Submitted:
18 February 2025
Posted:
19 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
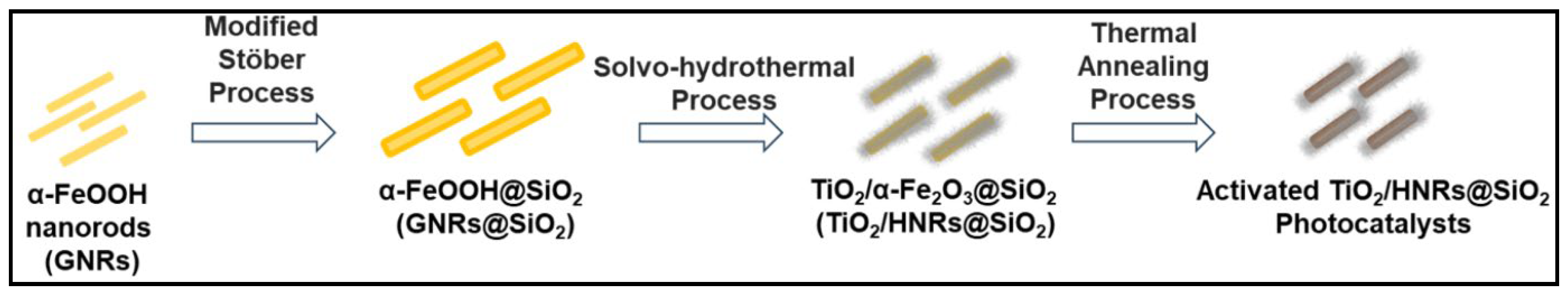
2.2. Preparation of Goethite (α-FeOOH) Nanorods (GNRs)
2.3. Preparation of SiO2 Coated GNRs (GNRs@SiO2)
2.4. Preparation of TiO2/SiO2 Coated Hematite (α-Fe2O3) Nanorods (TiO2/HNRs@SiO2)
2.5. Thermal Annealing of TiO2/HNRs@SiO2 for Photocatalytic Applications
2.6. Material Characterization
2.7. Photocatalytic Activity of Thermally Annealed TiO2/HNRs@SiO2
2.8. Statistical Analysis
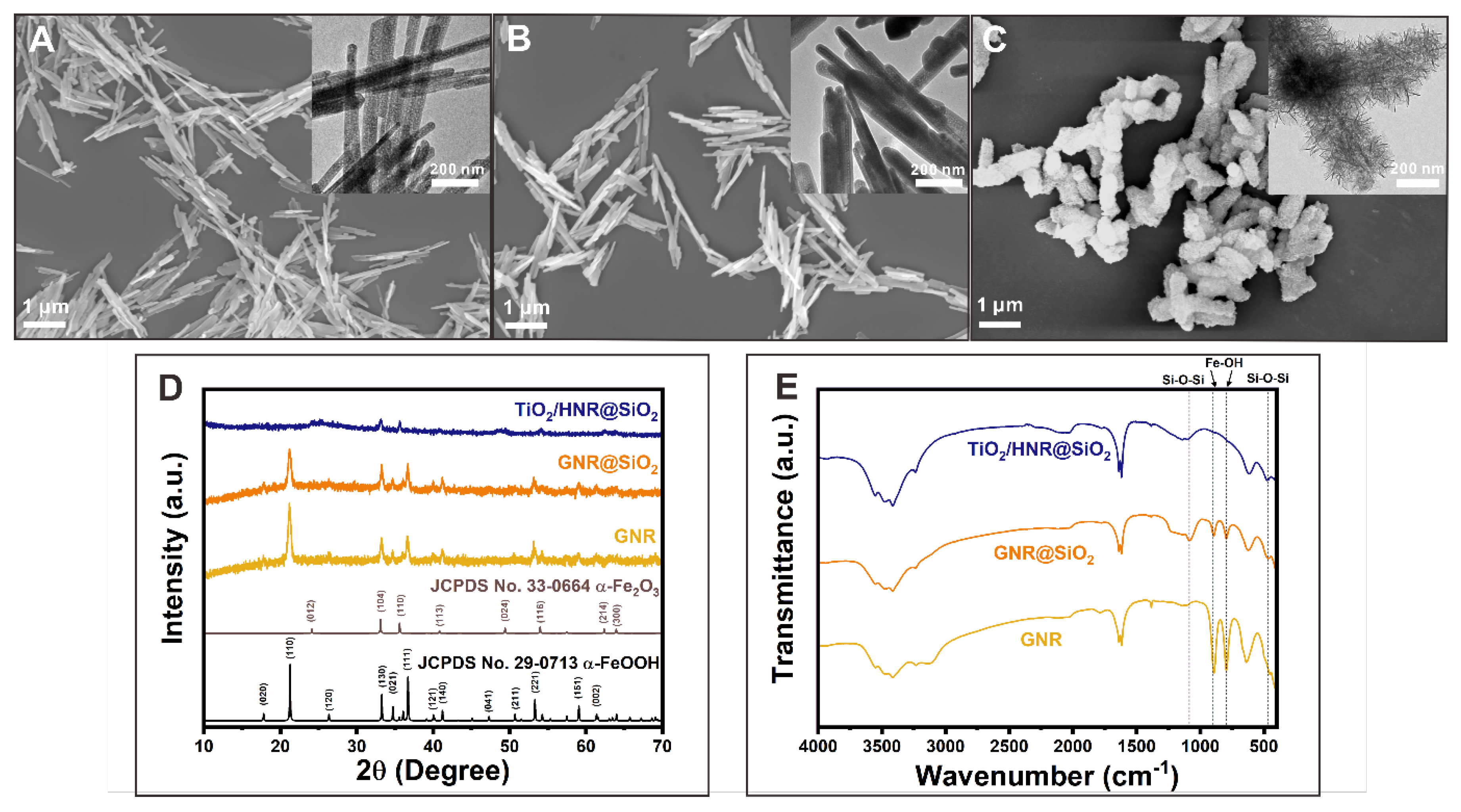
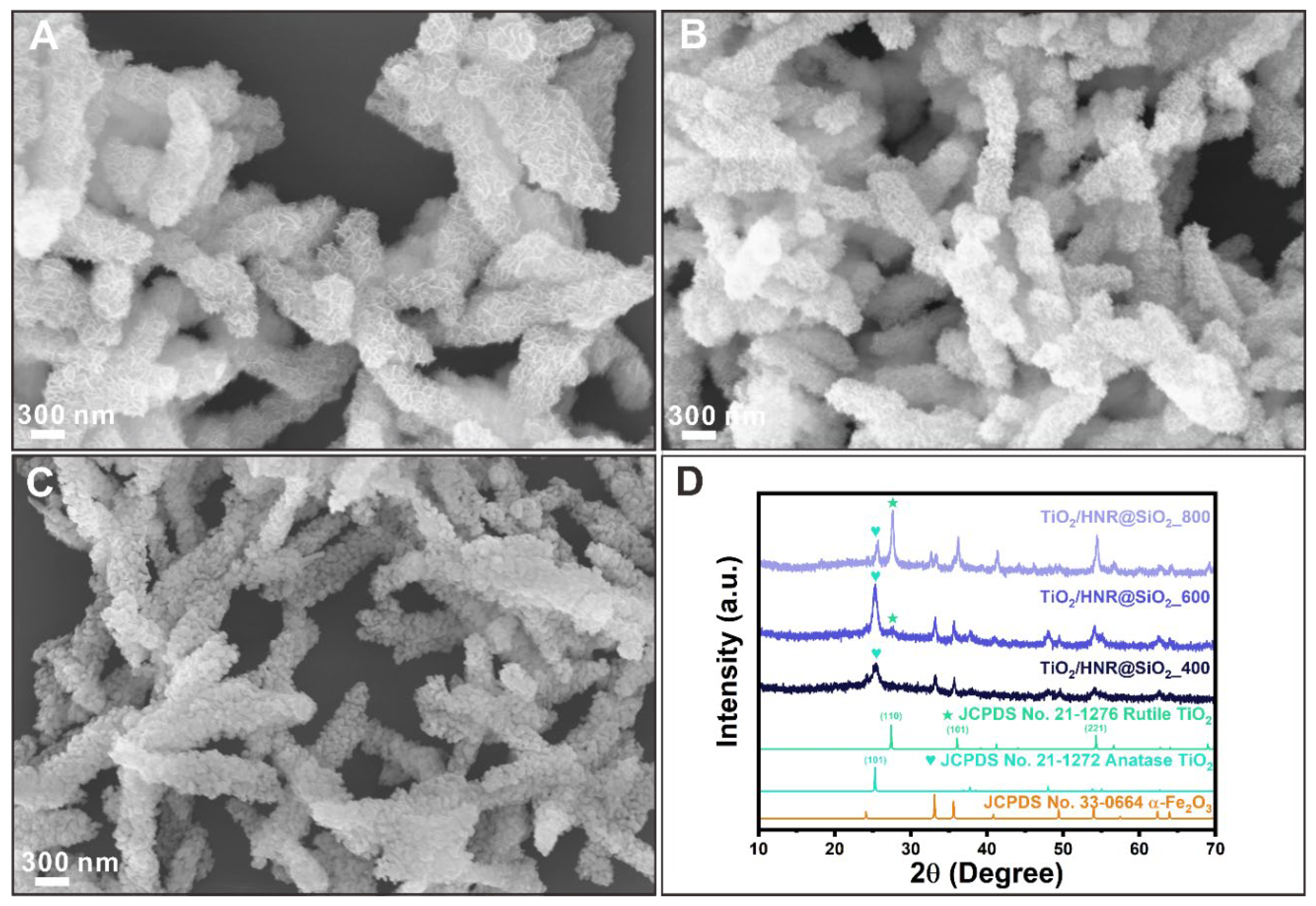
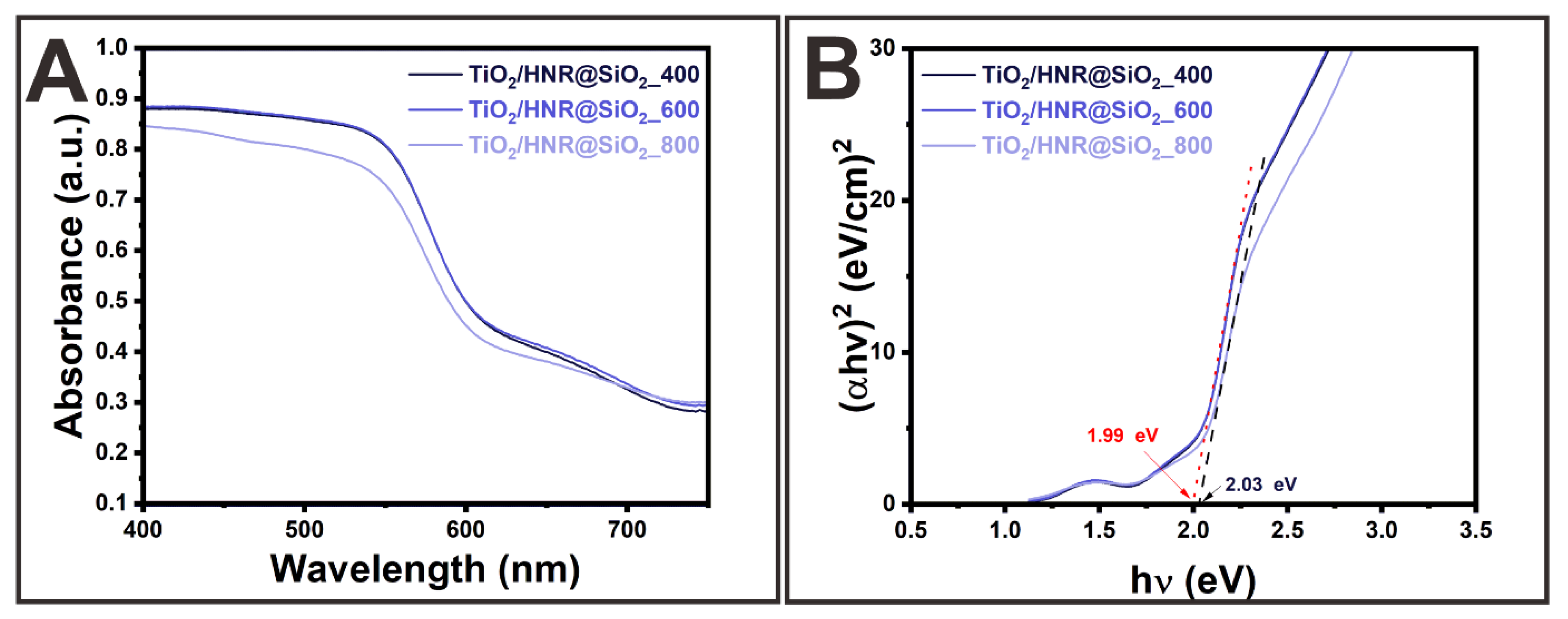
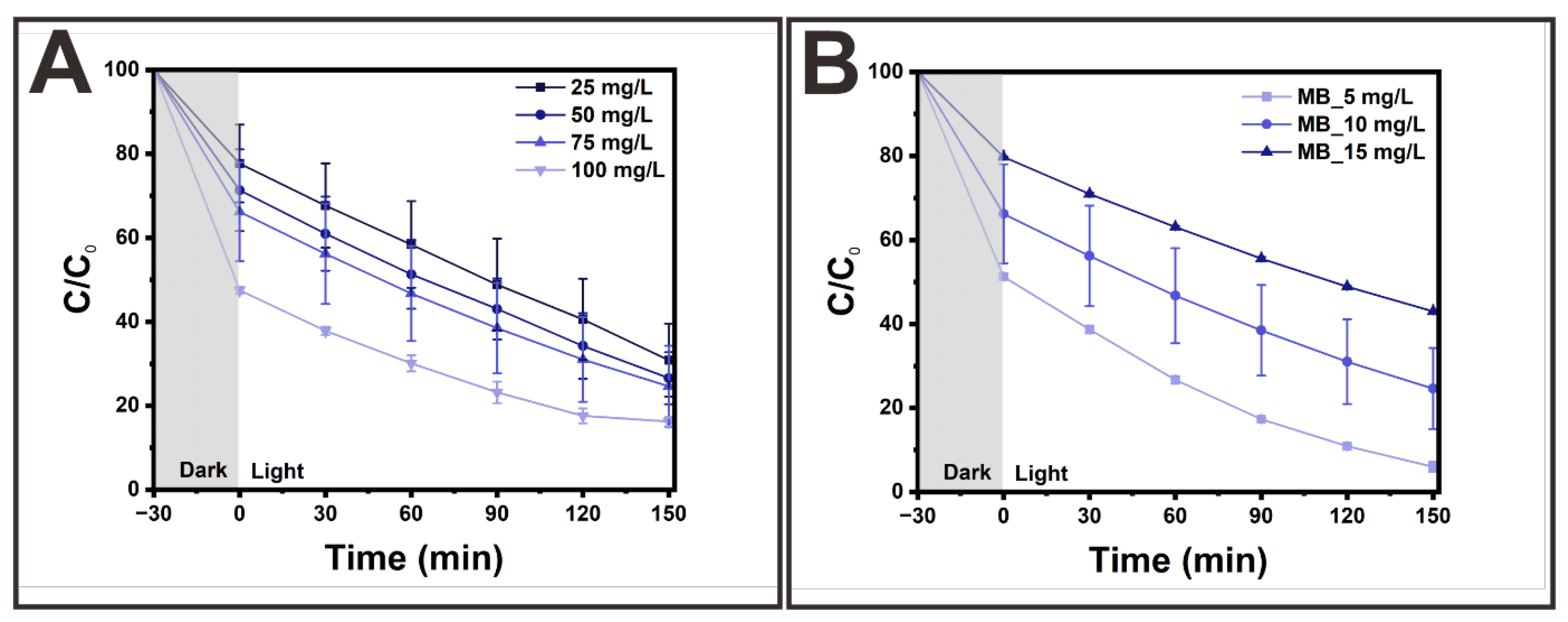
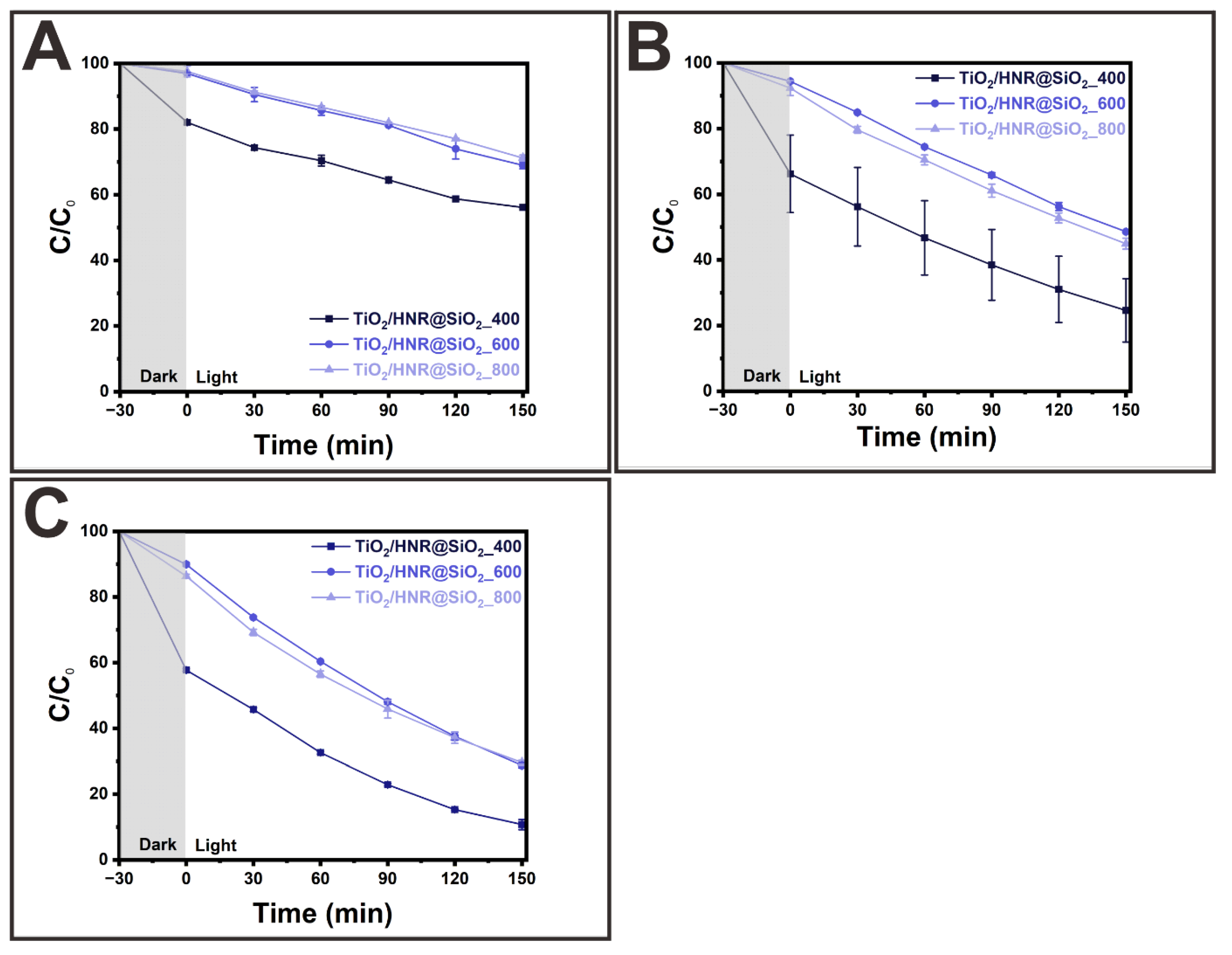
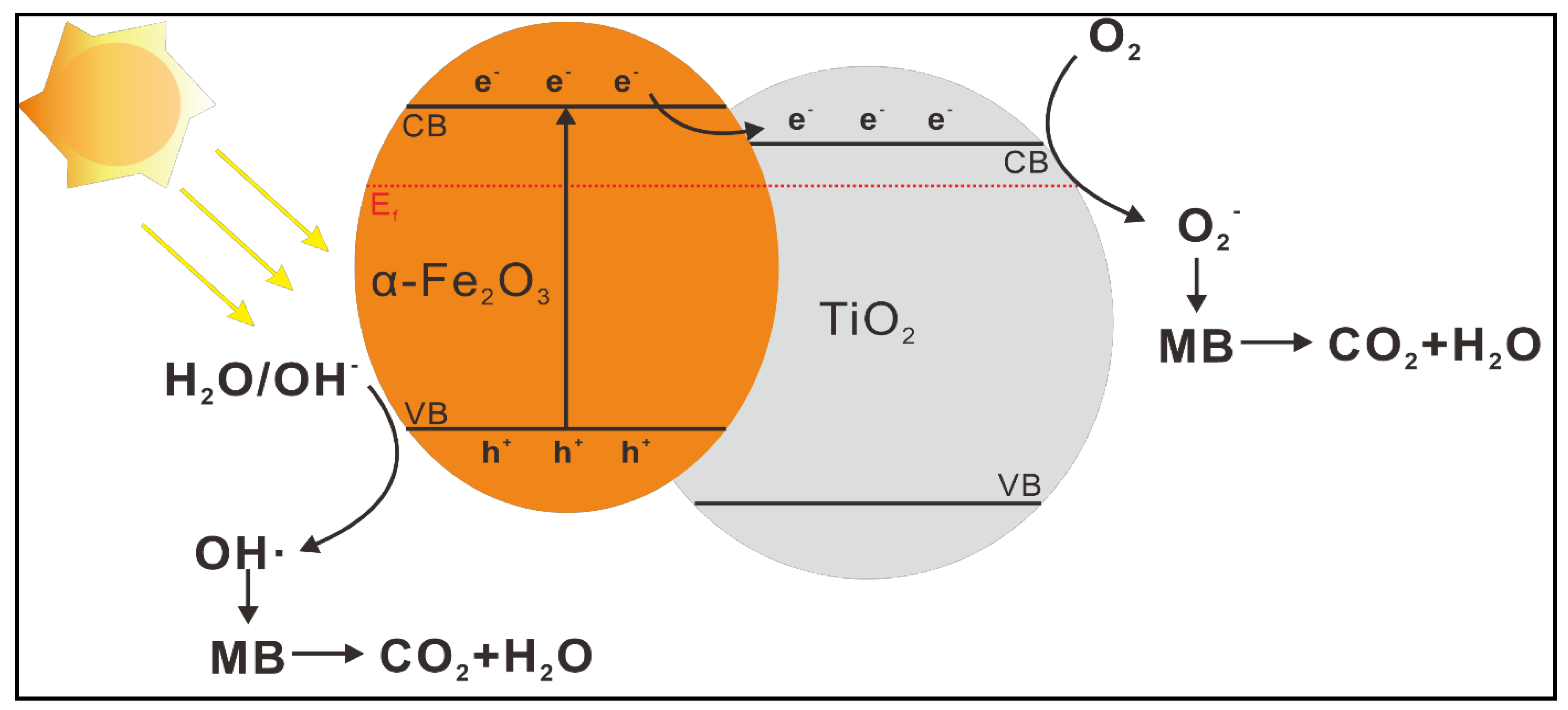
3. Results and Discussion
Morphologies and Crystal Structures
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
References
- Verma, N.; Chundawat, T.S.; Chandra, H.; Vaya, D. An efficient time reductive photocatalytic degradation of carcinogenic dyes by TiO2-GO nanocomposite. Materials Research Bulletin 2023, 158, 112043. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R. A comprehensive review of applications of magnetic graphene oxide based nanocomposites for sustainable water purification. Journal of environmental management 2019, 231, 622–634. [Google Scholar] [CrossRef]
- Fiaz, M.; Sohail, M.; Nafady, A.; Will, G.; Wahab, M.A. A facile two-step hydrothermal preparation of 2D/2D heterostructure of Bi2WO6/WS2 for the efficient photodegradation of methylene blue under sunlight. Environmental Research 2023, 234, 116550. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Wang, X.; He, Y.; Zhang, C.; Xue, G.; Liu, Z.; Lao, H.; Song, H.; Chen, W. Dyeing and finishing wastewater treatment in China: State of the art and perspective. Journal of Cleaner Production 2021, 326, 129353. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Wu, W.; Irfan, A.; Al-Sehemi, A.G. Silver nanoparticles engineered polystyrene-poly (n-isopropylmethacrylamide-acrylic acid) core shell hybrid polymer microgels for catalytic reduction of Congo red. Macromolecular Chemistry and Physics 2018, 219, 1800211. [Google Scholar] [CrossRef]
- Anjugam Vandarkuzhali, S.A.; Pugazhenthiran, N.; Mangalaraja, R.; Sathishkumar, P.; Viswanathan, B.; Anandan, S. Ultrasmall plasmonic nanoparticles decorated hierarchical mesoporous TiO2 as an efficient photocatalyst for photocatalytic degradation of textile dyes. ACS omega 2018, 3, 9834–9845. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the total environment 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresource technology 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Toxicity of Metal Oxides, Dyes, and Dissolved Organic Matter in Water: Implications for the Environment and Human Health. Toxics 2024, 12, 111. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent advances in the remediation of textile-dye-containing wastewater: prioritizing human health and sustainable wastewater treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. Journal of Industrial and Engineering Chemistry 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Ouda, M.; Kadadou, D.; Swaidan, B.; Al-Othman, A.; Al-Asheh, S.; Banat, F.; Hasan, S.W. Emerging contaminants in the water bodies of the Middle East and North Africa (MENA): A critical review. Science of the Total Environment 2021, 754, 142177. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.-Q.; Khan, A.; Doan, V.D.; Tran, V.A. Photocatalytic degradation of methyl orange dye by Ti3C2–TiO2 heterojunction under solar light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef] [PubMed]
- Motamedisade, A.; Heydari, A.; Osborn, D.; Alotabi, A.S.; Andersson, G.G. Au9 clusters deposited as co-catalysts on S-modified mesoporous TiO2 for photocatalytic degradation of methyl orange. Applied Surface Science 2024, 655, 159475. [Google Scholar] [CrossRef]
- Saeed, M.; Asghar, H.; Khan, I.; Akram, N.; Usman, M. Synthesis of TiO2-g-C3N4 for efficient photocatalytic degradation of Congo Red dye. Catalysis Today 2025, 447, 115154. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Popoola, L.T.; Gbadamosi, A.O.; Igbafe, A.I. Coal fly ash-supported ZnO-promoted TiO2 towards UV photocatalytic degradation of anthraquinone dye: Parametric optimization, kinetics and mechanism studies. Materials Today Communications 2024, 38, 107999. [Google Scholar] [CrossRef]
- Hamza, M.A.; El-Sayed, A.; El-Shazly, A.N.; Elmahgary, M.G. Efficient utilization of ceramic waste (cyclone dust waste) for enhancing the photocatalytic performance of TiO2 nanoparticles toward Rhodamine B photodegradation. Journal of Cleaner Production 2024, 434, 140341. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Advanced materials 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A review of synthesis methods, modifications, and mechanisms of ZnO/TiO2-based photocatalysts for photodegradation of contaminants. ACS omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.A.; Ashraf, W.M. Hydrogen production using TiO2-based photocatalysts: a comprehensive review. ACS omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder technology 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catalysis today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Degabriel, T.; Colaço, E.; Domingos, R.F.; El Kirat, K.; Brouri, D.; Casale, S.; Landoulsi, J.; Spadavecchia, J. Factors impacting the aggregation/agglomeration and photocatalytic activity of highly crystalline spheroid-and rod-shaped TiO2 nanoparticles in aqueous solutions. Physical Chemistry Chemical Physics 2018, 20, 12898–12907. [Google Scholar] [CrossRef]
- Amani-Ghadim, A.R.; Alizadeh, S.; Khodam, F.; Rezvani, Z. Synthesis of rod-like α-FeOOH nanoparticles and its photocatalytic activity in degradation of an azo dye: Empirical kinetic model development. Journal of Molecular Catalysis A: Chemical 2015, 408, 60–68. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Pasa, A.A.; Alcântara, C.C.; Acuña, J.J.; Bilmes, S.A.; Ricci, M.L.M.; Landers, R.; Fermino, T.Z.; Rodrigues-Filho, U.P. Enhanced photocatalytic properties of core@ shell SiO2@ TiO2 nanoparticles. Applied Catalysis B: Environmental 2015, 179, 333–343. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. Removal of hazardous pollutants from wastewaters: applications of TiO2-SiO2 mixed oxide materials. Journal of Nanomaterials 2014, 2014, 617405. [Google Scholar] [CrossRef]
- Periyat, P.; Baiju, K.; Mukundan, P.; Pillai, P.; Warrier, K. High temperature stable mesoporous anatase TiO2 photocatalyst achieved by silica addition. Applied Catalysis A: General 2008, 349, 13–19. [Google Scholar] [CrossRef]
- Liu, H.; Ji, S.; Yang, H.; Zhang, H.; Tang, M. Ultrasonic-assisted ultra-rapid synthesis of monodisperse meso-SiO2@Fe3O4 microspheres with enhanced mesoporous structure. Ultrasonics sonochemistry 2014, 21, 505–512. [Google Scholar] [CrossRef]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.C.; Archer, L.A.; Lou, X.W. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage. Journal of the American Chemical Society 2010, 132, 6124–6130. [Google Scholar] [CrossRef]
- Rodriguezcarvajal, J. Recent Advances in Magnetic-Structure Determination by Neutron Powder Diffraction. Physica B: Condensed Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J Applied Crystallography 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Sakata, M.; Cooper, M. An analysis of the Rietveld refinement method. J Applied Crystallography 1979, 12, 554–563. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, B.; Sathiyavahisan, L.P.; Mahadi, R.; Lee, K.; Jang, E.-H.; Choe, Y.; Oh, Y.-K.; Chung, S. Preparation of Two-Dimensional Fe3O4 Nanodisks and Nanosheets Transformed from α-Fe2O3 Analogues and Their Applications in Magnetic Field-Assisted Microalgal Harvesting Biorefinery Processes. ACS Sustainable Chemistry & Engineering 2023, 12, 2018–2027. [Google Scholar]
- Moon, G.; Lee, N.; Kang, S.; Park, J.; Kim, Y.-E.; Lee, S.-A.; Chitumalla, R.K.; Jang, J.; Choe, Y.; Oh, Y.-K. Hydrothermal synthesis of novel two-dimensional α-quartz nanoplates and their applications in energy-saving, high-efficiency, microalgal biorefineries. Chemical Engineering Journal 2021, 413, 127467. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Dang, V.-H.; Huang, H.J.; Wu, J.C. New viewpoints of magnetic-field influence on photocatalysis via 2-propanol oxidation. Catalysis Communications 2023, 179, 106691. [Google Scholar] [CrossRef]
- Ha, P.S.; Youn, H.-J.; Jung, H.S.; Hong, K.S.; Park, Y.H.; Ko, K.H. Anatase–rutile transition of precipitated titanium oxide with alcohol rinsing. Journal of Colloid and Interface Science 2000, 223, 16–20. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Modern powder diffraction 1989, 20, 277–308. [Google Scholar]
- Cao, J.; Song, X.-Z.; Kang, X.; Dai, Z.; Tan, Z. One-pot synthesis of oleic acid modified monodispersed mesoporous TiO2 nanospheres with enhanced visible light photocatalytic performance. Advanced Powder Technology 2018, 29, 1925–1932. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.; Hong, B. Effect of hydrogen plasma treatment on nano-structured TiO2 films for the enhanced performance of dye-sensitized solar cell. Applied surface science 2013, 274, 171–175. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Scientific reports 2016, 6, 32355. [Google Scholar] [CrossRef]
- Chong, R.; Fan, Y.; Du, Y.; Liu, L.; Chang, Z.; Li, D. Hydroxyapatite decorated TiO2 as efficient photocatalyst for selective reduction of CO2 with H2O into CH4. International Journal of Hydrogen Energy 2018, 43, 22329–22339. [Google Scholar] [CrossRef]
- Xiong, J.; Li, G.; Hu, C. Treatment of methylene blue by mesoporous Fe/SiO2 prepared from rice husk pyrolytic residues. Catalysis Today 2020, 355, 529–538. [Google Scholar] [CrossRef]
- Tissot, H.; Li, L.; Shaikhutdinov, S.; Freund, H.-J. Preparation and structure of Fe-containing aluminosilicate thin films. Physical Chemistry Chemical Physics 2016, 18, 25027–25035. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, W.; Zhang, Q.; Guan, Y.; Lu, Z. Synergistic Promotion of the Photocatalytic Preparation of Hydrogen Peroxide (H2O2) from Oxygen by Benzoxazine and Si─ O─ Ti Bond. Small 2023, 19, 2303907. [Google Scholar] [CrossRef]
- Zhong, N.; Shima, H.; Akinaga, H. Mechanism of the performance improvement of TiO2-x-based field-effect transistor using SiO2 as gate insulator. AIP advances 2011, 1. [Google Scholar] [CrossRef]
- Ma, H.-P.; Yang, J.-H.; Yang, J.-G.; Zhu, L.-Y.; Huang, W.; Yuan, G.-J.; Feng, J.-J.; Jen, T.-C.; Lu, H.-L. Systematic study of the SiOx film with different stoichiometry by plasma-enhanced atomic layer deposition and its application in SiOx/SiO2 super-lattice. Nanomaterials 2019, 9, 55. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Advances 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Materials research bulletin 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Bopape, D.A.; Tetana, Z.N.; Mabuba, N.; Motaung, D.E.; Hintsho-Mbita, N.C. Biosynthesis of TiO2 nanoparticles using Commelina benghanlensis for the photodegradation of methylene blue dye and antibiotics: Effect of plant concentration. Results in Chemistry 2023, 5, 100825. [Google Scholar] [CrossRef]
- Naffeti, M.; Zaïbi, M.A.; Nefzi, C.; García-Arias, A.V.; Chtourou, R.; Postigo, P.A. Highly efficient photodegradation of methylene blue by a composite photocatalyst of bismuth nanoparticles on silicon nanowires. Environmental Technology & Innovation 2023, 30, 103133. [Google Scholar]
- Khan, S.U.; Hussain, R.; Ali, Z.; Maryam, R.; Hussain, A.; Alajmi, M.F.; ur Rahman, S.; Zulfiqar, S.; Cochran, E.W. Facile synthesis of NiSe2–ZnO nanocomposites for enhanced photocatalysis and wastewater remediation. RSC advances 2024, 14, 28626–28637. [Google Scholar] [CrossRef]
- Zulfa, L.L.; Hidayat, A.R.P.; Utomo, W.P.; Subagyo, R.; Kusumawati, E.N.; Kusumawati, Y.; Hartanto, D.; Widyastuti, W.; Ediati, R. Facile synthesis of Ni-ZIF-8 with improved photodegradation performance for methylene blue. Case Studies in Chemical and Environmental Engineering 2024, 10, 100828. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure and applied chemistry 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustainable Environment Research 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Ding, L.; Yang, S.; Liang, Z.; Qian, X.; Chen, X.; Cui, H.; Tian, J. TiO2 nanobelts with anatase/rutile heterophase junctions for highly efficient photocatalytic overall water splitting. Journal of colloid and interface science 2020, 567, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.; Liu, X.; Qin, W.; Wang, M.; Pan, L. Metal-organic frameworks derived cake-like anatase/rutile mixed phase TiO2 for highly efficient photocatalysis. Journal of Alloys and Compounds 2017, 690, 640–646. [Google Scholar] [CrossRef]
- Xia, X.; Peng, S.; Bao, Y.; Wang, Y.; Lei, B.; Wang, Z.; Huang, Z.; Gao, Y. Control of interface between anatase TiO2 nanoparticles and rutile TiO2 nanorods for efficient photocatalytic H2 generation. Journal of Power Sources 2018, 376, 11–17. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-supported photocatalytic evidence of an extended type I electronic structure of the TiO2@Fe2O3 interface. ACS Applied Materials & Interfaces 2022, 14, 38255–38269. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




