GFP+ MSCs to promote HSCs quiescence , the use of nicotinic receptors agonists may protect them from the toxic effect of these anti-cancer therapies. On the other hand, an excessive and chronic stimulation of nicotinic receptors as it happens in the case of smoking may delay hematopoietic regeneration (Fielding et al. 2022).
Previous studies (Maestroni et al. 1992; Togni and Maestroni 1996; Maestroni et al. 1997: Maestroni et al. 1998) have also shown that alpha1-ARs expressed on lymphoid- ¨primed progenitor cells are involved in hematopoietic rescue and regeneration (
Figure 3, see also the Discussion section).
2.3. Hematopoiesis Under Stress and Ageing
Emergency myelopoiesis occurs when granulocytes are recruited to the site of an acute physical injury with inflammation and infection. This results in accumulation of myeloid-derived suppressor cells (MDSCs), reduced T cell functions and increased proliferation of HSCs and HSPCs (Scumpia et al. 2010). MDSCs have both inflammatory and immunosuppressive properties and play an important role in inflammation and cancer immunity (Ostrang-Rosemberg and Sinha 2009). Activation of SNS that is common in the stress responses plays a crucial role in this process by potentiating myelopoiesis (Loftus et al. 2018).
Chronic stress is integrated by brain structures that signal the periphery via the ANS and the HPA axis. The stress effects on hematopoiesis results in neurophilia, monocytosis and lymphopenia and hence in an upregulation of inflammation in peripheral tissues. This may increase the risk for many diseases including myocardial infarction and stroke (Heidt et al. 2014; Chan at al. 2023). The mechanism underlying these phenomena depends on increased adrenergic signalling to beta3-ARs on nestin+MSCs resulting in reduced CXCL12 production and finally in increased neutrophils and inflammatory monocytes generation (Heidt et al. 2014).
Ageing is associated with a loss of the regenerative capacities and myeloid-biased differentiation of HSCs, a condition that may favor the development of blood disorders ( Guidi and Geiger 2017). These alterations are associated with decreased lymphoid progenitors, lymphopenia and decreased immunity in the elderly. Hematopoietic ageing is often associated with clonal hematopoiesis and increased risk of both hematological malignancies and atherosclerosis (Groarke and Young 2019). Alteration of the sympathetic innervation of the hematopoietic niche provokes HSCs ageing. In fact, it has been reported that loss of SNS nerves and beta3-ARs signaling may induce ageing of the hematopoietic system (Maryanovich et al. 2018). At variance, another study suggests that ageing of the BM niche is associated with increased SNS innervation but confirmed, however, the reduced beta3-ARs signaling complemented by increased beta2-ARs activity leading to increased megacaryiopoiesis (Ho et al. 2019). Most interesting , both reports agree that beta3-ARs agonists may rejuvenate the HSCs niche (Maryanovich et al. 2018; Ho et al. 2019).
Interestingly, both stress and ageing seems to induce similar alterations in hematopoiesis and are linked to an increased or disturbed SNS activity.
2.3. Hematological Malignancies
Leukemia and myeloproliferative neoplasms (MPNs) modify the BM niche affecting migration and differentiation of normal HSCs. A fundamental study reports that MPNs patients and mice expressing the human mutation JAK2(V617F) in their HSCs show reduced sympathetic innervation , Schwann cells and nestin + MSCs in their BM. The MSCs reduction in mice does not derive from differentiation but is apparently caused by the neural damage caused by Schwann cells apoptosis induced by interleukin-1 beta produced by mutant HSCs. On the other hand, depletion of nestin+ MSCs or of their product CXCL12 promoted the expansion of the mutant HSCs (Arranz et al. 2014). Another report suggests that sympathetic neuropathy is a mechanism by which acute myeloid leukemia (AML) depletes normal BM niche cells while expanding more differentiated mesenchymal progenitors supporting leukemia progression. To investigate the mechanism of such effect, antagonists of both beta2- and beta3-ARs were injected in leukemic mice. The result was that blockade of beta2-ARs but not of beta3-ARs had a disease promoting effect. The opposite was found with a b beta2-ARs agonist (Hanoun et al. 2014). These studies suggested a possible novel therapeutic approach in hematological malignancies, however, rather surprisingly, remained isolated and did not foster any new insight in this interesting topic.
3. Discussion
The majority of the studies above reported indicate beta3-ARs and beta2-ARs expressed on BM MSCs as the major players of the adrenergic influence on the hematopoietic system. Recently, even alpah7-nicotinic receptors have been shown to cooperate with beta2-ARs in promoting HSCs quiescence, a condition necessary for their self-renewal. Nevertheless, the cholinergic role in regulating hematopoiesis remains largely unexplored. In addition, the current concept that only the modulation of beta-ARs activity may explain the SNS influence on hematopoiesis cannot compose all the available experimental evidences in the field. For example, catecholamines and acetylcholine may be synthesized by hematopoietic cells in the BM, adding further complexity to our understanding of the adrenergic/cholinergic modulation of the hematopoietic process (Maestroni 2000; Schloss et al.2022). Also the PSNS-dependent reduction of the SNS activity that according to García-García et al. (2020) should dampen beta3-ARs activation during the night in mice, is conflicting with the well known notion that the active phase of the photoperiod is dependent on an increased SNS activity in all species. Consistently, our previous findings indicating that in murine BM the SNS activity follows a circadian rhythmicity peaking during the night ( Maestroni et al. 1998). Furthermore, the mechanisms and the cell type(s) involved in the beta-ARs promotion of hematopoietic regeneration remain unknown. The same holds true for the regulation of the lymphoid/myeloid differentiation ratio. In fact, conditions associated to a myeloid-biased hematopoiesis are as different as stress and ageing, yet under stress conditions it was claimed that the effect is due to the stress-associated NE overproduction activating beta3-ARs on MSCs (Heidt et al. 2014; Fielding and Méndez-Ferrer 2020), while in the myeloid bias associated with ageing it was found that beta3-ARs activation is reduced and the effect was ascribed to beta2-ARs (Guidi and Geiger 2017; Marynovich et al. 2018; Ho etal. 2019;Fielding and Méndez-Ferrer 2020) . Thus is seems that the picture is far from being complete.
In the studies that pioneered the field and by various in vivo and in vitro experimental approaches, we repeatedly found that alpha1-ARs, possibly expressed on lymphoid progenitor cells, were involved in promotion of the lymphoid lineage while inhibiting myeloid differentiation. In fact, their pharmacological blockade in vivo favoured myelopoiesis at the expense of lymphopoiesis (Maestroni and Conti 1994; Maestroni 1995). Even the NE-induced hematopoietic rescue in mice injected with lethal doses of carboplatin was , at least in part, mediated by alpha1-ARs ( Maestroni et al. 1998). All these experiments were performed in mice, and therefore it is possible that the a1-ARs antagonist prazosin affected also pre-synaptic alpha2-ARs potentiating indirectly the activation of b-ARs. In fact, it has been reported that in rodents, prazosin may act also as an alpha2-ARs antagonist (Dong et al. 2002). In any case, in BM cultures, NE inhibited the growth of granulocytes and macrophages colonies (GM-CFU) and the effect was neutralized by prazosin (Maestroni et al. 1992; Maestroni et al. 1997: Maestroni et al. 1998]. In vitro, NE proved also to protect GM-CFU in BM cells expressing high affinity alpha-1ARs from the toxic effect of carboplatin and the effect was counteracted by low concentrations of prazosin (Togni and Maestroni 1996).
Nonetheless, alpha1-ARs have never been taken in consideration in the studies following our contributions in the field. This is rather surprising because , to my knowledge, no study has ruled out the presence of alpha1-ARs in BM cells, a probably impossible goal. In fact, apart from our findings, macrophages are known to play an important role in the HSCs niche(Kandarakov et al 2022) and to express both beta-ARs and alpha1-ARs (Freire et al.2022). In addition, it has been also reported that HSPCs do express alpha1- and alpha2 -ARs (Muthu et al. 2007). To the sake of gaining a better knowledge of the adrenergic influence on hematopoiesis it would be therefore desirable to re-address the role of alpha1-ARs in the hematopoietic system.
4. Conclusion and Prospects
The findings reported above ascertain that the SNS plays a major role in the physiology of the HSCs niche. Due to the major adaptive role of both systems, this fact should not be so surprising. In steady state situation, the SNS activation of beta3-ARs on nes-GFP+ MSCs during the resting phase of the circadian cycle results in decreased CXCL12 production and therefore in the leukocytes and HSCs egress from BM into the blood. The cycle is then closed by the E activation of beta2-ARs and possibly of alpha7-nicotinic receptors by cholinergic sympathetic fibers resulting in increased CXCL12 inducing leukocytes and HSCS homing. This cellular traffic is crucial for both HSCs differentiation and self-renewal. The SNS contributes also in the control of stress hematopoiesis after myeloablative treatments or severe physical injuries in order to regenerate an efficient hematopoiesis or replenish the site of injury with effector cells. The mechanisms inducing hematopoietic regeneration or myeloid-biased hematopoiesis are still unclear. Perhaps, in these cases it would be advisable to take in consideration also the hematopoietic role of alpha1-ARs beside that of beta-ARs. . On the basis of our original findings and of the recent evidence about the existence of lymphoid-biased multipotent progenitors cells capable of generating myeloid cells and CLPs (LMPs , see Fig 1), I would like to suggest that alpha1-ARs are expressed on LMPs and mediate the SNS effect on their differentiation and/or proliferation. In particular, their activation might promote the formation of CLPs while myeloid cells generation would be enhanced by their blockade. On the other hand, alpha-1ARs have been involved in osteoblastic stem cell differentiation (Choi et al. 2011) and erythroid differentiation in human chronic myelogenous leukemia( Ha et al. 2017), therefore their possible action on LMPs does not seem unreasonable.
This approach might also be useful to better uncover and perhaps control by adrenergic agents the myeloid promoting effect of psychogenic stress and of ageing. In addition, also the use of adrenergic drugs as therapeutic tools in hematological malignancies could be better devised. Likewise, preclinical experiments using limiting dilution assays in HSCs transplantation in presence of pharmacological activation or inhibition of the various ARs might provide useful information to improve the success of this therapeutic approach. Last but not least, the role of ARs in the central nervous system should also be taken in consideration as there are evidences about their possible involvement in the regulation of BM functions [
59].
References
- Akashi K ,Traver D, Miyamoto T , Weissman IL(2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages Nature Mar 9 404:(6774)193-7.
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntion S, Tzeng YS, Lai DM, Schwaller Jskoda RC, Mendez-Ferrer S (2014)Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms Nature 512(7512) :78-81. [CrossRef]
- Bianchi ME, Mezzapelle R(2020) The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration Front Immunol 11:2109 Published 2020 Aug 28. [CrossRef]
- Bozdağ SC, Yüksel MK, Demirer T (2018) Adult Stem Cells and Medicine Adv Exp Med Biol 1079: 17-36. [CrossRef] [PubMed]
- Buijs RM, Scheer FA, Kreier F, YI C, Bos N, Goncharuk VD, Kalsbeek A (2006) Organization of circadian functions: interaction with the body Prog Brain Res 153: 341-360. [CrossRef]
- Calvo W (1968) The innervation of the bone marrow in laboratory animals Am J Anat 123(2): 315-328. [CrossRef]
- Chan KL, Poller WC, Swirski FK, Russo SJ (2023) Central regulation of stress-evoked peripheral immune responses. Nat Rev Neurosci 24(10):591-604. [CrossRef]
- Cheshier SH Morrison SJ Liao X Weissman IL (1999) In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells Proc Natl Acad Sci U S A 96(6):3120-3125. [CrossRef]
- Cora MC Latimer K, Travlos GC (2018) Bone Marrow In: Suttie AW editor Boorman’s Pathology of the Rat 2nd ed, Elsevier Inc Academic Press Boston.
- Cossío I, Lucas D, Hidalgo A (2019) Neutrophils as regulators of the hematopoietic niche Blood 133(20): 2140-2148. [CrossRef]
- Dong J,Mrabet O, Moze E, Li K, Neveu PJ(2002) Lateralization and catecholaminergic neuroimmunomodulation: prazosin an alpha1/alpha2-adrenergic receptor antagonist suppresses interleukin-1 and increases interleukin-10 production induced bylipopolysaccharides Neuroimmunomodulation 10(3): 163-168. [CrossRef]
- Drinker C K , Drinker K R (1916) A method for maintaining an artificial circulation through the tibia of the dog with a demonstration of the vasomotor control of the marrow vessels Am J Physiol 40: 514-521.
- Fielding C, García-García A, Korn C GadomskiS Fang Z Reguera JL Perez-Simon JA GöttgensB Mendez-Ferrer S (2022) Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis Nat Commun13(1) 543: Published 2022 Jan 27. [CrossRef]
- Fielding C, Méndez-Ferrer S (2020)Neuronal regulation of bone marrow stem cell niches F1000Res 9 F1000 Faculty Rev-614 Jun 16. [CrossRef]
- Freire BM, de Melo FM, Basso AS (2022) Adrenergic signaling regulation of macrophage function: do we understand it yet? Immunother Adv 2(1) ltac010 Published 2022 Jun 1. [CrossRef]
- Gao X, Murphy MM, Peyer JG, Ni Y, Yang MZ, HangY, Guo J, Kara N, Embree C, Tasdogan A, Ubellacker JM, Crane GM, Fang S, Zhao Z, Shen B, Morrison SJ (2023) Leptin receptor+ cells promote bone marrow innervation and regeneration by synthesizing nerve growth factor Nat Cell Biol 25(12): 1746-1757. [CrossRef]
- García-García A, Korn C, García-Fernández M, Domingues O, Villadiego J, Martin-Perrz D, Iser J, Bejarano-Garcia JA, Zimmer J, Perez-Simon JA, Toledo-Aral JJ, Michel T, Airaksinen MS, Mendez-Ferrer S (2020) Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes [published correction appears in Blood Dec 17 136(25):2965. https://doi.org/10.1182/blood2020009650] Blood 2019133(3):224-236. [CrossRef]
- Graham RM (1990)Adrenergic receptors: structure and function Cleve Clin J Med 57(5): 481-491. [CrossRef]
- Groarke EM, Young NS (2019) Aging and Hematopoiesis. Clin Geriatr Med 35(3):285-293. [CrossRef]
- Guidi N, Geiger H (2017) Rejuvenation of aged hematopoietic stem cells Semin Hematol 54(1): 51-55. [CrossRef]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S,Pierce H, Kunisaki Y, Lacombe J, Armsrong SA, Dürsen U, Frenette PS(2014) Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche Cell Stem Cell 15(3) :365-375. [CrossRef]
- Heidt T, Sager HB, Courties G,Dutta P, Iwamoto Y, Zaltsman A, vor zur Muheln C, Bode C, Fricchione GL Denninger J Lin CP Vinegoni C Libby P Swirski FK Weissleder R Nahrendorf M (2014) Chronic variable stress activates hematopoietic stem cells Nat Med 20(7) :754-758. [CrossRef]
- Ho YH, Del Toro R, Rivera-Torres J, Rak J, Korn C, Garcia-Garcia A, Macia D, Gonzales-Gomez C, Del Monte A, Wittner M, Waller AK,Foster HR, Lopez-Otin C, Johnson RS, Nerlov C, Ghevaert C, Vainchenker W, Louache F, Andres V, Mendez-Ferrer S (2019) Remodeling of Bone Marrow Hematopoietic Stem Cell Niches Promotes Myeloid Cell Expansion during Premature or Physiological Aging Cell Stem Cell 25(3): 407-418e6. [CrossRef]
- Höfer T, Rodewald HR (2018) Differentiation-based model of hematopoietic stem cell functions and lineage pathways Blood 132(11):1106-1113. [CrossRef]
- Kandarakov O, Belyavsky A, Semenova E (2022)Bone Marrow Niches of Hematopoietic Stem and Progenitor Cells Int J Mol Sci 23(8):4462 Published 2022 Apr 18. [CrossRef]
- Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL (2008) Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages Blood Jun 15111(12): 5562-70. [CrossRef]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow Cell 124(2): 407-421. [CrossRef]
- Kaushansky K (2018) Hunting for hematopoietic transcriptional networks. Proc Natl Acad Sci U S A. 115(40):9818-9820. [CrossRef]
- Kokkaliaris KD, Kunz L, Cabezas-Wallscheid N, Christodoulu C, Renders S Camargo F, Trump A, Scadden DT, Schroeder T ( 2020) Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations Blood 136(20):2296-2307. [CrossRef]
- Loftus TJ, Mohr AM. Moldawer LL (2018) Dysregulated myelopoiesis and hematopoietic function following acute physiologic insult Curr Opin Hematol 25(1):37-43. [CrossRef]
- Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS (2013) Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration Nat Med Jun19(6): 695-703. [CrossRef]
- Maestroni G (2023) Circadian regulation of the immune-hematopoietic system Exploration of Neuroscience 2(3) :123-39. [CrossRef]
- Maestroni GJ (2000)Neurohormones and catecholamines as functional components of the bone marrow microenvironment Ann N Y Acad Sci 917 :29-37. [CrossRef]
- Maestroni GJ (1995) Adrenergic regulation of haematopoiesis Pharmacol Res 32(5): 249-253. [CrossRef]
- Maestroni GJ Conti A (1994) Modulation of hematopoiesis via alpha 1-adrenergic receptors on bone marrow cells Exp Hematol 22(3): 313-320.
- Maestroni GJ, Conti A, Pedrinis E (1992) Effect of adrenergic agents on hematopoiesis after syngeneic bone marrow transplantation in mice Blood 80(5):1178-82 PMID: 1515638.
- Maestroni GJ, Cosentino M. Marino F, Togni M, Conti A, Lecchini S, Frigo G (1998)Neural and endogenous catecholamines in the bone marrow Circadian association of norepinephrine with hematopoiesis? Exp Hematol Nov26(12):1172-7 PMID: 9808057.
- Maestroni GJ, Togni M, Covacci V (1997) Norepinephrine protects mice from acute lethal doses of carboplatin Exp Hematol Jun 25(6): 491-4 PMID: 9197326.
- Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, Wei Q, WangX,Ciero P, Xu J, Leftin A, Frenette PS (2018) Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche [published correction appears in Nat Med Apr25(4):701. doi: 10.1038/s41591-019-0425-3] Nat Med 24(6): 782-791 . [CrossRef]
- McCorry LK (2009) Physiology of the autonomic nervous system Am J Pharm Educ 71(4): 78. [CrossRef]
- Méndez-Ferrer S, Battista M, Frenette PS (2010) Cooperation of β2- and β3-adrenergic receptors in hematopoietic progenitor cell mobilization Ann N Y Acad Sci 1192: 139–44.
- Méndez-Ferrer S, Lucas D, Battista M, Frenette PS (2008) Haematopoietic stem cell release is regulated by circadian oscillations Nature 452(7186): 442-447. [CrossRef]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, MacArthur BD, Lira SA, Scadden DT Maayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche Nature 466(7308):829-834. [CrossRef]
- Morrison SJ, Scadden DT (2014) The bone marrow niche for haematopoietic stem cells Nature 505(7483): 327-334. [CrossRef]
- Muthu K, Iyer S, He LK, Szilagyi A, Gamelli RL, Shankar R, Jones SB (2007)Murine hematopoietic stem cells and progenitors express adrenergic receptors J Neuroimmunol 186(1-2) :27-36. [CrossRef]
- Nakorn TN, Miyamoto T, Weissman IL (2003) Characterization of mouse clonogenic megakaryocyte progenitors Proc Natl Acad Sci U S A Jan 7100(1): 205-10. [CrossRef]
- Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer J Immunol 182(8): 4499-4506. [CrossRef]
- Pinho S, Marchand T, Yang E, Wie Q, Nerlov C, Frenette PS (2018) Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches Dev Cell 44(5):634-641e4. [CrossRef]
- Pronk CJ, Rossi DJ, Månsson R, Attema JL, Norddahl GL, Chan CK (2007) Sigvardsson M Weissman IL Bryder D Elucidation of the phenotypic functional and molecular topography of a myeloerythroid progenitor cell hierarchy Cell Stem Cell Oct 111(4):428-42. [CrossRef]
- Psaila B, Mead AJ (2019) Single-cell approaches reveal novel cellular pathways for megakaryocyte and erythroid differentiation Blood133(13):1427-1435. [CrossRef]
- Schloss MJ, Hulsmans M, Rohde D, Lee B (2022) Lymphocyte-derived acetylcholine limits steady-state and emergency hematopoiesis [published correction appears in Nat Immunol Aug23(8):1285. https://doi.org/10.1038/s41590-022-01266-3] Nat Immunol(4):605-618. [CrossRef]
- Scott-Solomon E, Boehm E, Kuruvilla R (2021) The sympathetic nervous system in development and disease Nat Rev Neurosci 22: 685–702. [CrossRef]
- Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL (2010) Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling J Immunol 184(5) :2247-2251. [CrossRef]
- Skurikhin EG, Pershina OV, Minakova MY, Dygai A, Gol'dberg ED (2006) Monoaminergic regulation of proliferation and differentiation of granulomonocytopoietic precursors during neuroses Bull Exp Biol Med 141(6) :669-674. [CrossRef]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling Nat Immunol Oct 8(10): 1123-31. [CrossRef]
- Sukhtankar DD, Fung JJ, Kim MN, Cvayto T, Chiou V, Caculita NG, Zalicki P, Kim S, Jo Y, Kim S, Lee JM, Choi J, Mun SG, Chin A, Jang Y, Lee JY, Kim G, Kim EH, Huh WK, Jeong JY, Seen DS, Cardarelli PM( 2023) GPC-100 a novel CXCR4 antagonist improves in vivo hematopoietic cell mobilization when combined with propranolol PLoS One 18(10): e0287863 Published 2023 Oct 25. [CrossRef]
- Togni M; Maestroni G (1996) Hematopoietic rescue in mice via alpha 1-adrenoceptors on bone marrow B cell precursors Int J Oncol 9(2): 313-318. [CrossRef]
- Vivier E,Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H (2018) Innate Lymphoid Cells: 10 Years On Cell Aug 23 174(5):1054-1066. [CrossRef]
- Wang JC, Doedens M, Dick JE (1997) Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay Blood 89(11):3919-3924.
- Wei Q, Frenette PS (2018) Niches for Hematopoietic Stem Cells and Their Progeny Immunity 48(4):632-648. [CrossRef]
- Zhao Y, Liu M, Chan XY, Tan SY, Subramaniam S, Fan Y et al (2017) Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice Blood 130: 1995–2005.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).