Submitted:
29 January 2025
Posted:
30 January 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Test Procedures and Methods
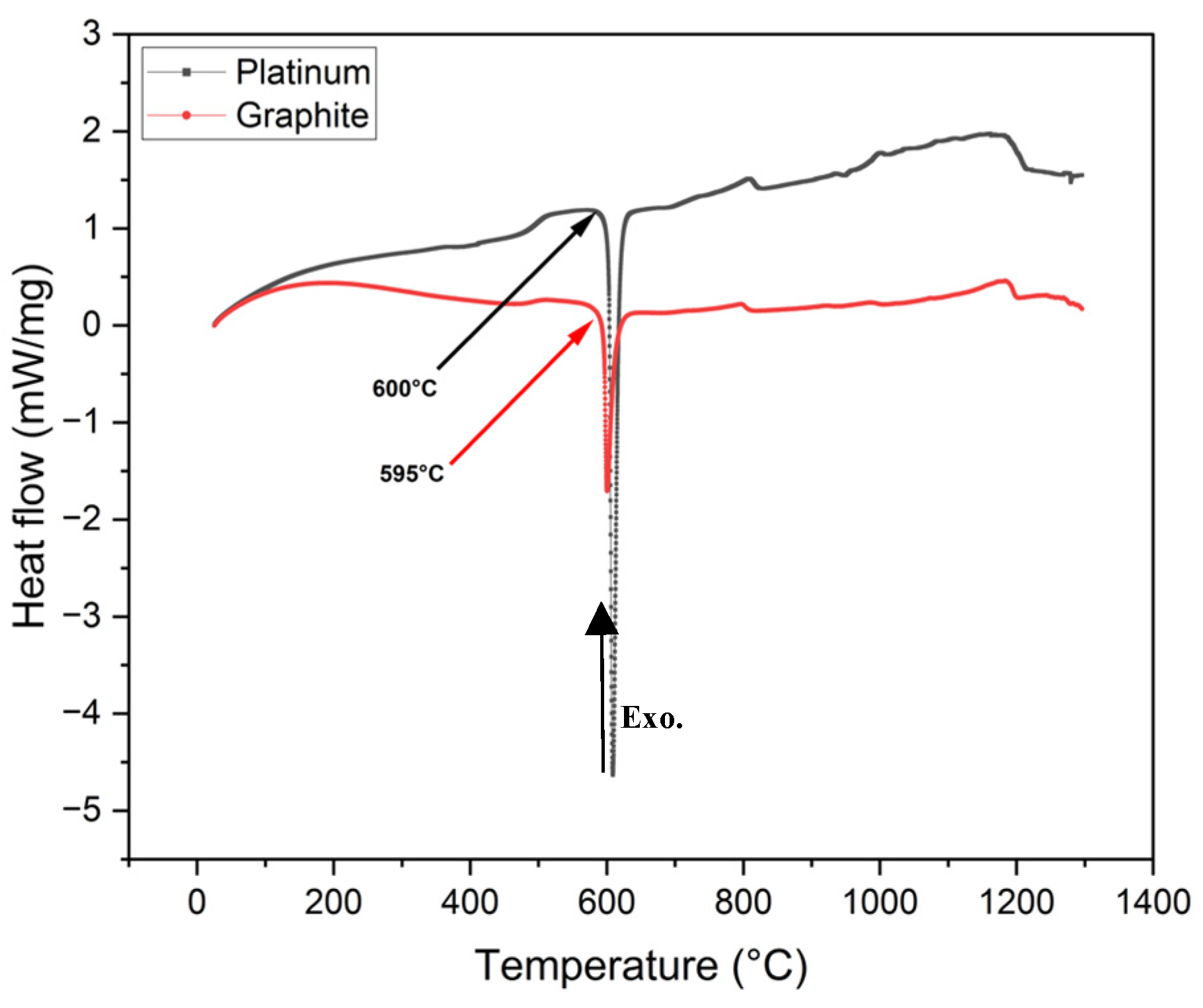
2.2.1. Crystallization Temperature Measurements Using Differential Scanning Calorimetry (DSC)
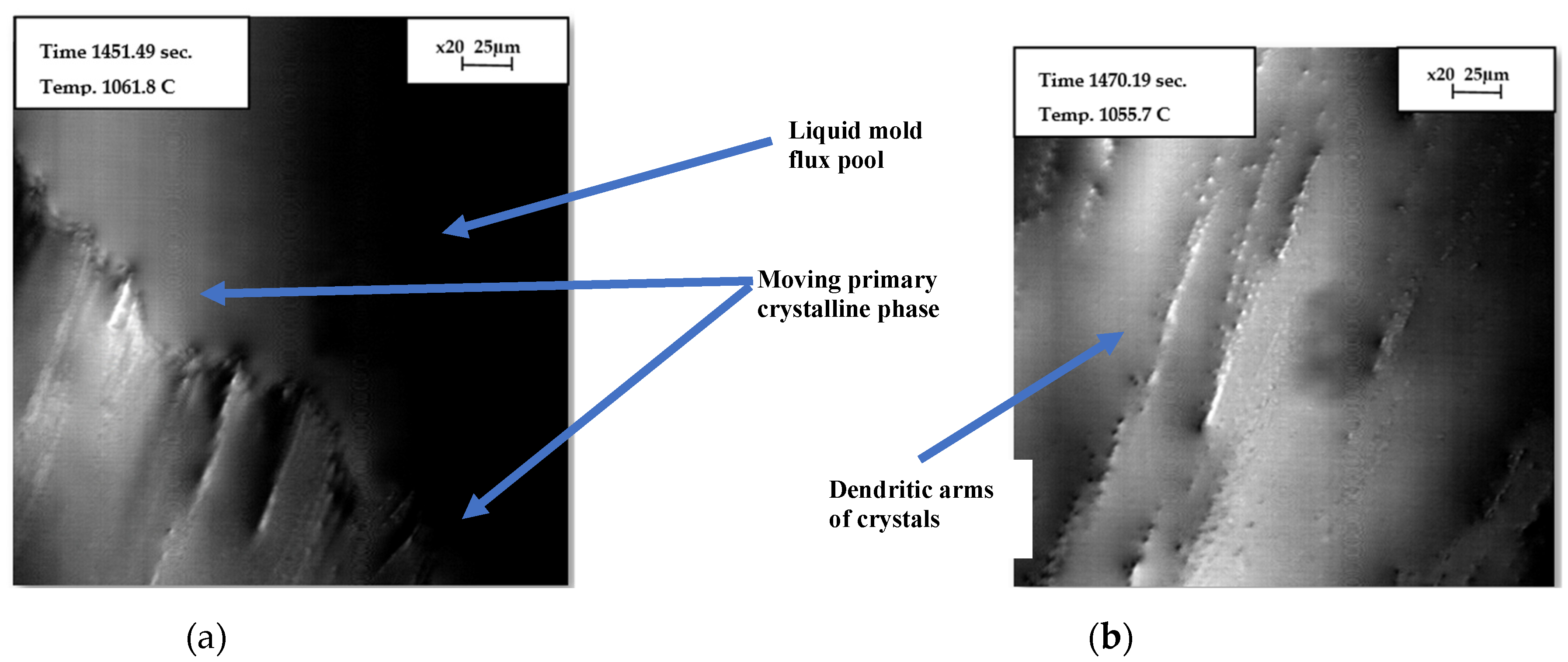
2.2.2. Observation of Onset of Crystallization Using Confocal Laser Scanning Microscopy (CLSM)
2.2.3. X-Ray Diffraction for Phase Detection
2.2.4. Computational Thermodynamic Analysis
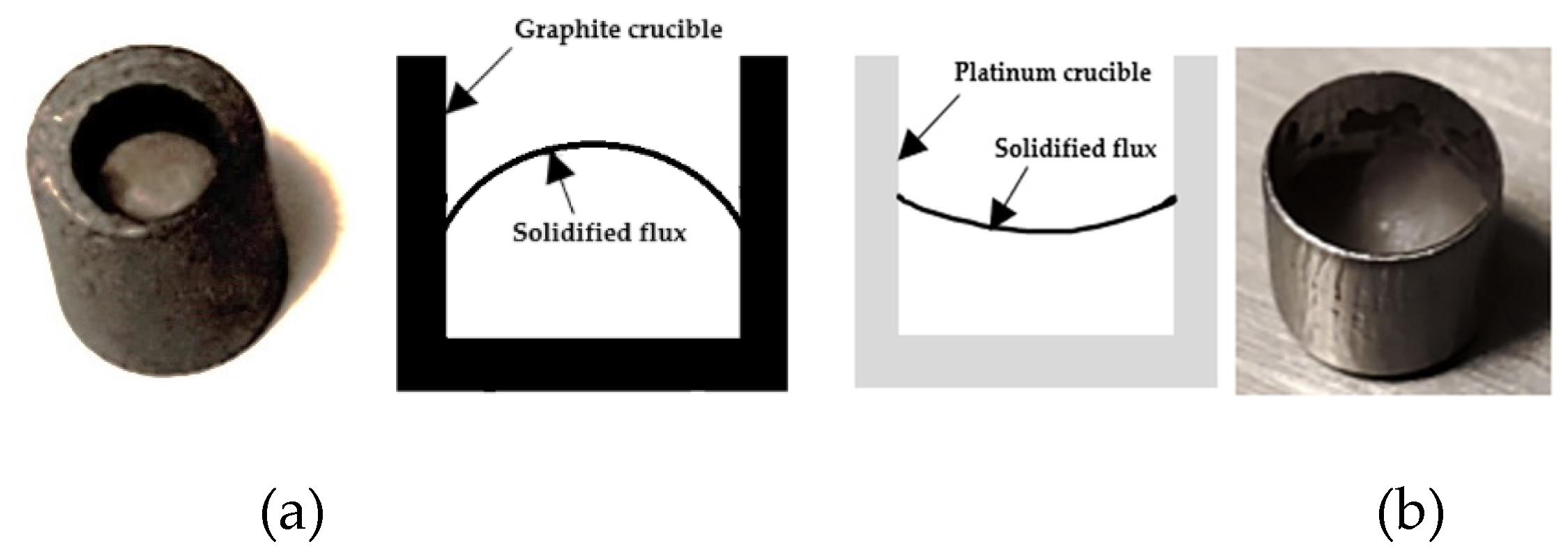
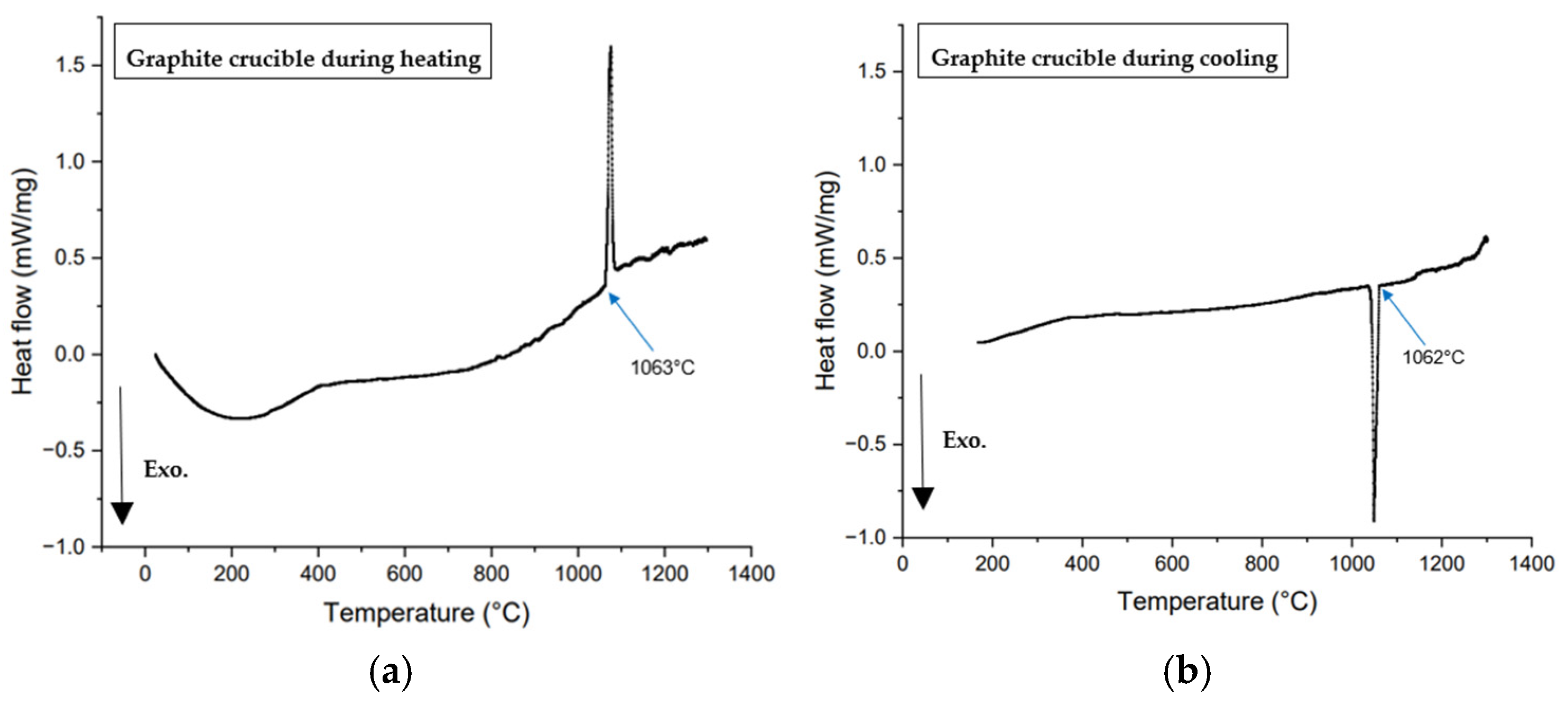
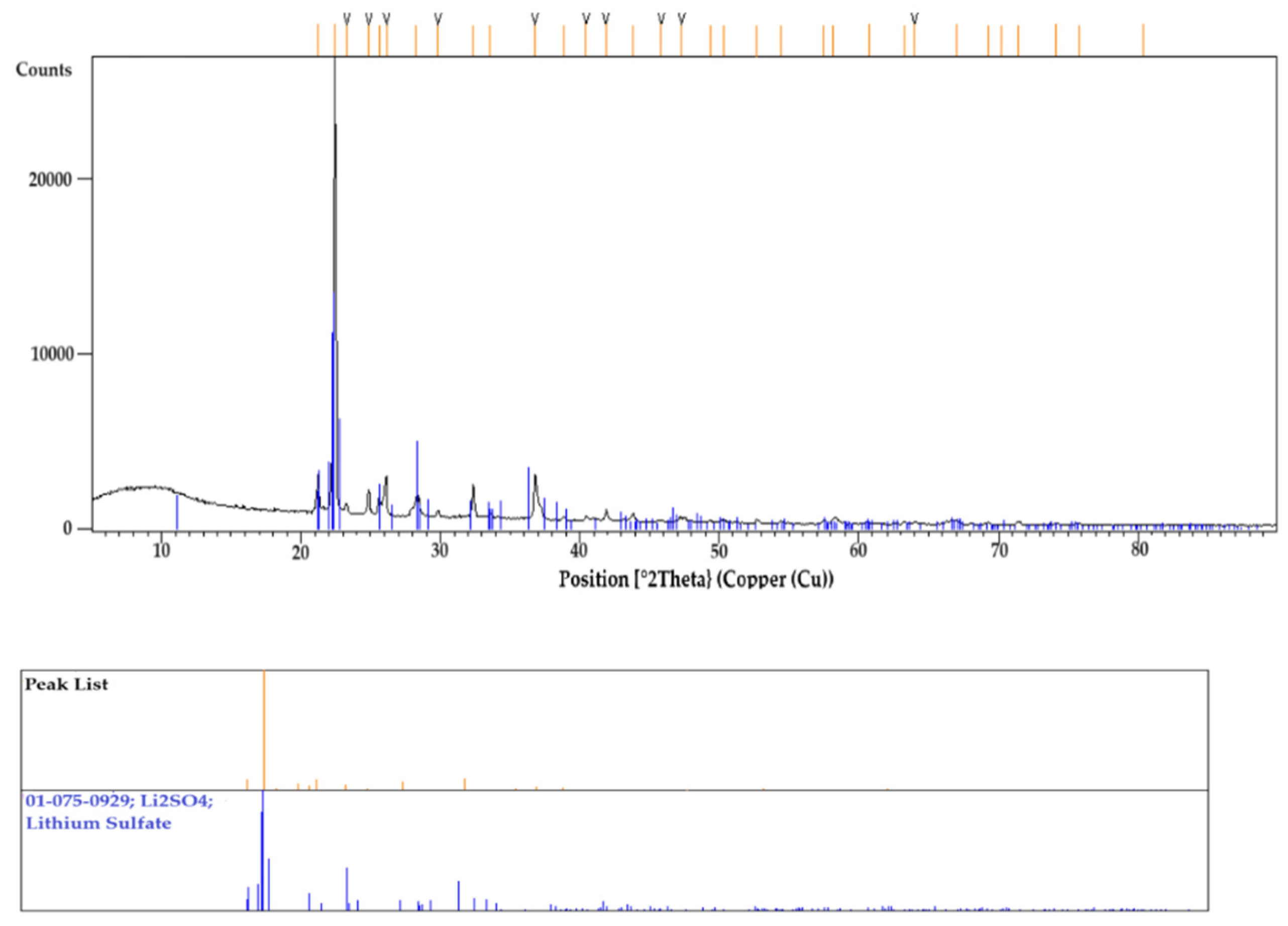
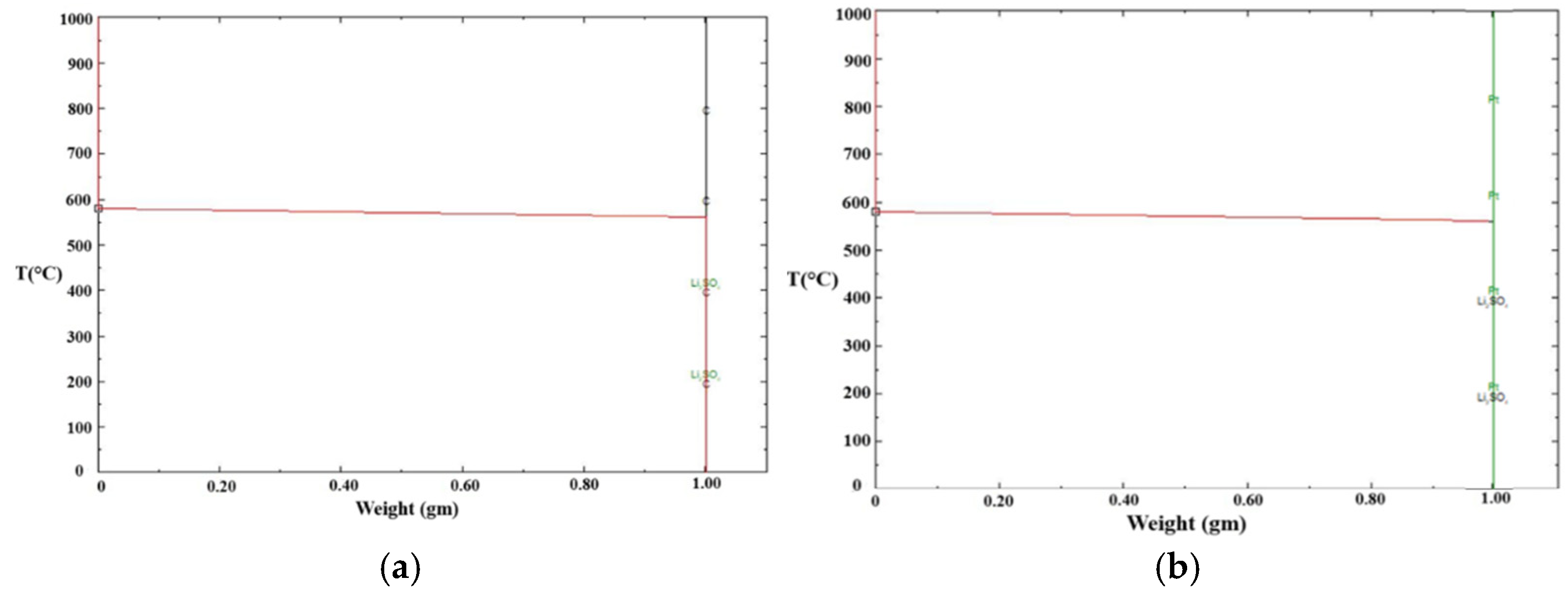
2.2.5. Calibration of Graphite Crucible Using Synthetic Slag, Pure Gold, and Li2SO4
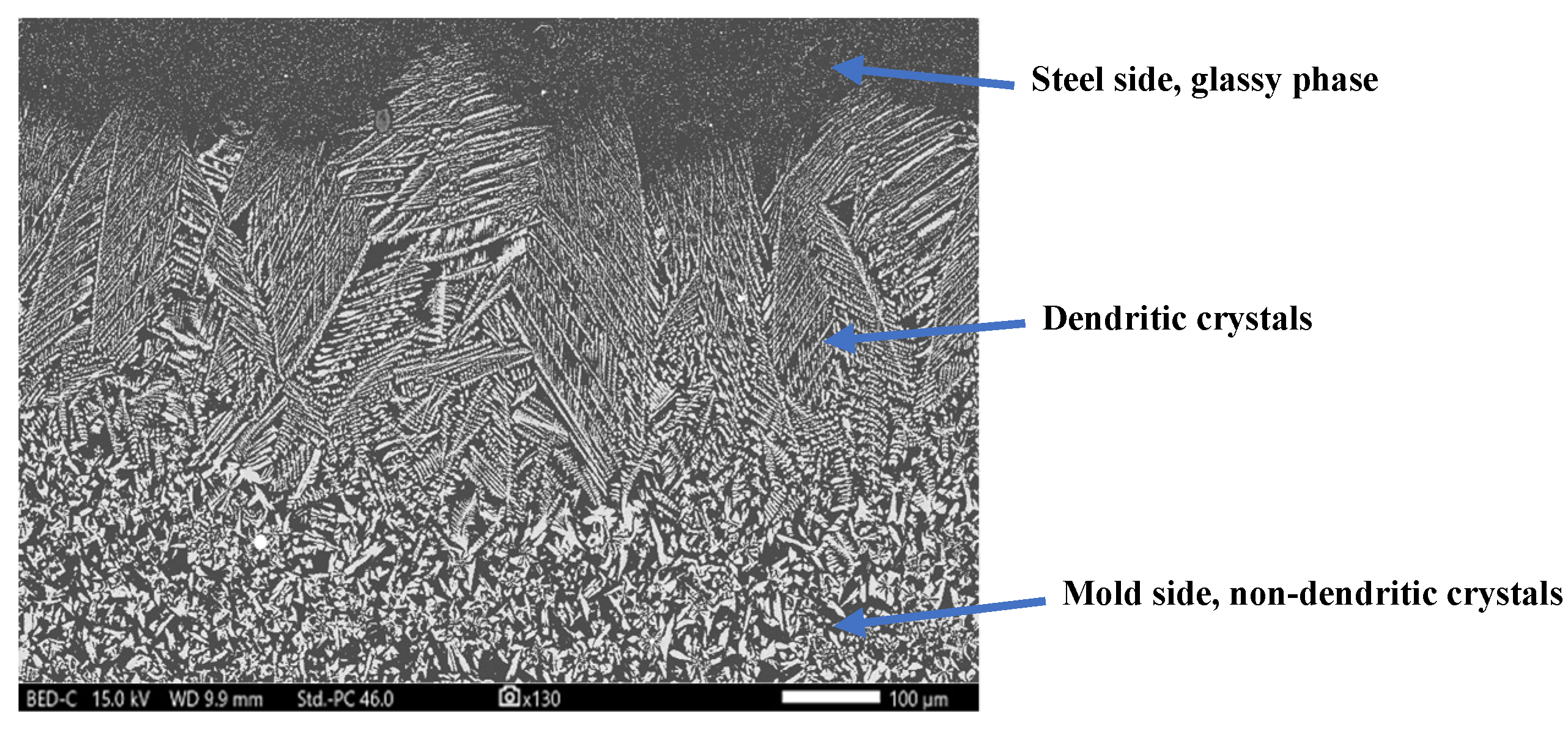
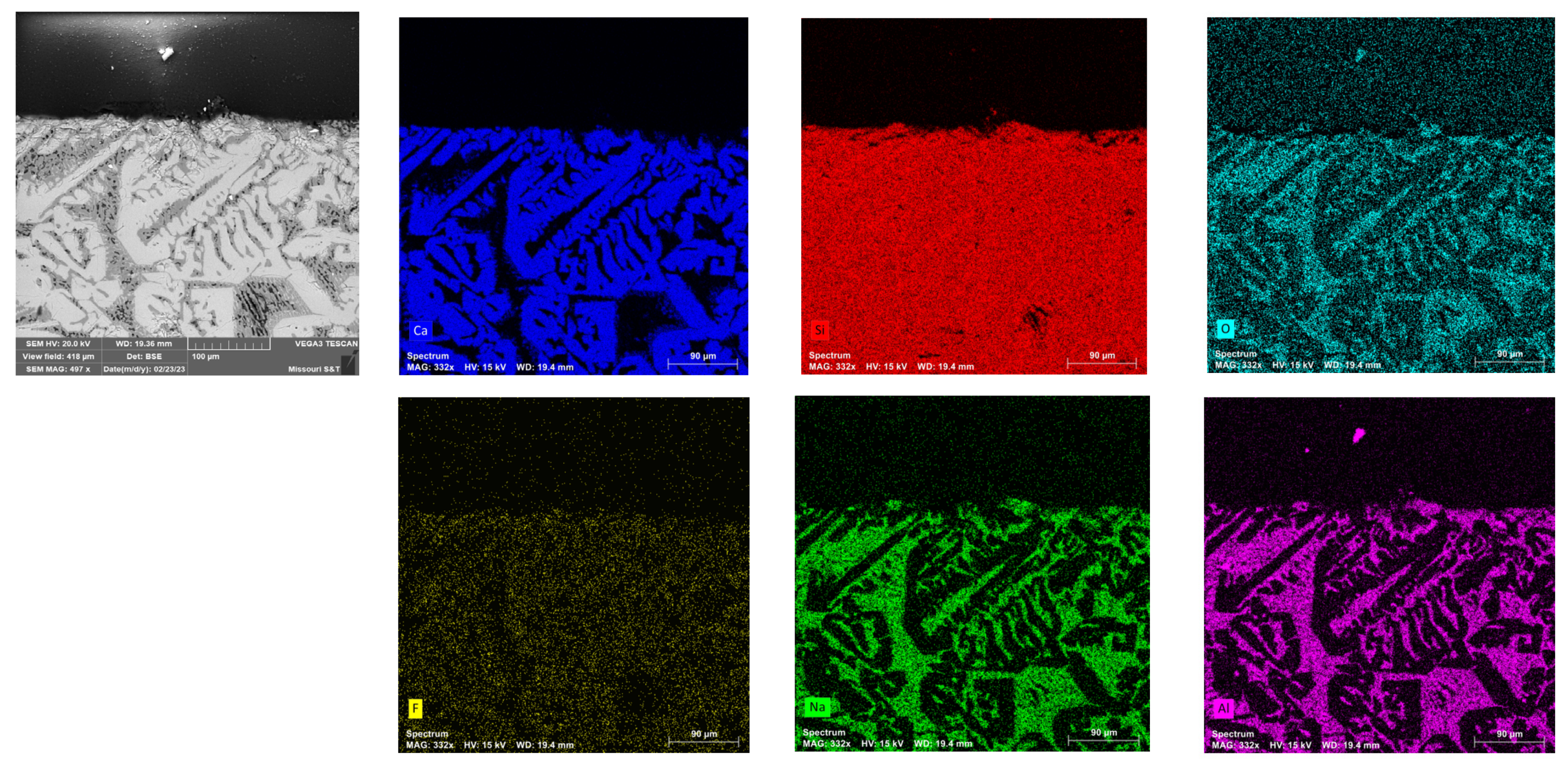
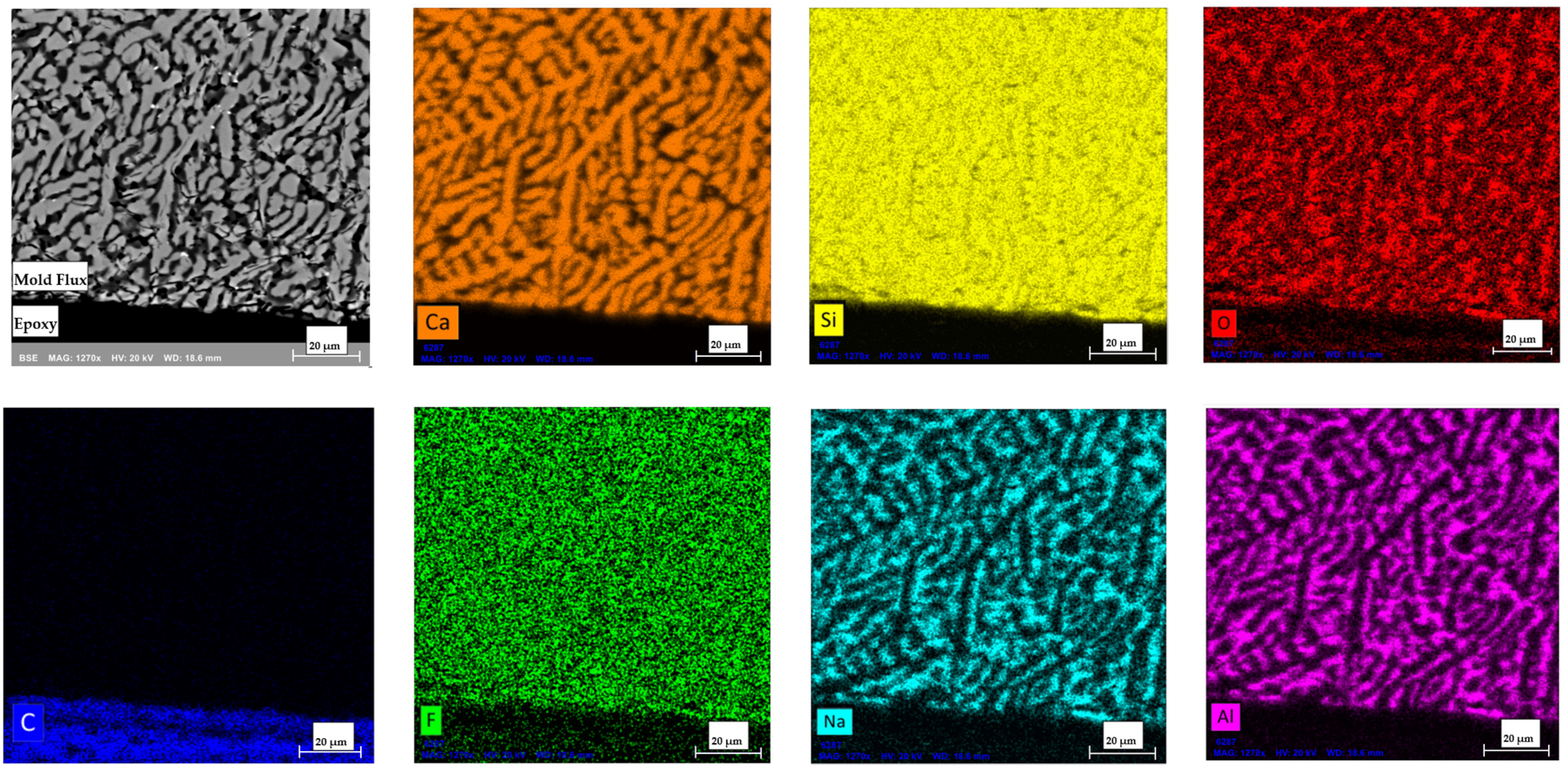
2.2.6. Elemental Map Using Scanning Electron Microscopy Coupled with Energy Dispersive X-Ray Spectroscopy (SEM-EDS)
3. Results and Discussion
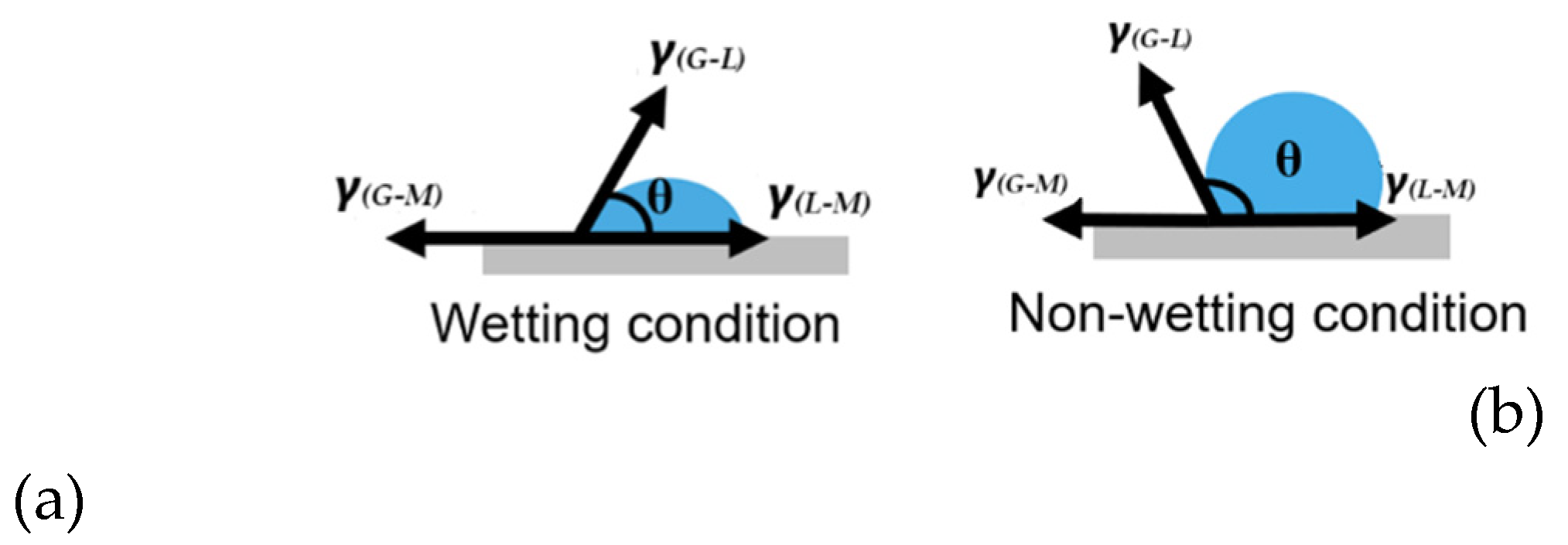
3.1. Crucible Material-Dependent Wetting Characteristics
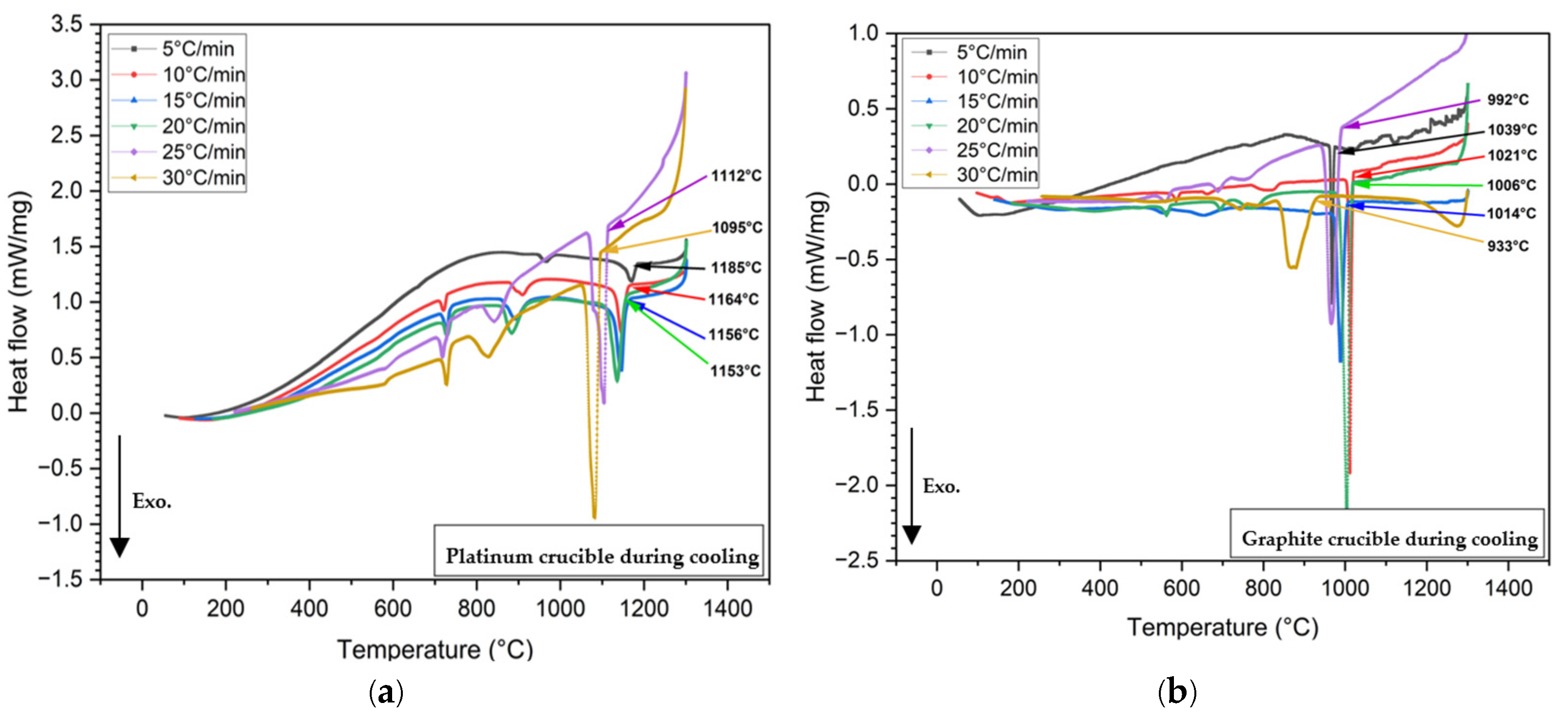
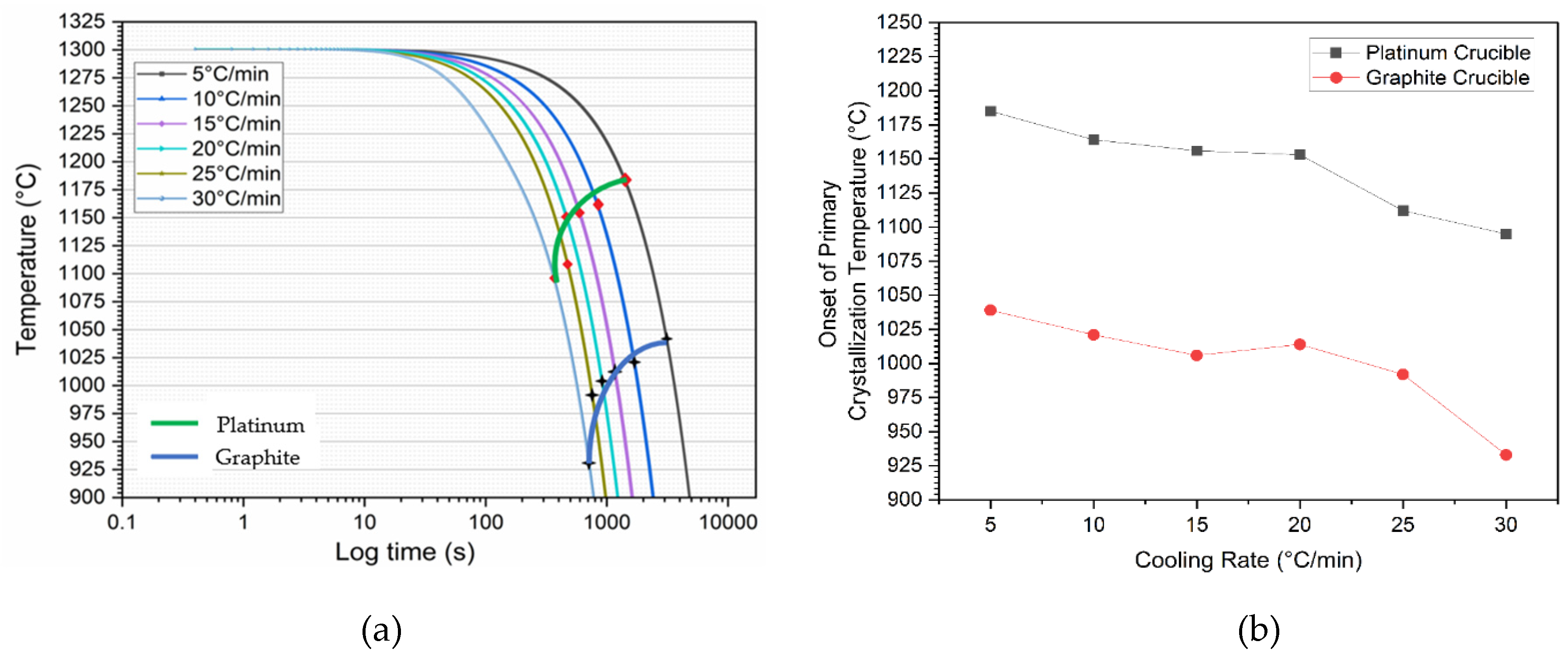
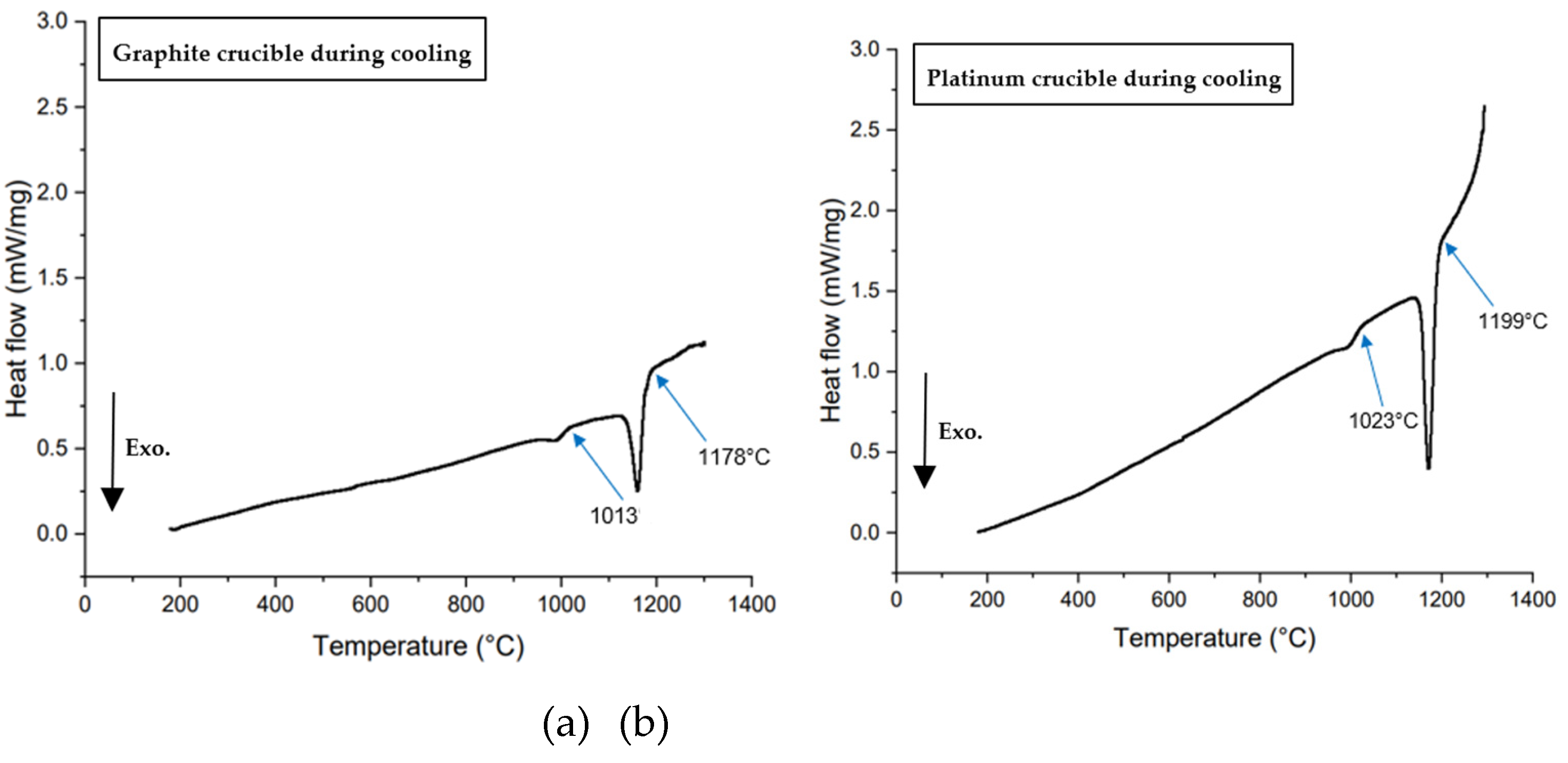
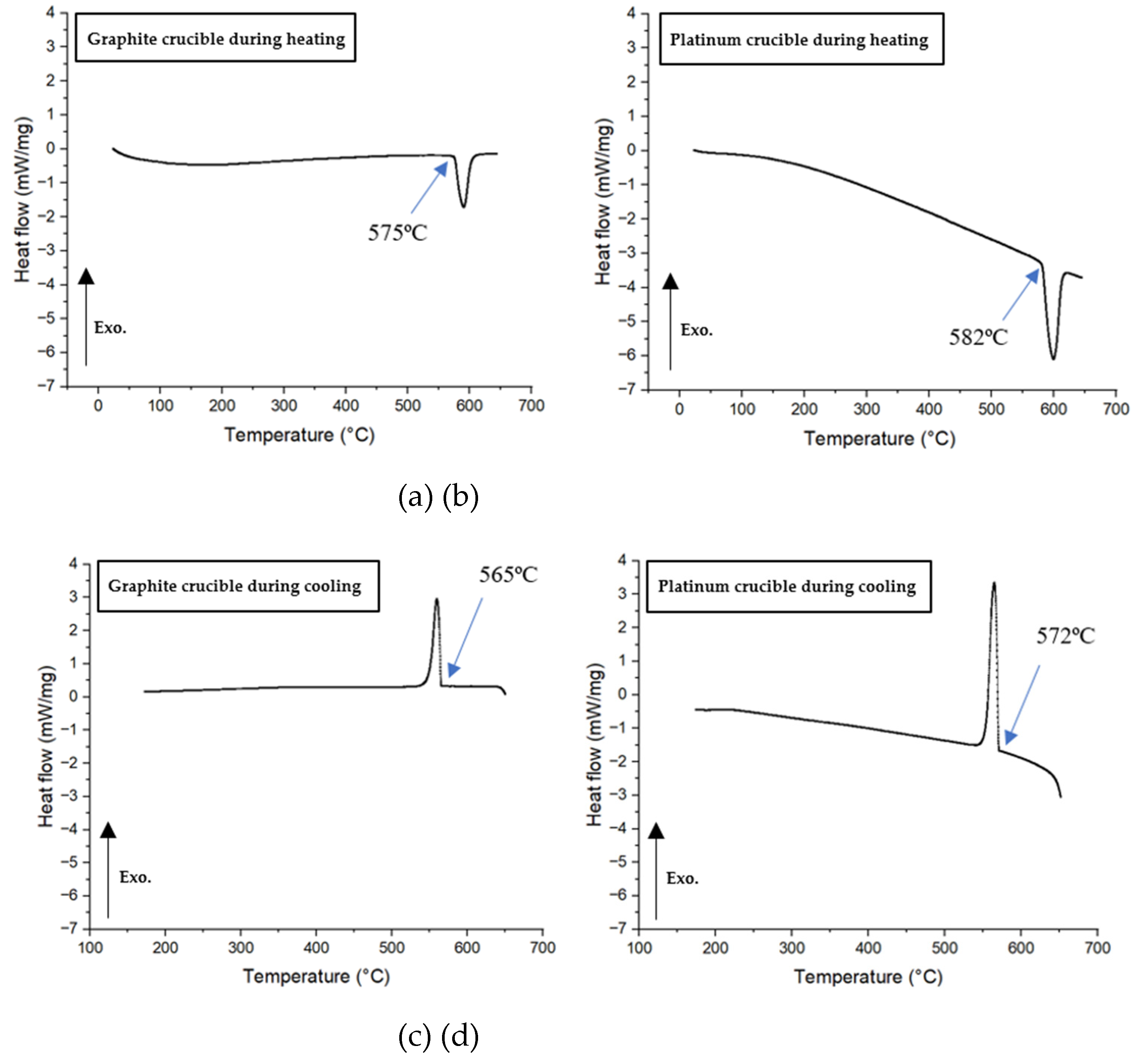
3.2. Measurements of Crystallization Temperature Depending on Crucible Materials in DSC
3.3. Observation of Onset of Primary Crystallization in CLSM
3.4. Identification of Primary Crystal Phase in XRD and SEM-EDS
3.5. Sensitivity of Graphite Crucible in DSC
3.6. Thermodynamic Equilibrium Simulations of Li2SO4 with Carbon and Platinum.
3.7. Detection of Potential Interactions at the Interface of Flux and Graphite Crucible in SEM-EDS
3.8. Wetting Conditions on Crystallization
5. Conclusions
- The conventional CaO-SiO₂-based mold flux exhibits strong wetting behavior with platinum crucibles but demonstrates a non-wetting condition when in contact with graphite crucibles.
- In all non-isothermal DSC experiments, mold flux samples in graphite crucibles consistently showed significantly lower primary crystallization temperatures compared to those in platinum crucibles across all tested cooling rates (5, 10, 15, 20, 25, and 30°C/min). The tendency for heterogeneous nucleation suppressed in a system that is non-wetting (graphite) than in a system that is wetting (platinum).
- In-situ solidification tests of mold flux in graphite crucibles using CLSM also revealed a decrease in the onset of primary crystallization temperature.
- Temperature measurements in the DSC with graphite and platinum crucibles using gold and Li2SO4 standards were found to be accurate to within a few degrees. DSC tests on synthetic slag exhibited a similar trend to mold flux, having lower crystallization temperatures in graphite crucibles compared to platinum crucibles.
- SEM-EDS analysis of the flux sample in the graphite crucible confirmed that no reactions occurred between the mold flux and the graphite crucible during the DSC experiments, clarifying that observed variations in crystallization behavior were due to the wetting characteristics of the crucible material rather than chemical interactions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DSC | Differential Scanning Calorimetry |
| CLSM | Confocal Laser Scanning Microscopy |
| SEM-EDS | Scanning Electron Microscopy Coupled with Energy Dispersive X-Ray Spectroscopy |
| XRD | X-ray Diffraction |
| CDMS | Copper Disc Mold Simulator |
| TGA | Thermal Gravimetric Analyzer |
| ICSD | Inorganic Crystal Structure Database |
| C | Carbon |
| Pt. | Platinum |
References
- Sarkar, R.; Li, Z. “Isothermal and Non-isothermal Crystallization Kinetics of Mold Fluxes used in Continuous Casting of Steel: A Review,” Metall. Mater. Trans. B 2021, 52, 1357–1378. [Google Scholar] [CrossRef]
- Leng, M.; Lai, F.; Li, J. “Effect of Cooling Rate on Phase and Crystal Morphology Transitions of CaO–SiO2-Based Systems and CaO–Al2O3-Based Systems,” Materials 2018, 12, 62. 12. [CrossRef]
- Shi, C.-B.; Seo, M.-D.; Wang, H.; Cho, J.-W.; Kim, S.-H. “Crystallization Kinetics and Mechanism of CaO–Al2O3-Based Mold Flux for Casting High-Aluminum TRIP Steels,” Metall. Mater. Trans. B 2015, 46, 345–356. [Google Scholar] [CrossRef]
- White, J. F.; Lee, J.; Hessling, O.; Glaser, B. “Reactions Between Liquid CaO–SiO₂ Slags and Graphite Substrates,” Metall. Mater. Trans. B 2017, 48, 506–515. [Google Scholar] [CrossRef]
- Kang, T. W.; Gupta, S.; Saha-Chaudhury, N.; Sahajwalla, V. “Wetting and Interfacial Reaction Investigations of Coke/Slag Systems and Associated Liquid Permeability of Blast Furnaces,” ISIJ Int. 2005, 45, 1526–1535. 45. [CrossRef]
- Blake, T. D. Wettability, J. C. Berg, Marcel Dekker Inc.: New York, USA, 1993.
- Chen, Z.; Yu, B.; Yao, T.; Du, W.; Ma, J. “Wetting and Reaction Behavior of TiN and TiO2 with Different Mold Fluxes for High Ti-Bearing Stainless Steel,” Metall. Mater. Trans. B 2024. [Google Scholar] [CrossRef]
- Si, X.; Wang, W.; Zhou, L.; Zeng, S.; Zhang, L.; Qi, J.; Liu, P. “Wettability of Mold Flux with Various Droplet Sizes on Stainless Steel Substrates,” Steel Res. Int. 2025, 96, 2400270. [Google Scholar] [CrossRef]
- Wang, W.; Gao, E.; Zhou, L.; Zhang, L.; Li, H. “Effect of Al₂O₃/SiO₂ and CaO/Al₂O₃ Ratios on Wettability and Structure of CaO–SiO₂–Al₂O₃-Based Mold Flux System,” J. Iron Steel Res. Int. 2019, 26, 355–364. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ostrovski, O.; Sasaki, Y.; Zhang, C.; Cai, D. “Dynamic Wetting of High-Al Steel by CaO–SiO₂- and CaO–Al₂O₃-Based Mold Fluxes,” Metall. Mater. Trans. B 2019, 50, 2175–2185. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Zhou, L.; Luo, H.; Zheng, Q.; Zhao, R. “Influence of Fluorine and CaO/Al₂O₃ Ratio on the Wetting Behavior Between CaO–Al₂O₃-Based Mold Flux and Steel Substrate,” Steel Res. Int. 2023, 94(3). 94(3). [CrossRef]
- Yuan, H.; Dan, Z.; Wang, Q.; He, S. “Contact Angle and Adhesion of CaO–SiO₂- and CaO–Al₂O₃-Based Mold Slags on Solid Steel of Various Compositions,” J. Mater. Res. Technol. 2020, 9(4), 7828–7837. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Wang, W.; Sohn, I. “Wetting Behavior of Mold Flux Droplet on Steel Substrate With or Without Interfacial Reaction,” Metall. Mater. Trans. B 2017, 48, 1943–1950. [Google Scholar] [CrossRef]
- Kromhout, J. ; “Mould powders for high speed continuous casting of steel,”. Doctoral Thesis, TU Delft, Netherlands, 2011. [Google Scholar]
- Park, J.-Y.; Sohn, I. “Evaluating the Heat Transfer Phenomena and the Interfacial Thermal Resistance of Mold Flux Using a Copper Disc Mold Simulator,” Int. J. Heat Mass Transf. 2017, 109, 1014–1025. [Google Scholar] [CrossRef]
- Park, J. Y.; Ko, E.; Choi, J.; Sohn, I. “Characteristics of Medium Carbon Steel Solidification and Mold Flux Crystallization Using the Multi-Mold Simulator,” Met. Mater. Int. 2014, 20, 1103–1114. [Google Scholar] [CrossRef]
- Ko, E. Y.; Choi, J.; Park, J. Y.; Sohn, I. “Simulation of Low Carbon Steel Solidification and Mold Flux Crystallization in Continuous Casting Using a Multi-Mold Simulator,” Met. Mater. Int. 2014, 20, 141–151. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Ma, F.; Zhang, H. “Study of Solidification and Heat Transfer Behavior of Mold Flux Through Mold Flux Heat Transfer Simulator Technique: Part I. Development of the Technique,” Metall. Mater. Trans. B 2015, 46, 1419–1430. [Google Scholar] [CrossRef]
- Ma, F.; Liu, Y.; Wang, W.; Zhang, H. “Study of Solidification and Heat Transfer Behavior of Mold Flux Through Mold Flux Heat Transfer Simulator Technique: Part II. Effect of Mold Oscillation on Heat Transfer Behaviors,” Metall. Mater. Trans. B 2015, 46, 1902–1911. [Google Scholar] [CrossRef]
- Wang, W.; Long, X.; Zhang, H.; Lyu, P. “Mold Simulator Study of Effect of Mold Oscillation Frequency on Heat Transfer and Lubrication of Mold Flux,” ISIJ Int. 2018, 58, 1695–1704. 58. [CrossRef]
- Zhang, H.; Wang, W. “Mold Simulator Study of Heat Transfer Phenomenon During the Initial Solidification in Continuous Casting Mold,” Metall. Mater. Trans. B 2017, 48, 779–793. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Xu, C.; Zhang, C. “An Investigation of the Mold-Flux Performance for the Casting of Cr12MoV Steel Using a Mold Simulator Technique,” Metall. Mater. Trans. B 2017, 48, 2017–2026. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Ma, F.; Zhou, L. “Mold Simulator Study of the Initial Solidification of Molten Steel in Continuous Casting Mold. Part I: Experiment Process and Measurement,” Metall. Mater. Trans. B 2015, 46, 2361–2373. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W. “Mold Simulator Study of the Initial Solidification of Molten Steel in Continuous Casting Mold: Part II. Effects of Mold Oscillation and Mold Level Fluctuation,” Metall. Mater. Trans. B 2016, 47, 920–931. [Google Scholar] [CrossRef]
- Lyu, P.; Wang, W.; Zhang, H. “Mold Simulator Study on the Initial Solidification of Molten Steel Near the Corner of Continuous Casting Mold,” Metall. Mater. Trans. B 2017, 48, 247–259. [Google Scholar] [CrossRef]
- Kamaraj, A.; Tripathy, S.; Chalavadi, G.; Sahoo, P. P.; Misra, S. “Characterization and Assessment of Mold Flux for Continuous Casting of Liquid Steel Using an Inverse Mold Simulator,” Steel Res. Int. 2022, 93, 1–13. [Google Scholar] [CrossRef]
- Nazim, M.; Buchely, M.; Emdadi, A.; Huang, J.; Saha, R. K.; O’Malley, R. “A Lab-Scale Mold Simulator Employing an Optical-Fiber-Instrumented Mold to Characterize Initial Steel Shell Growth Phenomena,” In proceedings of the AISTech, Columbus, USA 2024, 947–958. [CrossRef]
- O'Malley, R. J.; Neal, J. “An Examination of Mold Flux Film Structures and Mold Gap Behavior Using Mold Thermal Monitoring and Petrographic Analysis at Armco’s Mansfield Operations,” In proceedings of the METEC Congr. , Düsseldorf, Germany, 1–8. 1999. [Google Scholar]
- Novák, D.; Řeháčková, L.; Novák, V.; Matýsek, D.; Peikertová, P. “Wetting of Graphite and Platinum Substrate by Oxide System with Graded B2O3 Content,” Crystals 2023, 13, 1618. 13. [CrossRef]
- Kiser, K.; Athavale, V. A.; Bartlett, L.; Buchely, M.; O’Malley, R. “Growth Kinetics and Development of the Solid–Liquid Interface in Low-Carbon and High-Alloy Steel Castings Enabled by Confocal Microscopy,” Int. J. Metal-casting 2024. [CrossRef]
- Peterson, E. “Mold Flux Crystallization and Mold Thermal Behavior,”. Masters Thesis, Missouri University of Science and Technology, Rolla, Missouri, USA, 2017. [Google Scholar]
- Kölbl, N.; Marschall, I.; Harmuth, H. “Single Hot Thermocouple Technique for the Characterization of the Crystallization Behavior of Transparent or Translucent Liquids,” J. Mater. Sci. 2011, 46, 6248–6254. [Google Scholar] [CrossRef]
- Mu, W.; Hedström, P.; Shibata, H.; Jönsson, P. G.; Nakajima, K. “High-Temperature Confocal Laser Scanning Microscopy Studies of Ferrite Formation in Inclusion-Engineered Steels: A Review,” JOM 2018, 70, 2283–2295. 70. [CrossRef]
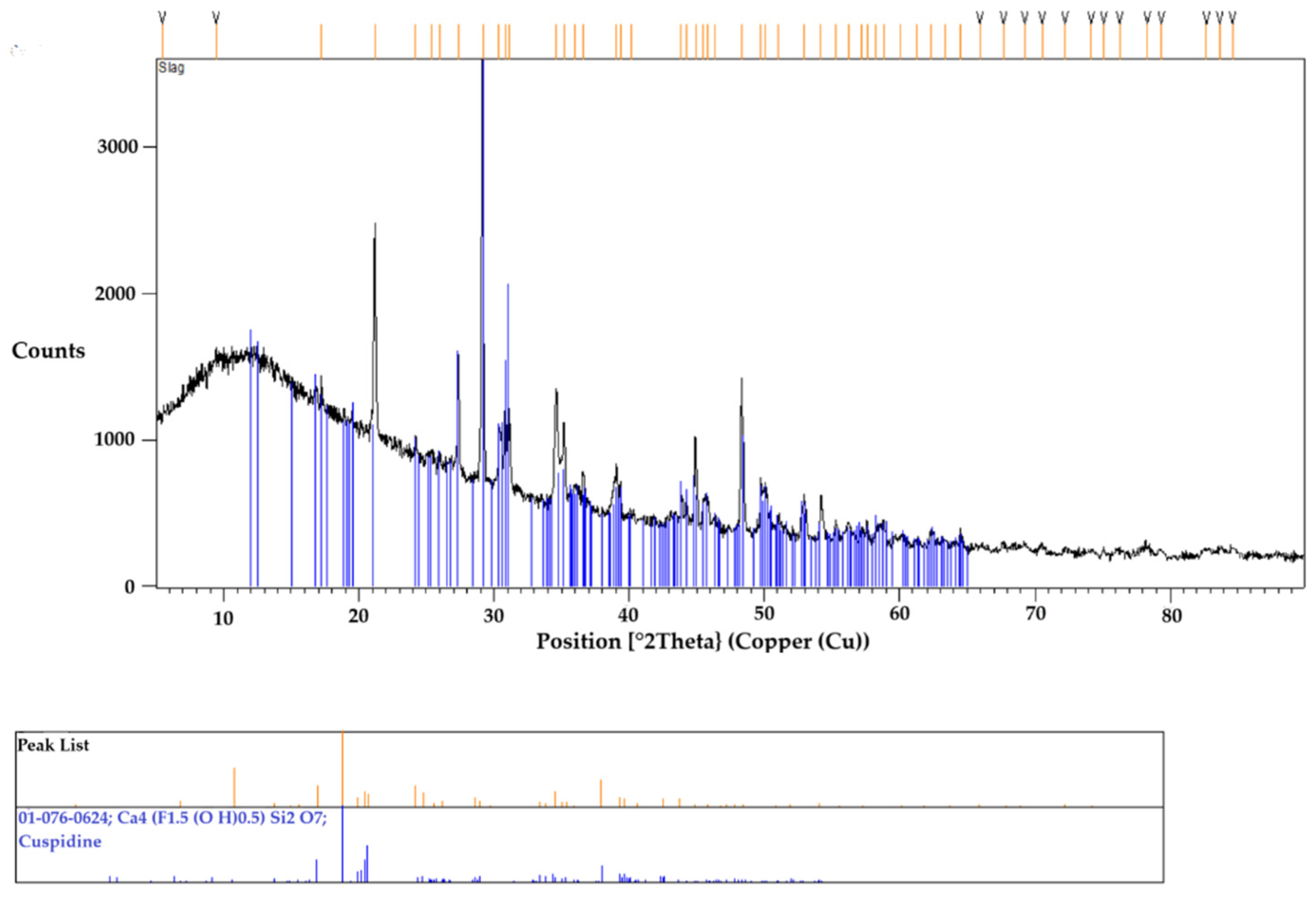
| CaO | SiO2 | Na2O | F | Al2O3 | MnO | Li2O | Fe2O3 | MgO | C(Total) |
|---|---|---|---|---|---|---|---|---|---|
| 31.7 | 27.5 | 9.2 | 7.6 | 5.2 | 2.8 | 0.7 | 0.5 | 0.3 | 9.4 |
| Basicity (CaO/ SiO2) | Solidification temperature | Viscosity at 1300°C |
|---|---|---|
| 1.15 | 1130°C | 0.7 Poise |
| Cooling rates | 5°C/min | 10°C/min | 15°C/min | 20°C/min | 25°C/min | 30°C/min |
| Crucibles | Primary Crystallization Temperatures | |||||
| Platinum | 1185°C | 1164°C | 1156°C | 1153°C | 1112°C | 1095°C |
| Graphite | 1039°C | 1021°C | 1006°C | 1014°C | 992°C | 933°C |
| Crystallization temperature difference | 146°C | 143°C | 150°C | 139°C | 120°C | 162°C |
| Cooling rates | 5°C/min | 10°C/min | 15°C/min | 20°C/min | 25°C/min | 30°C/min |
| Crucibles | Mass loss during the test | |||||
| Platinum | 5% | 3% | 2.5% | 2.5% | 2.5% | 2% |
| Graphite | 5% | 4% | 4% | 2% | 3% | 2% |
| Sample ID | CaO | SiO2 | Al2O3 | Na2O | F | Basicity |
|---|---|---|---|---|---|---|
| SA-3 | 39.3 | 34.1 | 10 | 7.5 | 9.1 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





