Submitted:
25 February 2025
Posted:
26 February 2025
You are already at the latest version
Abstract
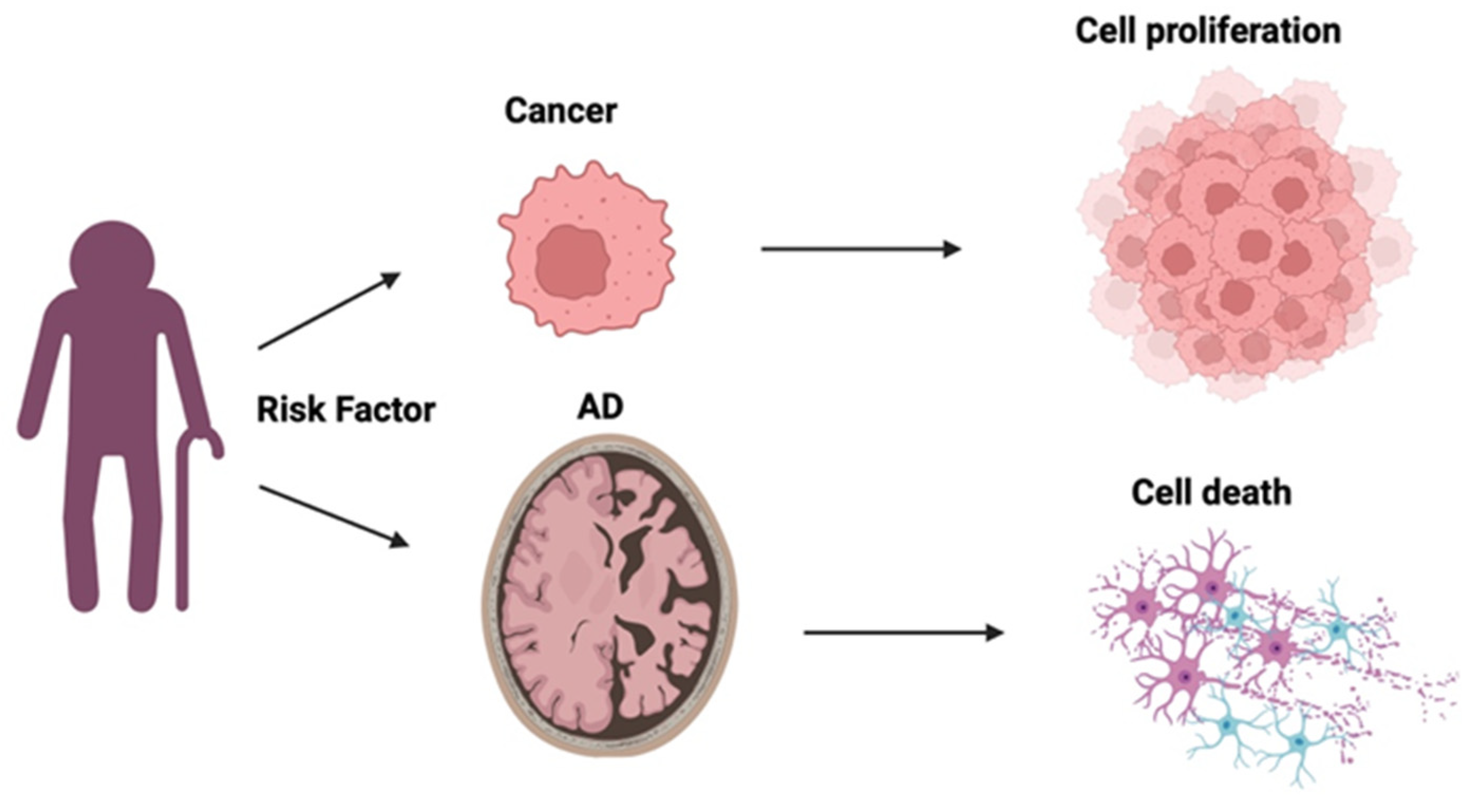
An apparent "inverse" relationship exists between two seemingly unconnected conditions: Alzheimer's disease (AD) and cancer, despite sharing similar risk factors, like increased age and obesity. AD is associated with amyloid beta (Aβ) plaques and neurofibrillary tau tangles that cause neural degeneration; cancer, in contrast, is characterised by enhanced cell survival and proliferation. Apolipoprotein E (ApoE) is the main lipoprotein found in the central nervous system and via its high affinity with lipoprotein receptors plays a critical role in cholesterol transport and uptake. ApoE has 3 protein isoforms: ApoE E2, ApoE E3, and ApoE E4, respectively encoded for by 3 allelic variants of APOE (ε2, ε3 and ε4). This review examines the characteristics and function of ApoE described in both AD and cancer, to assimilate evidence for its potential contribution to mechanisms that may underly the reported inverse association between the two conditions. Of the genetic risk factors relevant to most cases of AD, the most well-known with the strongest contribution to risk is APOE, specifically the ε4 variant, whereas for cancer risk, APOE has not featured as a significant genetic contributor to risk. Yet, at the protein level in both conditions, ApoE contributes to disease pathology via affecting lipid physiology and transport. In AD, Aβ-dependent and independent interactions have been suggested, whereas in cancer, ApoE plays a role in immunoregulation. Understanding the mechanism of action of ApoE in these diametrically opposed diseases, may enable differential targeting of therapeutics, to provide a beneficial outcome for both.
Keywords:
1. Introduction
The Molecular Biology and Function of ApoE
2. A Brief Introduction to Alzheimer’s Disease
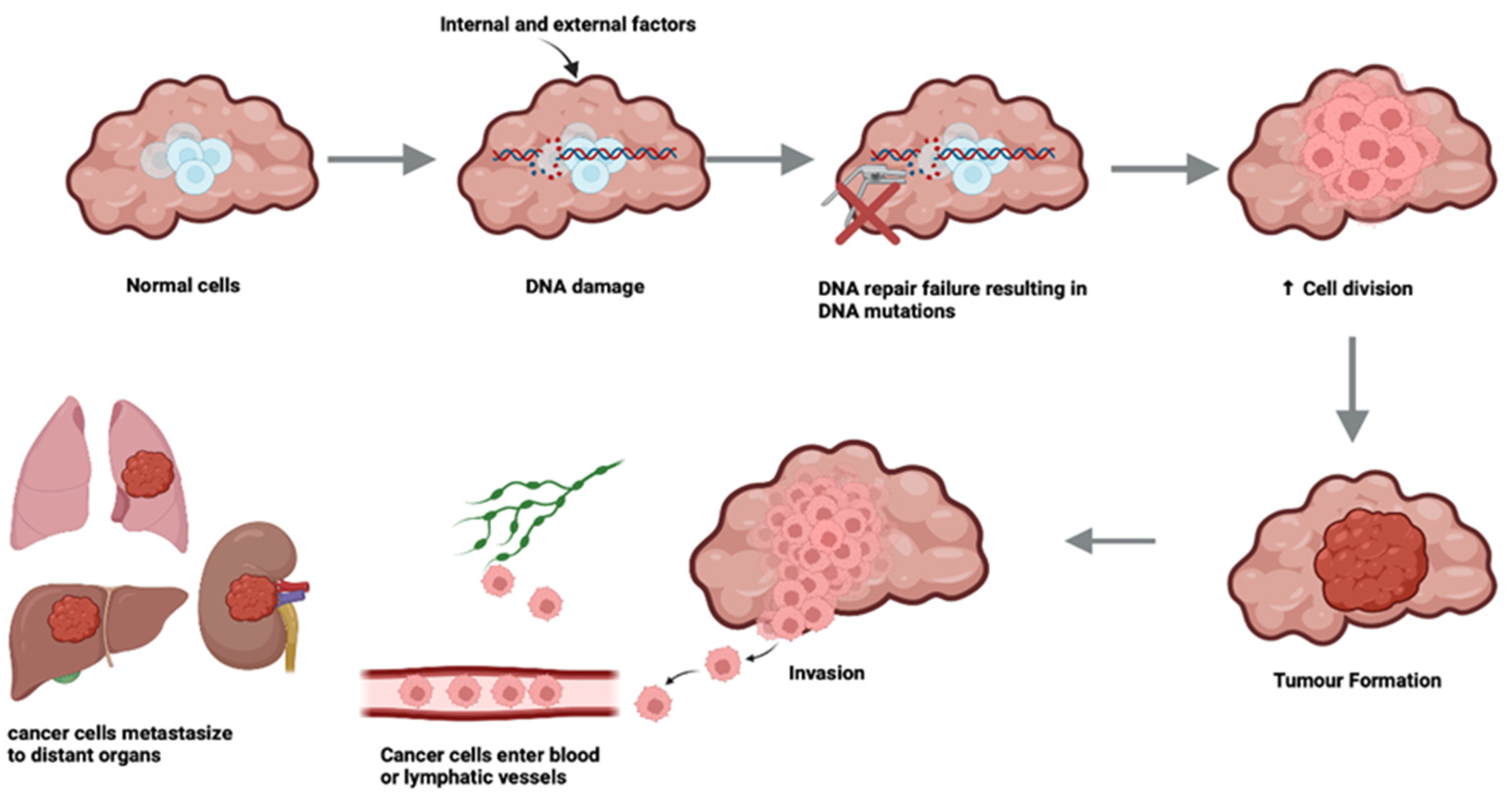
3. A Brief Overview of Cancers of Older Age
4. Curious Inverse Associations Between Alzheimer’s Disease and Cancer
5. ApoE in Alzheimer’s Disease
6. ApoE in Cancer
6.1. Prostate Cancer
6.2. Breast Cancer
6.3. Colorectal Cancer
6.4. Ovarian Cancer
7. Is ApoE a Mediator of Inverse Associations Between AD and Cancer?
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Abondio P, Sazzini M, Garagnani P, Boattini A, Monti D, Franceschi C, Luiselli D, Giuliani C: The Genetic Variability of APOE in Different Human Populations and Its Implications for Longevity. Genes (Basel) 2019, 10(3).
- Rebeck GW: The role of APOE on lipid homeostasis and inflammation in normal brains. J Lipid Res 2017, 58(8):1493-1499.
- Leduc V, Jasmin-Belanger S, Poirier J: APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol Med 2010, 16(10):469-477.
- Wetterau JR, Aggerbeck LP, Rall SC, Jr., Weisgraber KH: Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J Biol Chem 1988, 263(13):6240-6248.
- Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA: Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science 1991, 252(5014):1817-1822.
- Forstner M, Peters-Libeu C, Contreras-Forrest E, Newhouse Y, Knapp M, Rupp B, Weisgraber KH: Carboxyl-terminal domain of human apolipoprotein E: expression, purification, and crystallization. Protein Expr Purif 1999, 17(2):267-272.
- Weisgraber KH: Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res 1990, 31(8):1503-1511.
- Mahley RW, Weisgraber KH, Huang Y: Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res 2009, 50 Suppl(Suppl):S183-188.
- Lane-Donovan C, Herz J: ApoE, ApoE Receptors, and the Synapse in Alzheimer’s Disease. Trends Endocrinol Metab 2017, 28(4):273-284.
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH et al: The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res 2005, 46(8):1721-1731.
- Johnson LA, Olsen RH, Merkens LS, DeBarber A, Steiner RD, Sullivan PM, Maeda N, Raber J: Apolipoprotein E-low density lipoprotein receptor interaction affects spatial memory retention and brain ApoE levels in an isoform-dependent manner. Neurobiol Dis 2014, 64:150-162.
- Shinohara M, Tachibana M, Kanekiyo T, Bu G: Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J Lipid Res 2017, 58(7):1267-1281.
- Li Z, Shue F, Zhao N, Shinohara M, Bu G: APOE2: protective mechanism and therapeutic implications for Alzheimer’s disease. Mol Neurodegener 2020, 15(1):63.
- Strickland MR, Holtzman DM: Dr. Jekyll and Mr. Hyde: ApoE explains opposing effects of neuronal LRP1. J Clin Invest 2019, 129(3):969-971.
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J: Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 2002, 277(42):39944-39952.
- Lane-Donovan C, Herz J: The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease. J Lipid Res 2017, 58(6):1036-1043.
- Spuch C, Navarro C: Transport Mechanisms at the Blood-Cerebrospinal-Fluid Barrier: Role of Megalin (LRP2). Recent Patents on Endocrine, Metabolic & Immune Drug Discovery 2010, 4(3).
- Marzolo M-P, Farfán P: New Insights into the Roles of Megalin/LRP2 and the Regulation of its Functional Expression. Biological Research 2011, 44(1):89-105.
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV: apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008, 118(12):4002-4013.
- Zhang H, Chen W, Tan Z, Zhang L, Dong Z, Cui W, Zhao K, Wang H, Jing H, Cao R et al: A Role of Low-Density Lipoprotein Receptor-Related Protein 4 (LRP4) in Astrocytic Abeta Clearance. J Neurosci 2020, 40(28):5347-5361.
- Haas J, Beer AG, Widschwendter P, Oberdanner J, Salzmann K, Sarg B, Lindner H, Herz J, Patsch JR, Marschang P: LRP1b shows restricted expression in human tissues and binds to several extracellular ligands, including fibrinogen and apoE-carrying lipoproteins. Atherosclerosis 2011, 216(2):342-347.
- Real R, Martinez-Carrasco A, Reynolds RH, Lawton MA, Tan MMX, Shoai M, Corvol JC, Ryten M, Bresner C, Hubbard L et al: Association between the LRP1B and APOE loci and the development of Parkinson’s disease dementia. Brain 2023, 146(5):1873-1887.
- Yajima R, Tokutake T, Koyama A, Kasuga K, Tezuka T, Nishizawa M, Ikeuchi T: ApoE-isoform-dependent cellular uptake of amyloid-beta is mediated by lipoprotein receptor LR11/SorLA. Biochem Biophys Res Commun 2015, 456(1):482-488.
- DeTure MA, Dickson DW: The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 2019, 14(1):32.
- Sharma VK, Singh TG, Singh S, Garg N, Dhiman S: Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem Res 2021, 46(12):3103-3122.
- Goel P, Chakrabarti S, Goel K, Bhutani K, Chopra T, Bali S: Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front Mol Neurosci 2022, 15:937133.
- The Economic impact of dementia [internet] . Available online: https://www.alzheimers.org.uk/sites/default/files/2024-05/the-annual-costs-of-dementia.pdf.
- Association As: 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024, 20(5):3708-3821.
- Lopez-Lee C, Torres ERS, Carling G, Gan L: Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112(8):1208-1221.
- Mukadam N, Marston L, Lewis G, Mathur R, Lowther E, Rait G, Livingston G: South Asian, Black and White ethnicity and the effect of potentially modifiable risk factors for dementia: A study in English electronic health records. PLoS One 2023, 18(10):e0289893.
- Gleason CE, Zuelsdorff M, Gooding DC, Kind AJH, Johnson AL, James TT, Lambrou NH, Wyman MF, Ketchum FB, Gee A et al: Alzheimer’s disease biomarkers in Black and non-Hispanic White cohorts: A contextualized review of the evidence. Alzheimers Dement 2022, 18(8):1545-1564.
- Patel J, Baptiste BA, Kim E, Hussain M, Croteau DL, Bohr VA: DNA damage and mitochondria in cancer and aging. Carcinogenesis 2020, 41(12):1625-1634.
- Boutry J, Tissot S, Ujvari B, Capp JP, Giraudeau M, Nedelcu AM, Thomas F: The evolution and ecology of benign tumors. Biochim Biophys Acta Rev Cancer 2022, 1877(1):188643.
- Cooper GM: The Cell: A Molecular Approach, 2nd edition edn. Sunderland (MA): Sinauer Associates; 2000.
- Goel P, Chakrabarti S, Goel K, Bhutani K, Chopra T, Bali S: Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Frontiers in Molecular Neuroscience 2022, 15.
- United Nations DoEaSA, Population Division: World Population Ageing 2017 - Highlights. In., ST/ESA/SER.A/397 edn. New York: UN; 2017.
- Javaid SF, Giebel C, Khan MA, Hashim MJ, Javaid SF, Giebel C, Khan MA, Hashim MJ: Epidemiology of Alzheimer’s disease and other dementias: rising global burden and forecasted trends. F1000Research 2021 10:425 2021, 10.
- Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, Soerjomataram I: Global cancer incidence in older adults, 2012 and 2035: A population-based study. International Journal of Cancer 2019, 144(1).
- Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP, Seshadri S, Wolf PA: Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ 2012, 344.
- Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, Williams MM, Kopan R, Behrens MI, Morris JC: Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010, 74(2):106-112.
- Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC: Alzheimer disease and cancer. Neurology 2005, 64(5):895-898.
- Yamada M, Sasaki H, Mimori Y, Kasagi F, Sudoh S, Ikeda J, Hosoda Y, Nakamura S, Kodama K: Prevalence and Risks of Dementia in the Japanese Population: RERF’s Adult Health Study Hiroshima Subjects. Journal of the American Geriatrics Society 1999, 47(2).
- Prinelli F, Adorni F, Leite MLC, Pettenati C, Russo A, Di Santo S, Musicco M: Different Exposures to Risk Factors Do Not Explain the Inverse Relationship of Occurrence Between Cancer and Neurodegenerative Diseases: An Italian Nested Case-control Study. Alzheimer Disease & Associated Disorders 2018, 32(1).
- Musicco M, Adorni F, Di Santo S, Prinelli F, Pettenati C, Caltagirone C, Palmer K, Russo A: Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study - PubMed. Neurology 2013, 81(4).
- Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG: Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol 2004, 164(5):1727-1737.
- Yamada M, Sasaki H, Mimori Y, Kasagi F, Sudoh S, Ikeda J, Hosoda Y, Nakamura S, Kodama K: Prevalence and risks of dementia in the Japanese population: RERF’s adult health study Hiroshima subjects. Radiation Effects Research Foundation. J Am Geriatr Soc 1999, 47(2):189-195.
- Chamberlain JD, Rouanet A, Dubois B, Pasquier F, Hanon O, Gabelle A, Ceccaldi M, Krolak-Salmon P, Béjot Y, Godefroy O et al: Investigating the association between cancer and the risk of dementia: Results from the Memento cohort. Alzheimer’s & Dementia 2021, 17(9).
- Ording AG, Horváth-Puhó E, Veres K, Glymour MM, Rørth M, Sørensen HT, Henderson VW: Cancer and risk of Alzheimer’s disease: Small association in a nationwide cohort study. Alzheimer’s & Dementia 2020, 16(7).
- Nudelman KNH, Risacher SL, West JD, McDonald BC, Gao S, Saykin AJ, ftAsDNI: Frontiers | Association of cancer history with Alzheimer’s disease onset and structural brain changes. Frontiers in Physiology 2014, 5.
- Sun M, Wang Y, Sundquist J, Sundquist K, Ji J: The Association Between Cancer and Dementia: A National Cohort Study in Sweden - PubMed. Frontiers in oncology 2020, 10.
- Catalá-López F, Hutton B, Driver JA, Page MJ, Ridao M, Valderas JM, Alonso-Arroyo A, Forés-Martos J, Martínez S, Gènova-Maleras R et al: Cancer and central nervous system disorders: protocol for an umbrella review of systematic reviews and updated meta-analyses of observational studies. Systematic Reviews 2017 6:1 2017, 6(1).
- Shi H-b, Tang B, Liu Y-W, Wang X-F, Chen G-J, Shi H-b, Tang B, Liu Y-W, Wang X-F, Chen G-J: Alzheimer disease and cancer risk: a meta-analysis. Journal of Cancer Research and Clinical Oncology 2014 141:3 2014, 141(3).
- Ospina-Romero M, Glymour MM, Hayes-Larson E, Mayeda ER, Graff RE, Brenowitz WD, Ackley SF, Witte JS, Kobayashi LC: Association Between Alzheimer Disease and Cancer. JAMA Network Open 2020, 3(11).
- Zhang Q, Guo S, Zhang X, Tang S, Shao W, Han X, Wang L, Y D: Inverse relationship between cancer and Alzheimer’s disease: a systemic review meta-analysis - PubMed. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2015 Nov, 36(11).
- White RS, Lipton RB, Hall CB, Steinerman JR: Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology 2013, 80(21):1966-1972.
- Tirumalasetti F, Han L, Birkett DP: The Relationship between Cancer and Alzheimer’s Disease. Journal of the American Geriatrics Society 1991, 39(8).
- Feng Y-CA, Cho K, Lindstrom S, Kraft P, Cormack J, Liang L, Driver JA, Feng Y-CA, Cho K, Lindstrom S et al: Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Human Genetics 2017 136:10 2017, 136(10).
- Seddighi S, Houck AL, Rowe JB, Pharoah PDP, Seddighi S, Houck AL, Rowe JB, Pharoah PDP: Evidence of a Causal Association Between Cancer and Alzheimer’s Disease: a Mendelian Randomization Analysis. Scientific Reports 2019 9:1 2019, 9(1).
- Bassil DT, Zheng B, Su B, Kafetsouli D, Udeh-Momoh C, Tzoulaki I, Ahmadi-Abhari S, Muller DC, Riboli E, Middleton LT et al: Lower Incidence of Dementia Following Cancer Diagnoses: Evidence from a Large Cohort and Mendelian Randomization Study. The Journal of Prevention of Alzheimer’s Disease 2024, 11(5).
- Dong Z, Xu M, Sun X, Wang X: Mendelian randomization and transcriptomic analysis reveal an inverse causal relationship between Alzheimer’s disease and cancer. J Transl Med 2023, 21(1):527.
- Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A: Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses. PLOS Genetics 2014, 10(2).
- Willik KDvd, Schagen SB, Ikram MA: Cancer and dementia: Two sides of the same coin? European Journal of Clinical Investigation 2018, 48(11).
- Driver JA, Zhou XZ, Lu KP: Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochimica et biophysica acta 2015, 1850(10).
- Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP: The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999, 399(6738):784-788.
- Wulf G, Garg P, Liou Y-C, Iglehart D, Lu KP: Modeling breast cancer in vivo and ex vivo reveals an essential role of Pin1 in tumorigenesis. The EMBO Journal 2004 Jul 15, 23(16).
- Liou Y-C, Sun A, Ryo A, Zhou XZ, Yu Z-X, Huang H-K, Uchida T, Bronson R, Bing G, Li X et al: Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 2003 424:6948 2003, 424(6948).
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM et al: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Medicine 2017 23:6 2017, 23(6).
- Sigal A, Rotter V: Oncogenic Mutations of the p53 Tumor Suppressor: The Demons of the Guardian of the Genome Cancer Research 2000, 60(24):6788-6793.
- Kitamura Y, Shimohama S, Kamoshima W, Matsuoka Y, Nomura Y, Taniguchi T: Changes of p53 in the Brains of Patients with Alzheimer’s Disease. Biochemical and Biophysical Research Communications 1997, 232(2).
- de la Monte SM, Sohn YK, Wands JR: Correlates of p53- and Fas (CD95)-mediated apoptosis in Alzheimer’s disease. Journal of the Neurological Sciences 1997, 152(1).
- Parsons MJ, Tammela T, Dow LE: WNT as a Driver and Dependency in Cancer. Cancer Discovery 2021, 11(10).
- Zhan T, Rindtorff N, Boutros M, Zhan T, Rindtorff N, Boutros M: Wnt signaling in cancer. Oncogene 2017 36:11 2016, 36(11).
- Inestrosa NC, Toledo EM, Inestrosa NC, Toledo EM: The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Molecular Neurodegeneration 2008 3:1 2008, 3(1).
- Lu Z, Hunter T, Lu Z, Hunter T: Prolyl isomerase Pin1 in cancer. Cell Research 2014 24:9 2014, 24(9).
- Xiao Q, Werner J, Venkatachalam N, Boonekamp KE, Ebert MP, Zhan T, Xiao Q, Werner J, Venkatachalam N, Boonekamp KE et al: Cross-Talk between p53 and Wnt Signaling in Cancer. Biomolecules 2022, Vol 12, Page 453 2022, 12(3).
- Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G: Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nature reviews Neurology 2019, 15(9).
- Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hägg S, Athanasiu L et al: Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nature Genetics 2019, 51(3).
- Shafi O: Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: a systematic review. BMC Neurology 2016 16:1 2016, 16(1).
- Lanni C, Masi M, Racchi M, Govoni S, Lanni C, Masi M, Racchi M, Govoni S: Cancer and Alzheimer’s disease inverse relationship: an age-associated diverging derailment of shared pathways. Molecular Psychiatry 2020 26:1 2020, 26(1).
- Driver JA: Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence - PubMed. Biogerontology 2014, 15(6).
- Caruso C, Motolese M, Iacovelli L, Caraci F, Copani A, Nicoletti F, Terstappen GC, Gaviraghi G, Caricasole A: Inhibition of the canonical Wnt signaling pathway by apolipoprotein E4 in PC12 cells. Journal of neurochemistry 2006, 98(2).
- Lattanzio F, Carboni L, Carretta D, Rimondini R, Candeletti S, Romualdi P: Human apolipoprotein E4 modulates the expression of Pin1, Sirtuin 1, and Presenilin 1 in brain regions of targeted replacement apoE mice. Neuroscience 2014, 256.
- Ostendorf BN, Bilanovic J, Adaku N, Tafreshian KN, Tavora B, Vaughan RD, Tavazoie SF, Ostendorf BN, Bilanovic J, Adaku N et al: Common germline variants of the human APOE gene modulate melanoma progression and survival. Nat Med 2020, 26(7).
- Ponec M, te Pas MF, Havekes L, Boonstra J, Mommaas AM, Vermeer BJ: LDL receptors in keratinocytes. J Invest Dermatol 1992, 98(6 Suppl):50S-56S.
- Chieosilapatham P, Yue H, Ikeda S, Ogawa H, Niyonsaba F: Involvement of the lipoprotein receptor LRP1 in AMP-IBP5-mediated migration and proliferation of human keratinocytes and fibroblasts. J Dermatol Sci 2020, 99(3):158-167.
- Li Y, Wang Y, Zhang W, Jiang L, Zhou W, Liu Z, Li S, Lu H: Overexpression of Amyloid Precursor Protein Promotes the Onset of Seborrhoeic Keratosis and is Related to Skin Ageing. Acta Derm Venereol 2018, 98(6):594-600.
- Puig KL, Combs CK: Expression and function of APP and its metabolites outside the central nervous system. Exp Gerontol 2013, 48(7):608-611.
- Li T, Wen H, Brayton C, Laird FM, Ma G, Peng S, Placanica L, Wu TC, Crain BJ, Price DL et al: Moderate reduction of gamma-secretase attenuates amyloid burden and limits mechanism-based liabilities. J Neurosci 2007, 27(40):10849-10859.
- Zhang Y, Chen H, Li R, Sterling K, Song W: Amyloid beta-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct Target Ther 2023, 8(1):248.
- Pericak-Vance MA, Bebout JL, Gaskell PC, Jr., Yamaoka LH, Hung WY, Alberts MJ, Walker AP, Bartlett RJ, Haynes CA, Welsh KA et al: Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet 1991, 48(6):1034-1050.
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ et al: Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43(8):1467-1472.
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD: Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993, 90(20):9649-9653.
- Bellenguez C, Kucukali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, Campos-Martin R, Grenier-Boley B, Andrade V et al: New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet 2022, 54(4):412-436.
- Jansen IE, van der Lee SJ, Gomez-Fonseca D, de Rojas I, Dalmasso MC, Grenier-Boley B, Zettergren A, Mishra A, Ali M, Andrade V et al: Genome-wide meta-analysis for Alzheimer’s disease cerebrospinal fluid biomarkers. Acta Neuropathol 2022, 144(5):821-842.
- Le Guen Y, Belloy ME, Grenier-Boley B, de Rojas I, Castillo-Morales A, Jansen I, Nicolas A, Bellenguez C, Dalmasso C, Kucukali F et al: Association of Rare APOE Missense Variants V236E and R251G With Risk of Alzheimer Disease. JAMA Neurol 2022, 79(7):652-663.
- Husain MA, Laurent B, Plourde M: APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Frontiers in Neuroscience 2021, 15.
- Husain MA, Laurent B, Plourde M: APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front Neurosci 2021, 15:630502.
- Windham IA, Cohen S: The cell biology of APOE in the brain. Trends Cell Biol 2024, 34(4):338-348.
- Narasimhan S, Holtzman DM, Apostolova LG, Cruchaga C, Masters CL, Hardy J, Villemagne VL, Bell J, Cho M, Hampel H: Apolipoprotein E in Alzheimer’s disease trajectories and the next-generation clinical care pathway. Nat Neurosci 2024, 27(7):1236-1252.
- Safieh M, Korczyn AD, Michaelson DM: ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med 2019, 17(1):64.
- Wisniewski T, Drummond E: APOE-amyloid interaction: Therapeutic targets. Neurobiol Dis 2020, 138:104784.
- Wisniewski T, Frangione B: Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 1992, 135(2):235-238.
- Wisniewski T, Golabek A, Matsubara E, Ghiso J, Frangione B: Apolipoprotein E: binding to soluble Alzheimer’s beta-amyloid. Biochem Biophys Res Commun 1993, 192(2):359-365.
- Huang YA, Zhou B, Wernig M, Sudhof TC: ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell 2017, 168(3):427-441 e421.
- Tachibana M, Holm ML, Liu CC, Shinohara M, Aikawa T, Oue H, Yamazaki Y, Martens YA, Murray ME, Sullivan PM et al: APOE4-mediated amyloid-beta pathology depends on its neuronal receptor LRP1. J Clin Invest 2019, 129(3):1272-1277.
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D et al: Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 2000, 97(6):2892-2897.
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM et al: ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron 2004, 41(2):193-202.
- Hauser PS, Narayanaswami V, Ryan RO: Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res 2011, 50(1):62-74.
- Chen Y, Strickland MR, Soranno A, Holtzman DM: Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109(2):205-221.
- Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM: ApoE influences amyloid-beta (Abeta) clearance despite minimal apoE/Abeta association in physiological conditions. Proc Natl Acad Sci U S A 2013, 110(19):E1807-1816.
- Rodrigue KM, Kennedy KM, Park DC: Beta-Amyloid Deposition and the Aging Brain. Neuropsychology Review 2009, 19(4):436-450.
- Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES et al: Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330(6):512-527.
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S et al: Lecanemab in Early Alzheimer’s Disease. N Engl J Med 2023, 388(1):9-21.
- Rapp A, Gmeiner B, Hüttinger M: Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 2006, 88(5):473-483.
- Cancer Research UK (CRUK). Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk.
- Platz EA, Clinton SK, Giovannucci E: Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer 2008, 123(7):1693-1698.
- Grant WB: A multicountry ecological study of risk-modifying factors for prostate cancer: apolipoprotein E epsilon4 as a risk factor and cereals as a risk reduction factor. Anticancer Res 2010, 30(1):189-199.
- Steinmetz A, Jakobs C, Motzny S, Kaffarnik H: Differential distribution of apolipoprotein E isoforms in human plasma lipoproteins. Arteriosclerosis 1989, 9(3):405-411.
- Lehrer S: Possible relationship of the apolipoprotein E (ApoE) epsilon4 allele to prostate cancer. British journal of cancer 1998, 78(10):1398.
- Wessel N, Liestøl K, Maehlen J, Brorson SH: The apolipoprotein E epsilon4 allele is no risk factor for prostate cancer in the Norwegian population. In: British journal of cancer. Volume 85, edn. England; 2001: 1418.
- Liu H, Shui IM, Platz EA, Mucci LA, Giovannucci EL: No Association of ApoE Genotype with Risk of Prostate Cancer: A Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev 2015, 24(10):1632-1634.
- Yencilek F, Yilmaz SG, Yildirim A, Gormus U, Altinkilic EM, Dalan AB, Bastug Y, Turkmen S, Turkan S, Isbir T: Apolipoprotein E Genotypes in Patients with Prostate Cancer. Anticancer Res 2016, 36(2):707-711.
- Wang A, Shen J, Rodriguez AA, Saunders EJ, Chen F, Janivara R, Darst BF, Sheng X, Xu Y, Chou AJ et al: Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants. Nat Genet 2023, 55(12):2065-2074.
- Niemi M, Kervinen K, Kiviniemi H, Lukkarinen O, Kyllönen AP, Apaja-Sarkkinen M, Savolainen MJ, Kairaluoma MI, Kesäniemi YA: Apolipoprotein E phenotype, cholesterol and breast and prostate cancer. J Epidemiol Community Health 2000, 54(12):938-939.
- Craig EL, Stopsack KH, Evergren E, Penn LZ, Freedland SJ, Hamilton RJ, Allott EH: Statins and prostate cancer-hype or hope? The epidemiological perspective. Prostate Cancer Prostatic Dis 2022, 25(4):641-649.
- Cai C, Wen Z, Li L: The relationship between ApoE gene polymorphism and the efficacy of statins controlling hyperlipidemia. Am J Transl Res 2021, 13(6):6772-6777.
- Xia Z, Liu H, Fan S, Tu H, Jiang Y, Wang H, Gu P, Liu X: A Novel Four Mitochondrial Respiration-Related Signature for Predicting Biochemical Recurrence of Prostate Cancer. J Clin Med 2023, 12(2).
- Qian Y, Feng D, Wang J, Wei W, Wei Q, Han P, Yang L: Establishment of cancer-associated fibroblasts-related subtypes and prognostic index for prostate cancer through single-cell and bulk RNA transcriptome. Sci Rep 2023, 13(1):9016.
- Tong Y, Tan Z, Wang P, Gao X: A Machine Learning Method for Predicting Biomarkers Associated with Prostate Cancer. Front Biosci (Landmark Ed) 2023, 28(12):333.
- Che L, Li D, Wang J, Tuo Z, Yoo KH, Feng D, Ou Y, Wu R, Wei W: Identification of circadian clock-related immunological prognostic index and molecular subtypes in prostate cancer. Discov Oncol 2024, 15(1):429.
- Fan S, Liu H, Hou J, Zheng G, Gu P, Liu X: Characterizing adipocytokine-related signatures for prognosis prediction in prostate cancer. Front Cell Dev Biol 2024, 12:1475980.
- Zhu Z, Wen Y, Xuan C, Chen Q, Xiang Q, Wang J, Liu Y, Luo L, Zhao S, Deng Y et al: Identifying the key genes and microRNAs in prostate cancer bone metastasis by bioinformatics analysis. FEBS Open Bio 2020, 10(4):674-688.
- Venanzoni MC, Giunta S, Muraro GB, Storari L, Crescini C, Mazzucchelli R, Montironi R, Seth A: Apolipoprotein E expression in localized prostate cancers. Int J Oncol 2003, 22(4):779-786.
- Ding L, Wang Y, Tang Z, Ni C, Zhang Q, Zhai Q, Liang C, Li J: Exploration of vitamin D metabolic activity-related biological effects and corresponding therapeutic targets in prostate cancer. Nutr Metab (Lond) 2024, 21(1):17.
- Celhay O, Bousset L, Guy L, Kemeny JL, Leoni V, Caccia C, Trousson A, Damon-Soubeyrant C, De Haze A, Sabourin L et al: Individual Comparison of Cholesterol Metabolism in Normal and Tumour Areas in Radical Prostatectomy Specimens from Patients with Prostate Cancer: Results of the CHOMECAP Study. Eur Urol Oncol 2019, 2(2):198-206.
- Nguyen D, Dhanasekaran P, Nickel M, Mizuguchi C, Watanabe M, Saito H, Phillips MC, Lund-Katz S: Influence of domain stability on the properties of human apolipoprotein E3 and E4 and mouse apolipoprotein E. Biochemistry 2014, 53(24):4025-4033.
- Ifere GO, Desmond R, Demark-Wahnefried W, Nagy TR: Apolipoprotein E gene polymorphism influences aggressive behavior in prostate cancer cells by deregulating cholesterol homeostasis. Int J Oncol 2013, 43(4):1002-1010.
- Haapala K, Lehtimäki T, Ilveskoski E, Koivisto PA: Apolipoprotein E genotype is not linked to locally recurrent hormone-refractory prostate cancer. Prostate Cancer Prostatic Dis 2000, 3(2):107-109.
- Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB: Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS One 2012, 7(1):e30062.
- Pelton K, Freeman MR, Solomon KR: Cholesterol and prostate cancer. Curr Opin Pharmacol 2012, 12(6):751-759.
- Bea AM, Larrea-Sebal A, Marco-Benedi V, Uribe KB, Galicia-Garcia U, Lamiquiz-Moneo I, Laclaustra M, Moreno-Franco B, Fernandez-Corredoira P, Olmos S et al: Contribution of APOE Genetic Variants to Dyslipidemia. Arterioscler Thromb Vasc Biol 2023, 43(6):1066-1077.
- Dunk MM, Driscoll I, Alzheimer’s Disease Neuroimaging I: Total Cholesterol and APOE-Related Risk for Alzheimer’s Disease in the Alzheimer’s Disease Neuroimaging Initiative. J Alzheimers Dis 2022, 85(4):1519-1528.
- Bancaro N, Calì B, Troiani M, Elia AR, Arzola RA, Attanasio G, Lai P, Crespo M, Gurel B, Pereira R et al: Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell 2023, 41(3):602-619.e611.
- Hui B, Lu C, Li H, Hao X, Liu H, Zhuo D, Wang Q, Li Z, Liu L, Wang X et al: Inhibition of APOE potentiates immune checkpoint therapy for cancer. Int J Biol Sci 2022, 18(14):5230-5240.
- Wang J, Guo T, Mi Y, Meng X, Xu S, Dai F, Sun C, Huang Y, Zhu L, Hou J et al: A tumour-associated macrophage-based signature for deciphering prognosis and immunotherapy response in prostate cancer. IET Syst Biol 2024, 18(5):155-171.
- Shamash J, Stebbing J, Sweeney C, Sonpavde G, Harland S, Dawkins G, Brock C, Abelman W, Wilson P, Sanitt A et al: A validated prognostic index predicting response to dexamethasone and diethylstilbestrol in castrate-resistant prostate cancer. Cancer 2010, 116(15):3595-3602.
- Haeno S, Maeda N, Yamaguchi K, Sato M, Uto A, Yokota H: Adrenal steroidogenesis disruption caused by HDL/cholesterol suppression in diethylstilbestrol-treated adult male rat. Endocrine 2016, 52(1):148-156.
- Lukkahatai N, Patel S, Gucek M, Hsiao CP, Saligan LN: Proteomic serum profile of fatigued men receiving localized external beam radiation therapy for non-metastatic prostate cancer. J Pain Symptom Manage 2014, 47(4):748-756.e744.
- Gallardo G, Schlüter OM, Südhof TC: A molecular pathway of neurodegeneration linking alpha-synuclein to ApoE and Abeta peptides. Nat Neurosci 2008, 11(3):301-308.
- Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, Zeng H, Zhou J, Wei W: Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond) 2021, 41(11):1183-1194.
- Sevinsky CJ, Khan F, Kokabee L, Darehshouri A, Maddipati KR, Conklin DS: NDRG1 regulates neutral lipid metabolism in breast cancer cells. Breast Cancer Res 2018, 20(1):55.
- Yang LG, March ZM, Stephenson RA, Narayan PS: Apolipoprotein E in lipid metabolism and neurodegenerative disease. Trends Endocrinol Metab 2023, 34(8):430-445.
- Liu YL, Zhang HM, Pan HM, Bao YH, Xue J, Wang TC, Dong XC, Li XL, Bao HG: The relationship between apolipoprotein E gene epsilon2/epsilon3/epsilon4 polymorphism and breast cancer risk: a systematic review and meta-analysis. Onco Targets Ther 2016, 9:1241-1249.
- Rao V, Bhushan R, Kumari P, Cheruku SP, Ravichandiran V, Kumar N: Chemobrain: A review on mechanistic insight, targets and treatments. Adv Cancer Res 2022, 155:29-76.
- Ahles TA, Root JC: Cognitive Effects of Cancer and Cancer Treatments. Annu Rev Clin Psychol 2018, 14:425-451.
- Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H: Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol 2019, 30(12):1925-1940.
- Holstege H, Hulsman M, Charbonnier C, Grenier-Boley B, Quenez O, Grozeva D, van Rooij JGJ, Sims R, Ahmad S, Amin N et al: Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat Genet 2022, 54(12):1786-1794.
- Zhang H, Ahearn TU, Lecarpentier J, Barnes D, Beesley J, Qi G, Jiang X, O’Mara TA, Zhao N, Bolla MK et al: Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat Genet 2020, 52(6):572-581.
- Xu X, Wan J, Yuan L, Ba J, Feng P, Long W, Huang H, Liu P, Cai Y, Liu M et al: Serum levels of apolipoprotein E correlates with disease progression and poor prognosis in breast cancer. Tumour Biol 2016.
- Ben Hassen C, Gutierrez-Pajares JL, Guimaraes C, Guibon R, Pinault M, Fromont G, Frank PG: Apolipoprotein-mediated regulation of lipid metabolism induces distinctive effects in different types of breast cancer cells. Breast Cancer Res 2020, 22(1):38.
- Cibeira GH, Giacomazzi J, Aguiar E, Schneider S, Ettrich B, CI DES, Camey S, Caleffi M, Weber B, Ashton-Prolla P et al: Apolipoprotein E genetic polymorphism, serum lipoprotein levels and breast cancer risk: A case-control study. Mol Clin Oncol 2014, 2(6):1009-1015.
- El Roz A, Bard JM, Valin S, Huvelin JM, Nazih H: Macrophage apolipoprotein E and proliferation of MCF-7 breast cancer cells: role of LXR. Anticancer Res 2013, 33(9):3783-3789.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3):209-249.
- Kervinen K, Sodervik H, Makela J, Lehtola J, Niemi M, Kairaluoma MI, Kesaniemi YA: Is the development of adenoma and carcinoma in proximal colon related to apolipoprotein E phenotype? Gastroenterology 1996, 110(6):1785-1790.
- Watson MA, Gay L, Stebbings WS, Speakman CT, Bingham SA, Loktionov A: Apolipoprotein E gene polymorphism and colorectal cancer: gender-specific modulation of risk and prognosis. Clin Sci (Lond) 2003, 104(5):537-545.
- Fernandez-Rozadilla C, Timofeeva M, Chen Z, Law P, Thomas M, Schmit S, Diez-Obrero V, Hsu L, Fernandez-Tajes J, Palles C et al: Deciphering colorectal cancer genetics through multi-omic analysis of 100,204 cases and 154,587 controls of European and east Asian ancestries. Nat Genet 2023, 55(1):89-99.
- Zhao Z, Zou S, Guan X, Wang M, Jiang Z, Liu Z, Li C, Lin H, Liu X, Yang R et al: Apolipoprotein E Overexpression Is Associated With Tumor Progression and Poor Survival in Colorectal Cancer. Front Genet 2018, 9:650.
- He L, Shi M, Ren S, Zhang J, Tian Y, Yang X, Liu H: Jun-APOE-LRP1 axis promotes tumor metastasis in colorectal cancer. Biomol Biomed 2023, 23(6):1026-1037.
- Liu Y, Liu C, Huang D, Ge C, Chen L, Fu J, Du J: Identification and prognostic analysis of candidate biomarkers for lung metastasis in colorectal cancer. Medicine (Baltimore) 2024, 103(11):e37484.
- Tanaka T, Oyama T, Sugie S, Shimizu M: Different Susceptibilities between Apoe- and Ldlr-Deficient Mice to Inflammation-Associated Colorectal Carcinogenesis. Int J Mol Sci 2016, 17(11).
- Boulagnon-Rombi C, Schneider C, Leandri C, Jeanne A, Grybek V, Bressenot AM, Barbe C, Marquet B, Nasri S, Coquelet C et al: LRP1 expression in colon cancer predicts clinical outcome. Oncotarget 2018, 9(10):8849-8869.
- Lee KJ, Ko EJ, Park YY, Park SS, Ju EJ, Park J, Shin SH, Suh YA, Hong SM, Park IJ et al: A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials 2020, 255:120151.
- Caruso MG, Osella AR, Notarnicola M, Berloco P, Leo S, Bonfiglio C, Di Leo A: Prognostic value of low density lipoprotein receptor expression in colorectal carcinoma. Oncol Rep 1998, 5(4):927-930.
- Kim BK, Yoo HI, Lee AR, Choi K, Yoon SK: Decreased expression of VLDLR is inversely correlated with miR-200c in human colorectal cancer. Mol Carcinog 2017, 56(6):1620-1629.
- Lai H, Zhao X, Qin Y, Ding Y, Chen R, Li G, Labrie M, Ding Z, Zhou J, Hu J et al: FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J Exp Clin Cancer Res 2018, 37(1):32.
- Chen Y-C, Pohl G, Wang T-L, Morin PJ, Risberg Br, Kristensen GB, Yu A, Davidson B, Shih I-M: Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer research 2005, 65(1):331-337.
- Poersch A, Grassi ML, Carvalho VP, Lanfredi GP, Palma CS, Greene LJ, de Sousa CB, Carrara HHA, Candido Dos Reis FJ, Faça VM: A proteomic signature of ovarian cancer tumor fluid identified by highthroughput and verified by targeted proteomics. J Proteomics 2016, 145:226-236.
- Zhang W, Peng P, Ou X, Shen K, Wu X: Ovarian cancer circulating extracelluar vesicles promote coagulation and have a potential in diagnosis: an iTRAQ based proteomic analysis. BMC Cancer 2019, 19(1):1095.
- Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ: Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000, 60(22):6281-6287.
- Yu S, Qian L, Ma J: Comprehensive analysis of the expression and prognosis for APOE in malignancies: A pan-cancer analysis. Oncol Res 2022, 30(1):13-22.
- Umemori Y, Chiba H, Tokusashi Y, Miyokawa N: [Apolipoprotein E immunoreactivities in normal human ovary and ovarian neoplasms]. Rinsho Byori 1998, 46(1):69-72.
- Ahmed M, Makinen VP, Mulugeta A, Shin J, Boyle T, Hypponen E, Lee SH: Considering hormone-sensitive cancers as a single disease in the UK biobank reveals shared aetiology. Commun Biol 2022, 5(1):614.
- Dareng EO, Coetzee SG, Tyrer JP, Peng PC, Rosenow W, Chen S, Davis BD, Dezem FS, Seo JH, Nameki R et al: Integrative multi-omics analyses to identify the genetic and functional mechanisms underlying ovarian cancer risk regions. Am J Hum Genet 2024, 111(6):1061-1083.
- Phelan CM, Kuchenbaecker KB, Tyrer JP, Kar SP, Lawrenson K, Winham SJ, Dennis J, Pirie A, Riggan MJ, Chornokur G et al: Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat Genet 2017, 49(5):680-691.
- Ao W, Kim HI, Tommarello D, Conrads KA, Hood BL, Litzi T, Abulez T, Teng PN, Dalgard CL, Zhang X et al: Metronomic dosing of ovarian cancer cells with the ATR inhibitor AZD6738 leads to loss of CDC25A expression and resistance to ATRi treatment. Gynecol Oncol 2023, 177:60-71.
- Ferri-Borgogno S, Zhu Y, Sheng J, Burks JK, Gomez JA, Wong KK, Wong STC, Mok SC: Spatial Transcriptomics Depict Ligand-Receptor Cross-talk Heterogeneity at the Tumor-Stroma Interface in Long-Term Ovarian Cancer Survivors. Cancer Res 2023, 83(9):1503-1516.
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S et al: TREM2 variants in Alzheimer’s disease. N Engl J Med 2013, 368(2):117-127.
- Hardy J, Salih D: TREM2-mediated activation of microglia breaks link between amyloid and tau. Lancet Neurol 2021, 20(6):416-417.
- Pike CJ, Carroll JC, Rosario ER, Barron AM: Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 2009, 30(2):239-258.
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H: The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci 2004, 1019:24-28.
- Fleisher A, Grundman M, Jack CR, Jr., Petersen RC, Taylor C, Kim HT, Schiller DH, Bagwell V, Sencakova D, Weiner MF et al: Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 2005, 62(6):953-957.
- Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimaki T, Laippala P, Roytta M, Viitanen M, Wahlund L, Winblad B et al: Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer’s disease in women. Neurosci Lett 2000, 282(1-2):45-48.
- Taxier LR, Philippi SM, York JM, LaDu MJ, Frick KM: The detrimental effects of APOE4 on risk for Alzheimer’s disease may result from altered dendritic spine density, synaptic proteins, and estrogen receptor alpha. Neurobiol Aging 2022, 112:74-86.
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM: Free testosterone and risk for Alzheimer disease in older men. Neurology 2004, 62(2):188-193.
- Hogervorst E, Lehmann DJ, Warden DR, McBroom J, Smith AD: Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer’s disease in men. Int J Geriatr Psychiatry 2002, 17(10):938-940.
- Van Dyk K, Zhou X, Small BJ, Ahn J, Zhai W, Ahles T, Graham D, Jacobsen PB, Jim H, McDonald BC et al: Protective Effects of APOE epsilon2 Genotype on Cognition in Older Breast Cancer Survivors: The Thinking and Living With Cancer Study. JNCI Cancer Spectr 2021, 5(2).
- Harrison RA, Rao V, Kesler SR: The association of genetic polymorphisms with neuroconnectivity in breast cancer patients. Sci Rep 2021, 11(1):6169.
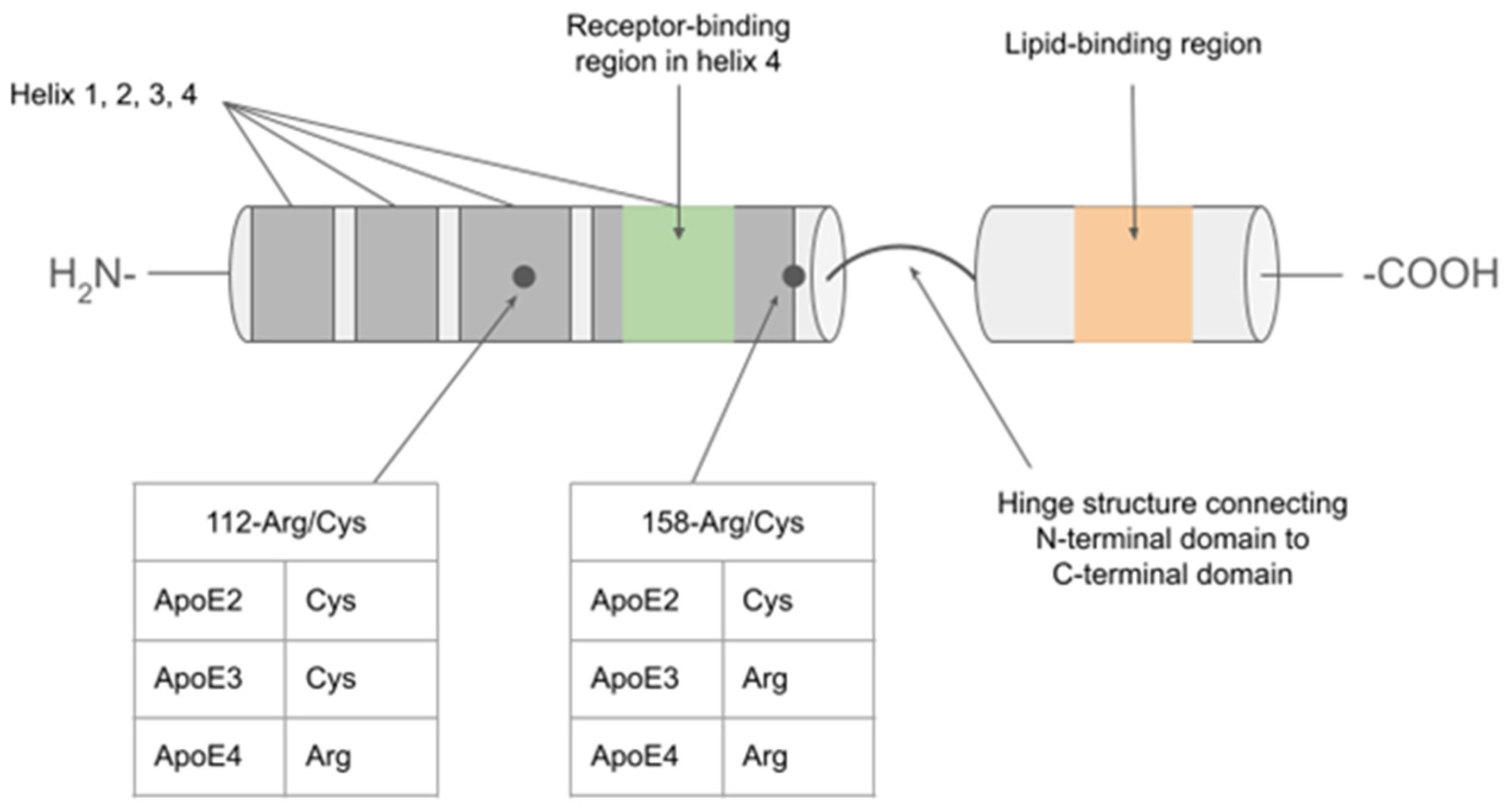
| Isoform-specific effects | ||||
| Receptor | Role of ApoE-receptor complex in CNS | ApoE2 | ApoE3 | ApoE4 |
| Low-density lipoprotein receptor (LDLR) | Mediates uptake of lipids and cholesterol into cells; key component in cholesterol homeostasis, participates in cell signaling [9]. | Extremely poor binding affinity results in decreased reuptake into cells. Associated with type III hyperlipidemia [10]. | Normal binding and reuptake activity [10]. | Increased binding affinity has been proposed but resulting in the “trapping” of E4 protein, leading to its decreased availability and impaired lipid uptake. Contributes to hypercholesterolemia and atherosclerosis. [11]. |
| LDL-receptor related protein 1 (LRP1) | Aids lipid and cholesterol metabolism, functions in cell signalling, and mediates amyloid-β (Aβ) reuptake [12]. | Potential protective activity from certain neurogenic diseases. Supports cell signalling pathways, positive neurotrophy, and decreased Aβ aggregation [13]. | May have similar profile of functions to ApoE2 but not to a similar degree. [12]. | Increased receptor binding affinity. Promotes excess accumulation of Aβ through several proposed but still unclear mechanisms [12,14]. |
| Very-low-density lipoprotein receptor (VLDLR) | ApoE binding impacts the Reelin signalling pathway, critical in cerebellar development and adult neural plasticity. Some potential roles in relation Aβ handling in cells [15]. | Mildly impairs the receptor recycling back to cell-surface following endocytosis [16]. | Moderately impairs receptor recycling back to cell surface post-endocytosis [16]. | Severely impairs receptor recycling back to the cell surface and reduces availability of Reelin receptors, negatively impacting neural health [16]. |
| Apolipoprotein E receptor 2 (ApoEr2 or LRP8) | Similar role to VLDLR in the Reelin pathway, and important to cortical and hippocampal development [15]. | Mildly impairs the receptor recycling back to cell-surface following endocytosis [16]. | Moderately impairs receptor recycling back to cell surface post-endocytosis [16]. | Severely impairs receptor recycling back to the cell surface and reduces availability of Reelin receptors, negatively impacting neural health [16]. |
| Megalin (LRP2) | Supports endocytic uptake of lipids e.g. cholesterol, and promotes regenerative and neuroprotective functions, implicated in Aβ clearance from cells [17,18]. | Research on ApoE E2-Megalin interactions is very limited. | Research on ApoE E3-Megalin interactionss is very limited. | ApoE E4-Megalin shown to hinder Aβ clearance from cells, but the mechanism is unknown [19]. |
| Low-density lipoprotein receptor-related protein 4 (LRP4) | Astrocytic LRP4 shown to promote uptake of Aβ into astroctyes by binding ApoE [20]. | ApoE E2-LRP4 interactions have not currently been extensively studied, thus data is limited. | Higher binding affinity results in “normal” Aβ uptake activity [20]. | Reduced binding affinity compared to ApoE3 yields lower Aβ uptake and thus reduced Aβ-clearance via astrocytes [20]. |
| Low-density lipoprotein receptor-related protein 1b (LRP1b) | Possible role in endocytic metabolism of ApoE-bound lipoproteins (i.e. cholesterol) - lower expression/limited tissue distribution compared to other LDL receptors suggests a less important role or more specific role, e.g. such as cell signaling due to a large cytoplasmic tail [21]. | ApoE2-LRP1b interactions have not been extensively studied, thus data is limited. | ApoE3-LRP1b interactions have not = been extensively studied, thus data is limited. | Limited protein isoform information but in Parkinson’s Disease (PD), presence of the APOE ε4allele and LRP1b rs80306347 variants was associated with increased progression to PD dementia, proposed as a result of, but not tested, impaired metabolism of amyloid precursor protein (APP) [22]. |
| LR11/SorLA | Mediates cellular uptake of both ApoE and Aβ in an ApoE isoform-dependent manner. | No enhancement of uptake of ApoE E2 and Aβ by LR11/SorLA. | Enhanced uptake of ApoE E3 and Aβ associated with LR11/SorLA. | Enhanced uptake of ApoE E4 and Aβ associated with LR11/SorLA [23]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





