Submitted:
03 March 2025
Posted:
04 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Troponin C Is Essential for Ca2+-Regulation of Striated Muscle Contraction
1.2. TnC Function as a Ca2+-Sensor Is Intimately Related to Its Structure
1.3. TnC EF-Hand Affinity and Selectivity Determines Function

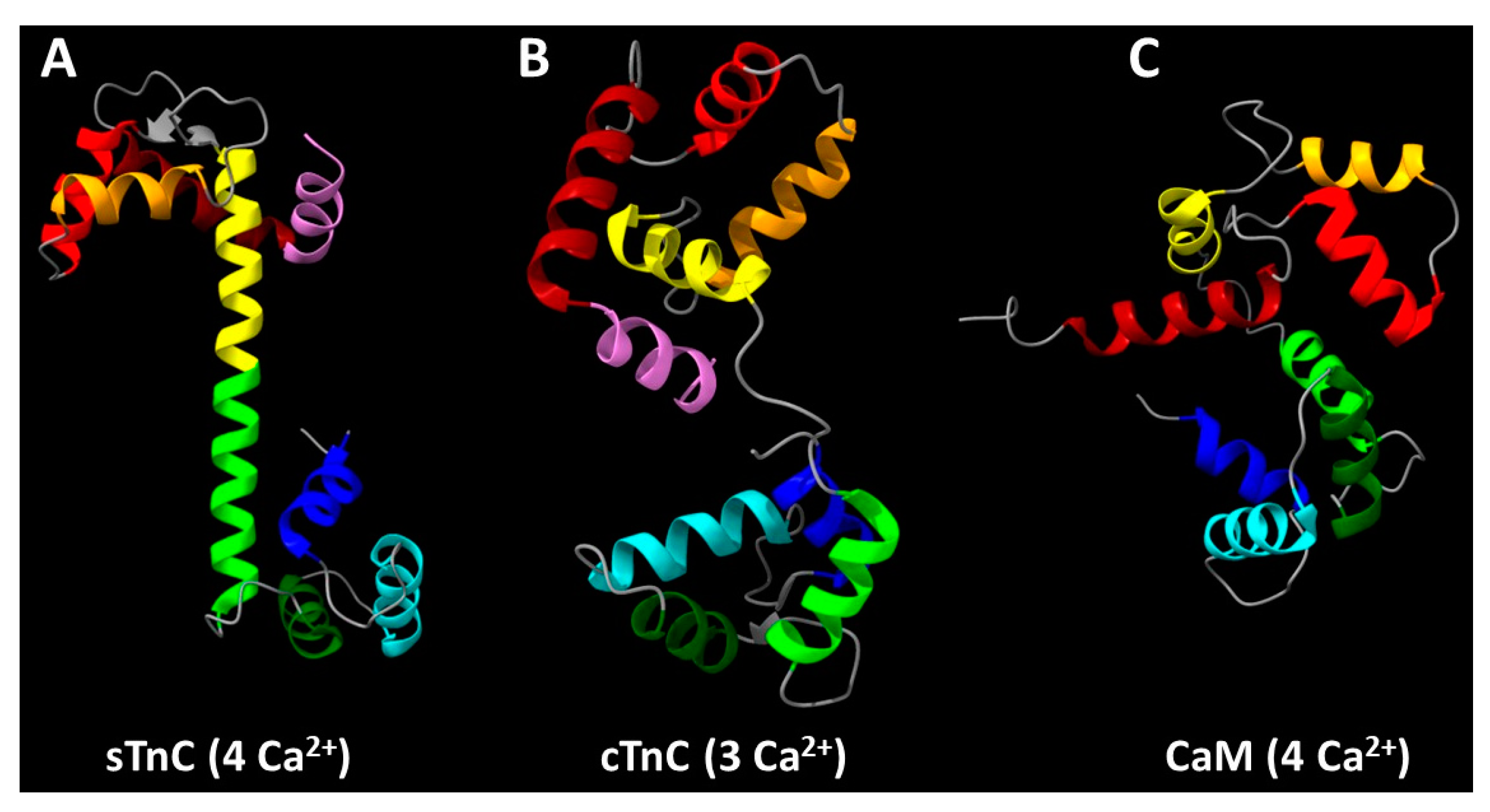
2. Structural and Functional Studies Suggest a Critical Role for the N-Helix of Cardiac Troponin C in Ca2+-Regulation of Cardiac Muscle
2.1. Evolutionary Significance of TnC N-Helix
2.2. Structural Evidence for the Functional Significance of the TnC N-Helix
2.3. Biophysical Studies Demonstrate a Critical Role for the N-Helix of sTnC in Normal Ca2+-Regulation of Skeletal Muscle, But Evidence for That of cTnC Is Lacking
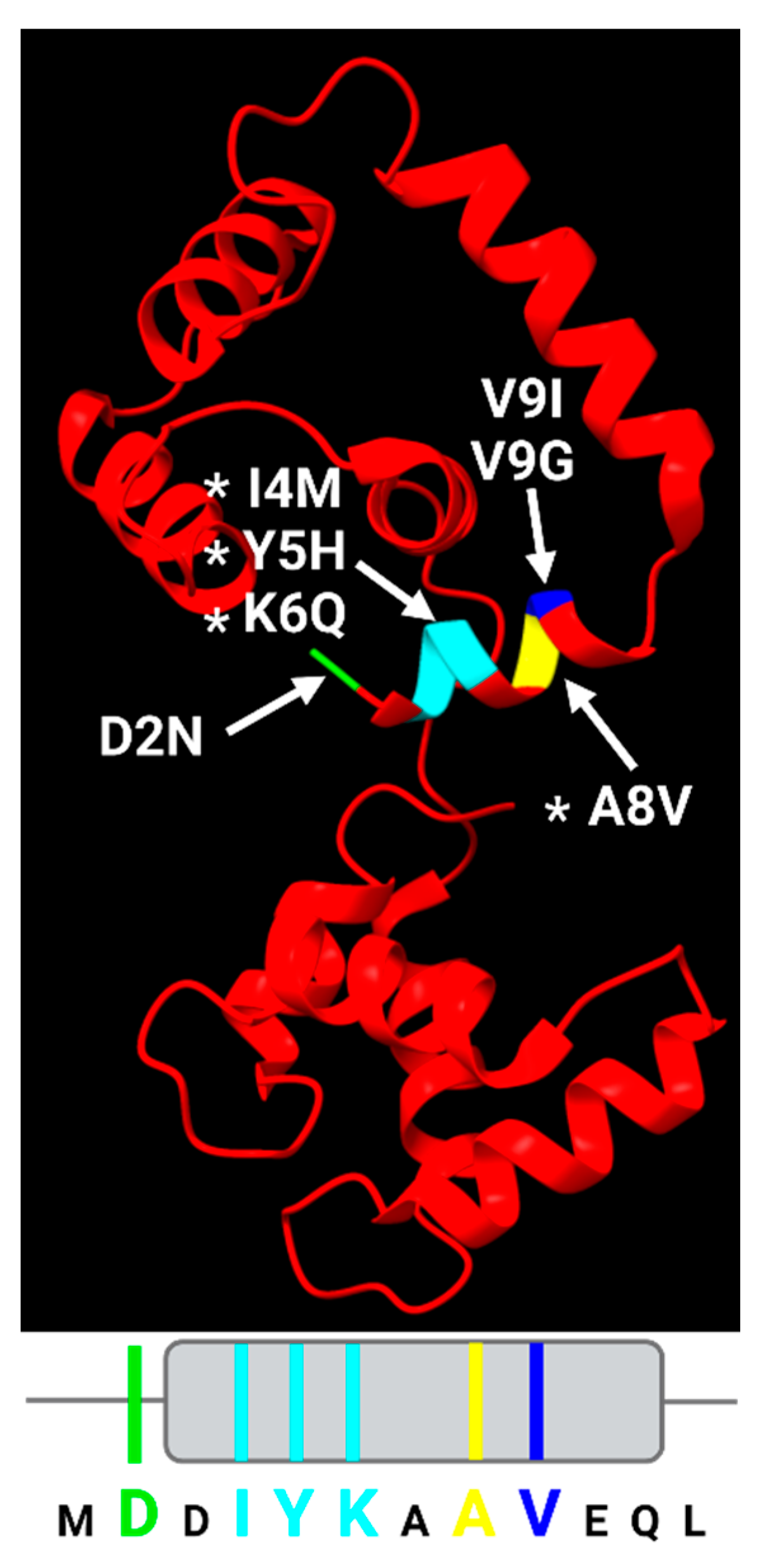
2.4. Pathophysiological Evidence for the Significance of the TnC N-Helix: Variants in the N-Helix of Troponin C Are Associated with Human Cardiomyopathies
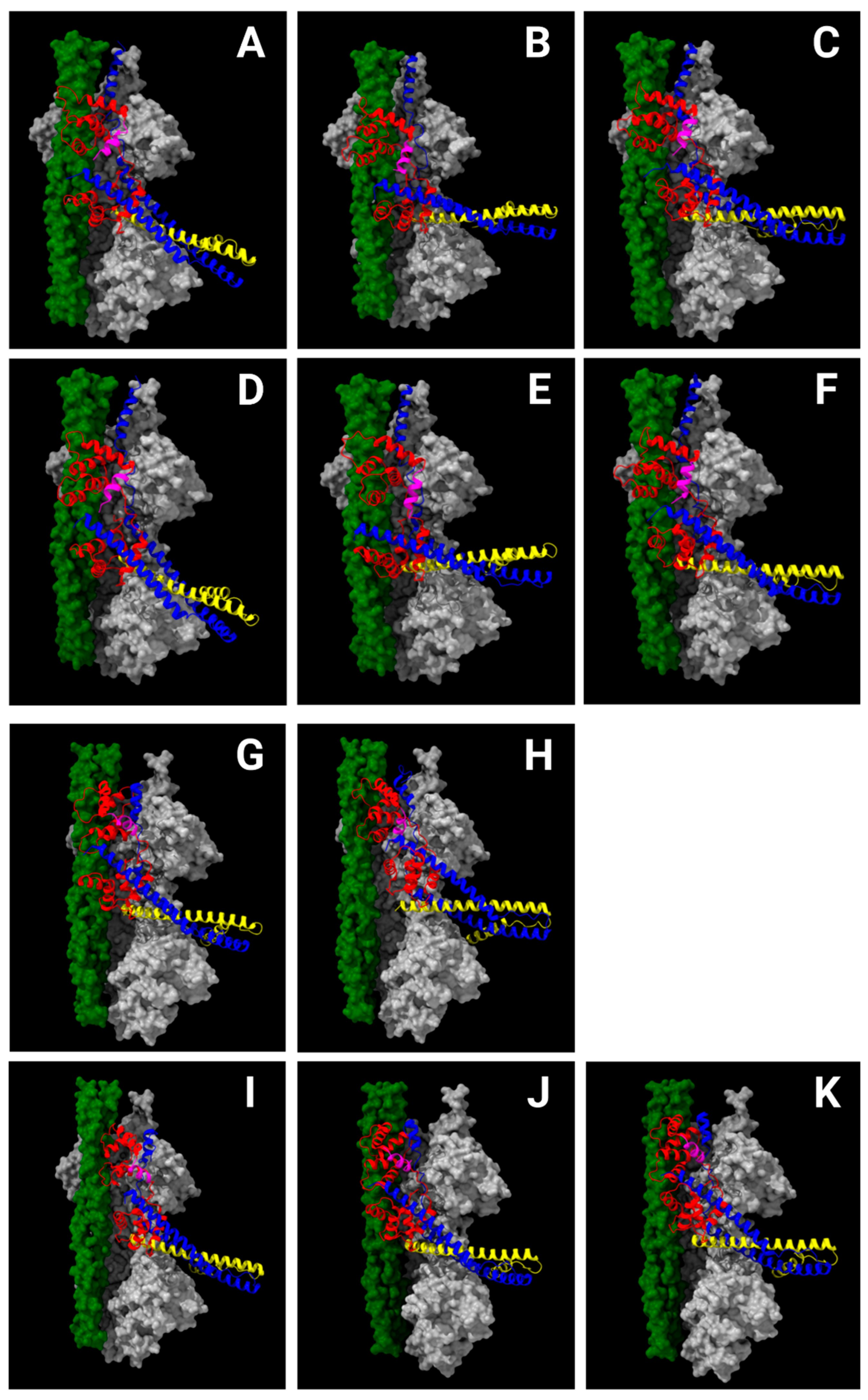
2.5. Cardiomyopathy Variants May Alter Communication Between the cTnC N-Helix and Other Parts of Troponin
3. Conclusions
4. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of Contraction in Striated Muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Rall, J.A. Discovery of the regulatory role of calcium ion in muscle contraction and relaxation: Setsuro Ebashi and the international emergence of Japanese muscle research. Adv. Physiol. Educ. 2022, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Brunello, E.; Fusi, L. Regulating Striated Muscle Contraction: Through Thick and Thin. Annu. Rev. Physiol. 2024, 86, 255–275. [Google Scholar] [CrossRef]
- Ebashi, S.; Endo, M. , Calcium ion and muscle contraction. Prog Biophys Mol Biol 1968, 18, 123–183. [Google Scholar] [PubMed]
- Potter, J. D. , The content of troponin, tropomyosin, actin, and myosin in rabbit skeletal muscle myofibrils. Arch. Biochem. Biophys. 1974, 162, 436–441. [Google Scholar]
- Parmacek, M.S.; Leiden, J.M. Structure, function, and regulation of troponin C. Circulation 1991, 84, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Solaro, R. J.; Rarick, H. M. , Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ. Res. 1998, 83, 471–80. [Google Scholar]
- Gomes, A.V.; Potter, J.D.; Szczesna-Cordary, D. The Role of Troponins in Muscle Contraction. IUBMB Life 2002, 54, 323–333. [Google Scholar] [CrossRef]
- Li, M.X.; Hwang, P.M. Structure and function of cardiac troponin C (TNNC1): Implications for heart failure, cardiomyopathies, and troponin modulating drugs. Gene 2015, 571, 153–166. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Cao, T.; Thongam, U.; Jin, J.-P. Invertebrate troponin: Insights into the evolution and regulation of striated muscle contraction. Arch. Biochem. Biophys. 2019, 666, 40–45. [Google Scholar] [CrossRef]
- Joyce, W.; Ripley, D.M.; Gillis, T.; Black, A.C.; A Shiels, H.; Hoffmann, F.G. A Revised Perspective on the Evolution of Troponin I and Troponin T Gene Families in Vertebrates. Genome Biol. Evol. 2022, 15. [Google Scholar] [CrossRef]
- Russell, B.; Solís, C. Mechanosignaling pathways alter muscle structure and function by post-translational modification of existing sarcomeric proteins to optimize energy usage. J. Muscle Res. Cell Motil. 2021, 42, 367–380. [Google Scholar] [CrossRef] [PubMed]
- McKillop, D.; Geeves, M. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys. J. 1993, 65, 693–701. [Google Scholar] [CrossRef]
- Araujo, A.; Walker, J. Phosphate release and force generation in cardiac myocytes investigated with caged phosphate and caged calcium. Biophys. J. 1996, 70, 2316–2326. [Google Scholar] [CrossRef]
- Sun, Y.-B.; Brandmeier, B.; Irving, M. Structural changes in troponin in response to Ca 2+ and myosin binding to thin filaments during activation of skeletal muscle. Proc. Natl. Acad. Sci. 2006, 103, 17771–17776. [Google Scholar] [CrossRef]
- Sun, Y.; Lou, F.; Irving, M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J. Physiol. 2009, 587, 155–163. [Google Scholar] [CrossRef]
- Fusi, L.; Brunello, E.; Sevrieva, I.R.; Sun, Y.-B.; Irving, M. Structural dynamics of troponin during activation of skeletal muscle. Proc. Natl. Acad. Sci. 2014, 111, 4626–4631. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Risi, C.M.; Pepper, I.; Belknap, B.; Landim-Vieira, M.; White, H.D.; Dryden, K.; Pinto, J.R.; Chase, P.B.; Galkin, V.E. The structure of the native cardiac thin filament at systolic Ca 2+ levels. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef]
- Brunello, E.; Marcucci, L.; Irving, M.; Fusi, L. Activation of skeletal muscle is controlled by a dual-filament mechano-sensing mechanism. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef]
- Risi, C.M.; Belknap, B.; Atherton, J.; Coscarella, I.L.; White, H.D.; Chase, P.B.; Pinto, J.R.; Galkin, V.E. Troponin Structural Dynamics in the Native Cardiac Thin Filament Revealed by Cryo Electron Microscopy. J. Mol. Biol. 2024, 436, 168498–168498. [Google Scholar] [CrossRef]
- Davis, J.P.; Tikunova, S.B.; Janssen, P.M. L. Mechanisms of Muscle Contraction and Relaxation. In Muscle and Exercise Physiology, Zoladz, J. A., Ed. Academic Press: London, 2019; pp 39-50.
- Kreutziger, K.L.; Gillis, T.E.; Davis, J.P.; Tikunova, S.B.; Regnier, M. Influence of enhanced troponin C Ca2+-binding affinity on cooperative thin filament activation in rabbit skeletal muscle. J. Physiol. 2007, 583, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, L.S. Troponin Revealed: Uncovering the Structure of the Thin Filament On-Off Switch in Striated Muscle. Biophys. J. 2020, 120, 1–9. [Google Scholar] [CrossRef]
- Davis, J.P.; Tikunova, S.B. Ca2+ exchange with troponin C and cardiac muscle dynamics. Cardiovasc. Res. 2007, 77, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.; Neher, E.; Taschenberger, H.; Smith, G. Physiology of intracellular calcium buffering. Physiol. Rev. 2023, 103, 2767–2845. [Google Scholar] [CrossRef]
- Bers, D.M.; Zhu, Y. Excitation-Contraction Coupling and Cardiac Contractile Force. J. Cardiovasc. Dis. Res. 2010, 1, 45. [Google Scholar] [CrossRef]
- Smith, G.L.; Eisner, D.A. Calcium Buffering in the Heart in Health and Disease. Circulation 2019, 139, 2358–2371. [Google Scholar] [CrossRef]
- Fakuade, F.E.; Hubricht, D.; Möller, V.; Sobitov, I.; Liutkute, A.; Döring, Y.; Seibertz, F.; Gerloff, M.; Pronto, J.R.D.; Haghighi, F.; et al. Impaired Intracellular Calcium Buffering Contributes to the Arrhythmogenic Substrate in Atrial Myocytes From Patients With Atrial Fibrillation. Circulation 2024, 150, 544–559. [Google Scholar] [CrossRef]
- Fine, R.; Lehman, W.; Head, J.; Blitz, A. , Troponin C in brain. Nature 1975, 258, 260–7. [Google Scholar] [CrossRef]
- Reddy, K.K.; Oitomen, F.M.; Patel, G.P.; Bag, J. Perinuclear localization of slow troponin C m RNA in muscle cells is controlled by a cis-element located at its 3′ untranslated region. RNA 2005, 11, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Alkass, K.; Druid, H.; Bernard, S.; Frisén, J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp. Cell Res. 2011, 317, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Asumda, F.Z.; Chase, P.B. Nuclear cardiac troponin and tropomyosin are expressed early in cardiac differentiation of rat mesenchymal stem cells. Differentiation 2012, 83, 106–115. [Google Scholar] [CrossRef]
- Chase, P.B.; Szczypinski, M.P.; Soto, E.P. Nuclear tropomyosin and troponin in striated muscle: new roles in a new locale? J. Muscle Res. Cell Motil. 2013, 34, 275–284. [Google Scholar] [CrossRef]
- Zhang, T.; Taylor, J.; Jiang, Y.; Pereyra, A.S.; Messi, M.L.; Wang, Z.-M.; Hereñú, C.; Delbono, O. Troponin T3 regulates nuclear localization of the calcium channel Cavβ1a subunit in skeletal muscle. Exp. Cell Res. 2015, 336, 276–286. [Google Scholar] [CrossRef]
- Lopez, Y.O.N.; Messi, M.L.; Pratley, R.E.; Zhang, T.; Delbono, O. Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs. Exp. Gerontol. 2018, 108, 35–40. [Google Scholar] [CrossRef]
- Johnston, J.R.; Chase, P.B.; Pinto, J.R. Troponin through the looking-glass: emerging roles beyond regulation of striated muscle contraction. Oncotarget 2017, 9, 1461–1482. [Google Scholar] [CrossRef]
- Kharitonov, A.V.; Shubina, M.Y.; Nosov, G.A.; Mamontova, A.V.; Arifulin, E.A.; Lisitsyna, O.M.; Nalobin, D.S.; Musinova, Y.R.; Sheval, E.V. Switching of cardiac troponin I between nuclear and cytoplasmic localization during muscle differentiation. Biochim. et Biophys. Acta (BBA) - Mol. Cell Res. 2020, 1867, 118601. [Google Scholar] [CrossRef]
- Solís, C.; Solaro, R.J. Novel insights into sarcomere regulatory systems control of cardiac thin filament activation. J. Gen. Physiol. 2021, 153. [Google Scholar] [CrossRef]
- Zhang, T.; Birbrair, A.; Delbono, O. Nonmyofilament-associated troponin T3 nuclear and nucleolar localization sequence and leucine zipper domain mediate muscle cell apoptosis. Cytoskeleton 2013, 70, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, A.; Lin, A.J.; Vijayan, V.; Pokhrel, S.; Kraemer, B.R.; Bechara, L.R.G.; Larus, I.; Sun, J.; Baena, V.; Syed, Z.A.; et al. Cardiac troponin I directly binds and inhibits mitochondrial ATP synthase with a noncanonical role in the post-ischemic heart. Nat. Cardiovasc. Res. 2024, 3, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lee, J.; Vincent, L.G.; Wang, Q.; Gu, M.; Lan, F.; Churko, J.M.; Sallam, K.I.; Matsa, E.; Sharma, A.; et al. Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised β-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy. Cell Stem Cell 2015, 17, 89–100. [Google Scholar] [CrossRef]
- Schreier, T.; Kedes, L.; Gahlmann, R. Cloning, structural analysis, and expression of the human slow twitch skeletal muscle/cardiac troponin C gene. J. Biol. Chem. 1990, 265, 21247–21253. [Google Scholar] [CrossRef] [PubMed]
- Gahlmann, R.; Kedes, L. Cloning, structural analysis, and expression of the human fast twitch skeletal muscle troponin C gene. J. Biol. Chem. 1990, 265, 12520–12528. [Google Scholar] [CrossRef]
- Gahlmann, R.; Wade, R.; Gunning, P.; Kedes, L. Differential expression of slow and fast skeletal muscle troponin C. J. Mol. Biol. 1988, 201, 379–391. [Google Scholar] [CrossRef]
- Collins, J.H.; Greaser, M.L.; Potter, J.D.; Horn, M.J. Determination of the amino acid sequence of troponon C from rabbit skeletal muscle. J. Biol. Chem. 1977, 252, 6356–6362. [Google Scholar] [CrossRef]
- Kobayashi, T.; Takagi, T.; Konishi, K.; Morimoto, S.; Ohtsuki, I. Amino Acid Sequence of Porcine Cardiac Muscle Troponin CJ. Biochem. 1989, 106, 55–59. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Leiden, J.M. Structure and Expression of the Murine Slow/Cardiac Troponin C Gene. J. Biol. Chem. 1989, 264, 13217–13225. [Google Scholar] [CrossRef]
- Ding, X.-L.; Akella, Á. B.; Su, H.; Gulati, J. , The role of glycine (residue 89) in the central helix of EF-hand protein troponin-C exposed following amino-terminal α-helix deletion. Protein Sci 1994, 3, 2089–96. [Google Scholar]
- Smith, L.; Greenfield, N.J.; Hitchcock-DeGregori, S.E. Mutations in the N- and D-Helices of the N-Domain of Troponin C Affect the C-Domain and Regulatory Function. Biophys. J. 1999, 76, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; da Silva, E.; Sorenson, M.; Ferro, J.; Pearlstone, J.; Nash, B.; Borgford, T.; Kay, C.; Smillie, L. The effects of N helix deletion and mutant F29W on the Ca2+ binding and functional properties of chicken skeletal muscle troponin. J. Biol. Chem. 1994, 269, 14988–14994. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Greenfield, N.; Hitchcock-DeGregori, S. The effects of deletion of the amino-terminal helix on troponin C function and stability. J. Biol. Chem. 1994, 269, 9857–9863. [Google Scholar] [CrossRef]
- Herzberg, O.; James, M.N.G. Structure of the calcium regulatory muscle protein troponin-C at 2.8 Å resolution. Nature 1985, 313, 653–659. [Google Scholar] [CrossRef]
- Herzberg, O.; James, M.N. G, Refined crystal structure of troponin C from turkey skeletal muscle at 2. 0 Å resolution. J. Mol. Biol. 1988, 203, 761–779. [Google Scholar] [CrossRef] [PubMed]
- A Satyshur, K.; Rao, S.T.; Pyzalska, D.; Drendel, W.; Greaser, M.; Sundaralingam, M. Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-A resolution. J. Biol. Chem. 1988, 263, 1628–1647. [Google Scholar] [CrossRef]
- Putkey, J.; Liu, W.; Sweeney, H. Function of the N-terminal calcium-binding sites in cardiac/slow troponin C assessed in fast skeletal muscle fibers. 2021, 266, 14881–14884. [Google Scholar] [CrossRef]
- Slupsky, C. M.; Sykes, B. D. , NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry 1995, 34, 15953–64. [Google Scholar] [CrossRef]
- Houdusse, A.; Love, M. L.; Dominguez, R.; Grabarek, Z.; Cohen, C. , Structures of four Ca2+-bound troponin C at 2. 0 Å resolution: further insights into the Ca2+-switch in the calmodulin superfamily. Structure 1997, 5, 1695–711. [Google Scholar]
- Sia, S.K.; Li, M.X.; Spyracopoulos, L.; Gagné, S.M.; Liu, W.; Putkey, J.A.; Sykes, B.D. Structure of Cardiac Muscle Troponin C Unexpectedly Reveals a Closed Regulatory Domain. J. Biol. Chem. 1997, 272, 18216–18221. [Google Scholar] [CrossRef]
- Takeda, S.; Yamashita, A.; Maeda, K.; Maéda, Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature 2003, 424, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bers, D. M. , Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar]
- Westerblad, H.; Allen, D.G. Relaxation, [Ca2+]i and [Mg2+]i during prolonged tetanic stimulation of intact, single fibres from mouse skeletal muscle. J. Physiol. 1994, 480, 31–43. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Brinkmeier, H.; Müntener, M. Calcium Ion in Skeletal Muscle: Its Crucial Role for Muscle Function, Plasticity, and Disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef] [PubMed]
- Kushmerick, M.J.; Dillon, P.F.; A Meyer, R.; Brown, T.R.; Krisanda, J.M.; Sweeney, H.L. 31P NMR spectroscopy, chemical analysis, and free Mg2+ of rabbit bladder and uterine smooth muscle. J. Biol. Chem. 1986, 261, 14420–14429. [Google Scholar] [CrossRef] [PubMed]
- Godt, R.E.; Maughan, D.W. On the composition of the cytosol of relaxed skeletal muscle of the frog. Am. J. Physiol. Physiol. 1988, 254, C591–C604. [Google Scholar] [CrossRef]
- Homsher, E.; Kean, C. J. , Skeletal muscle energetics and metabolism. Annu. Rev. Physiol. 1978, 40, 93–131. [Google Scholar]
- Hou, T.T.; Johnson, J.D.; A Rall, J. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J. Physiol. 1991, 441, 285–304. [Google Scholar] [CrossRef]
- Potter, J.D.; Gergely, J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J. Biol. Chem. 1975, 250, 4628–4633. [Google Scholar] [CrossRef]
- Negele, J.C.; Dotson, D.G.; Liu, W.; Sweeney, H.L.; A Putkey, J. Mutation of the high affinity calcium binding sites in cardiac troponin C. J. Biol. Chem. 1992, 267, 825–831. [Google Scholar] [CrossRef]
- Badr, M. A.; Pinto, J. R.; Davidson, M. W.; Chase, P. B. , Fluorescent protein-based Ca2+ sensor reveals global, divalent cation-dependent conformational changes in cardiac troponin C. PLoS One 2016, 11, e0164222. [Google Scholar]
- Davis, J.P.; Rall, J.A.; Reiser, P.J.; Smillie, L.B.; Tikunova, S.B.; Wesche, J.; Małecki, J.; Więdłocha, A.; Skjerpen, C.S.; Claus, P.; et al. Engineering Competitive Magnesium Binding into the First EF-hand of Skeletal Troponin C. J. Biol. Chem. 2002, 277, 49716–49726. [Google Scholar] [CrossRef]
- Skowronsky, R.A.; Schroeter, M.; Baxley, T.; Li, Y.; Chalovich, J.M.; Spuches, A.M. Thermodynamics and molecular dynamics simulations of calcium binding to the regulatory site of human cardiac troponin C: evidence for communication with the structural calcium binding sites. JBIC J. Biol. Inorg. Chem. 2012, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rayani, K.; Seffernick, J.; Li, A.Y.; Davis, J.P.; Spuches, A.M.; Van Petegem, F.; Solaro, R.J.; Lindert, S.; Tibbits, G.F. Binding of calcium and magnesium to human cardiac troponin C. J. Biol. Chem. 2021, 296, 100350. [Google Scholar] [CrossRef]
- van Eerd, J.-P.; Takahashi, K. , Determination of the complete amino acid sequence of bovine cardiac troponin C. Biochemistry 1976, 15, 1171–1180. [Google Scholar]
- Sweeney, H.L.; Brito, R.M.; Rosevear, P.R.; A Putkey, J. The low-affinity Ca2(+)-binding sites in cardiac/slow skeletal muscle troponin C perform distinct functions: site I alone cannot trigger contraction. Proc. Natl. Acad. Sci. 1990, 87, 9538–9542. [Google Scholar] [CrossRef] [PubMed]
- Marques, M. d. A.; Pinto, J. R.; Moraes, A. H.; Iqbal, A.; de Magalhães, M. T. Q.; Monteiro, J.; Pedrote, M. M.; Sorenson, M. M.; Silva, J. L.; de Oliveira, G. A. P. , Allosteric Transmission along a Loosely Structured Backbone Allows a Cardiac Troponin C Mutant to Function with Only One Ca2+ Ion. J. Biol. Chem. 2017, 292, 2379–2394. [Google Scholar]
- Vinogradova, M. V.; Stone, D. B.; Malanina, G. G.; Karatzaferi, C.; Cooke, R.; Mendelson, R. A.; Fletterick, R. J. , Ca2+-regulated structural changes in troponin. Proc. Natl. Acad. Sci. USA 2005, 102, 5038–43. [Google Scholar]
- Ikura, M.; Clore, G. M.; Gronenborn, A. M.; Zhu, G.; Klee, C. B.; Bax, A. , Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science 1992, 256, 632–638. [Google Scholar]
- Collins, J.H. Myosin light chains and troponin C: Structural and evolutionary relationships revealed by amino acid sequence comparisons. J. Muscle Res. Cell Motil. 1991, 12, 3–25. [Google Scholar] [CrossRef]
- Houdusse, A.; Silver, M.; Cohen, C. A model of Ca2+-free calmodulin binding to unconventional myosins reveals how calmodulin acts as a regulatory switch. Structure 1996, 4, 1475–1490. [Google Scholar] [CrossRef]
- Gillis, T. E.; Marshall, C. R.; Tibbits, G. F. , Functional and evolutionary relationships of troponin C. Physiol. Genomics 2007, 32, 16–27. [Google Scholar] [PubMed]
- Shi, Y.; Bethea, J. P.; Hetzel-Ebben, H. L.; Landim-Vieira, M.; Mayper, R. J.; Williams, R. L.; Kessler, L. E.; Ruiz, A. M.; Gargiulo, K.; Rose, J. S. M.; Platt, G.; Pinto, J. R.; Washburn, B. K.; Chase, P. B. , Mandibular muscle troponin of the Florida carpenter ant Camponotus floridanus: extending our insights into invertebrate Ca2+ regulation. J. Muscle Res. Cell Motil. 2021, 42, 399–417. [Google Scholar] [CrossRef]
- Agianian, B.; Kržič, U.; Qiu, F.; A Linke, W.; Leonard, K.; Bullard, B. A troponin switch that regulates muscle contraction by stretch instead of calcium. EMBO J. 2004, 23, 772–779. [Google Scholar] [CrossRef]
- Qiu, F.; Lakey, A.; Agianian, B.; Hutchings, A.; Butcher, G.W.; Labeit, S.; Leonard, K.; Bullard, B. Troponin C in different insect muscle types: identification of two isoforms in Lethocerus, Drosophila and Anopheles that are specific to asynchronous flight muscle in the adult insect. Biochem. J. 2003, 371, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.H.; Theibert, J.L.; Francois, J.M.; Ashley, C.C.; Potter, J.D. Amino acid sequences and calcium-binding properties of two isoforms of barnacle troponin C. Biochemistry 1991, 30, 702–707. [Google Scholar] [CrossRef] [PubMed]
- van Eerd, J.-P.; Takahashi, K. , The amino acid sequence of bovine cardiac troponin-C. Comparison with rabbit skeletal troponin-C. Biochem. Biophys. Res. Commun. 1975, 64, 122–127. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Troponin C from Rabbit Slow Skeletal and Cardiac Muscle Is the Product of a Single Gene. Eur. J. Biochem. 1980, 103, 179–188. [Google Scholar] [CrossRef]
- Roher, A.; Lieska, N.; Spitz, W. The amino acid sequence of human cardiac troponin-C. Muscle Nerve 1986, 9, 73–77. [Google Scholar] [CrossRef]
- Wilkinson, J. The amino acid sequence of troponin C from chicken skeletal muscle. FEBS Lett. 1976, 70, 254–256. [Google Scholar] [CrossRef]
- Romero-Herrera, A. E.; Castillo, O.; Lehmann, H. , Human skeletal muscle proteins. The primary structure of troponin C. J. Mol. Evol. 1976, 8, 251–70. [Google Scholar] [PubMed]
- Strasburg, G.; Greaser, M.; Sundaralingam, M. X-ray diffraction studies of troponin-C crystals from rabbit and chicken skeletal muscles. J. Biol. Chem. 1980, 255, 3806–3808. [Google Scholar] [CrossRef]
- McKay, R.T.; Tripet, B.P.; Hodges, R.S.; Sykes, B.D. Interaction of the Second Binding Region of Troponin I with the Regulatory Domain of Skeletal Muscle Troponin C as Determined by NMR Spectroscopy. 1997; 272, 2849. [Google Scholar] [CrossRef]
- Mckay, R.; Pearlstone, J.; Corson, D.; Gagne, S.; Smillie, L.; Sykes, B. ; Structure and interaction site of the regulatory domain of troponin-C when complexed with the 96-148 region of troponin-I. Biochemistry 1998, 37, 12419–30. [Google Scholar]
- McKay, R.T.; Tripet, B.P.; Pearlstone, J.R.; Smillie, L.B.; Sykes, B.D. Defining the Region of Troponin-I that Binds to Troponin-C. Biochemistry 1999, 38, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Li, M. X.; Spyracopoulos, L.; Sykes, B. D. , Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry 1999, 38, 8289–98. [Google Scholar]
- Vassylyev, D.G.; Takeda, S.; Wakatsuki, S.; Maeda, K.; Maéda, Y. Crystal structure of troponin C in complex with troponin I fragment at 2.3-Å resolution. Proc. Natl. Acad. Sci. 1998, 95, 4847–4852. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Takeda, S.; Scherer, A.; Kobayashi, T.; Maeda, Y.; Taniguchi, H.; Yao, M.; Wakatsuki, S. , Production, crystallization, and preliminary X-ray analysis of rabbit skeletal muscle troponin complex consisting of troponin C and fragment (1-47) of troponin I. Protein Sci 1997, 6, 916–8. [Google Scholar]
- Findlay, W.A.; Sykes, B.D. Proton NMR resonance assignments, secondary structure, and global fold of the TR1C fragment of turkey skeletal troponin C in the calcium-free state. Biochemistry 1993, 32, 3461–3467. [Google Scholar] [CrossRef]
- Li, M.X.; Gagne, S.M.; Tsuda, S.; Kay, C.M.; Smillie, L.B.; Sykes, B.D. Calcium Binding to the Regulatory N-Domain of Skeletal Muscle Troponin C Occurs in a Stepwise Manner. Biochemistry 1995, 34, 8330–8340. [Google Scholar] [CrossRef]
- Spyracopoulos, L.; Li, M.X.; Sia, S.K.; Gagné, S.M.; Chandra, M.; Solaro, R.J.; Sykes, B.D. Calcium-Induced Structural Transition in the Regulatory Domain of Human Cardiac Troponin C, Biochemistry 1997, 36, 12138–12146. [Google Scholar] [CrossRef]
- Findlay, W.; Soennichsen, F.; Sykes, B.D. Solution structure of the TR1C fragment of skeletal muscle troponin-C. J. Biol. Chem. 1994, 269, 6773–8. [Google Scholar] [PubMed]
- Oda, T.; Yanagisawa, H.; Wakabayashi, T. Cryo-EM structures of cardiac thin filaments reveal the 3D architecture of troponin. J. Struct. Biol. 2020, 209, 107450. [Google Scholar] [CrossRef]
- Risi, C. M.; Belknap, B.; White, H. D.; Dryden, K.; Pinto, J. R.; Chase, P. B.; Galkin, V. E. , High-resolution cryo-EM structure of the junction region of the native cardiac thin filament in relaxed state. PNAS Nexus 2023, 2, pgac298. [Google Scholar]
- Regnier, M.; Rivera, A.; Chase, P.; Smillie, L.; Sorenson, M. Regulation of Skeletal Muscle Tension Redevelopment by Troponin C Constructs with Different Ca2+ Affinities. Biophys. J. 1999, 76, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Gulati, J.; Babu, A.; Su, H.; Zhang, Y. Identification of the regions conferring calmodulin-like properties to troponin C. J. Biol. Chem. 1993, 268, 11685–11690. [Google Scholar] [CrossRef] [PubMed]
- Pearlstone, J.R.; Borgford, T.; Chandra, M.; Oikawa, K.; Kay, C.M.; Herzberg, O.; Moult, J.; Herklotz, A.; Reinach, F.C.; Smillie, L.B. Construction and characterization of a spectral probe mutant of troponin C: application to analyses of mutants with increased calcium affinity. Biochemistry 1992, 31, 6545–6553. [Google Scholar] [CrossRef]
- da Silva, E.F.; Sorenson, M.M.; Smillie, L.B.; Barrabin, H.; Scofano, H.M. Comparison of calmodulin and troponin C with and without its amino-terminal helix (residues 1-11) in the activation of erythrocyte Ca(2+)-ATPase. J. Biol. Chem. 1993, 268, 26220–26225. [Google Scholar] [CrossRef]
- Babu, A.; Orr, G.; Gulati, J. Calmodulin supports the force-generating function in desensitized muscle fibers. J. Biol. Chem. 1988, 263, 15485–15491. [Google Scholar] [CrossRef]
- Hill, A. V. , The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Reinoso, T.R.; Landim-Vieira, M.; Shi, Y.; Johnston, J.R.; Chase, P.B.; Parvatiyar, M.S.; Landstrom, A.P.; Pinto, J.R.; Tadros, H.J. A comprehensive guide to genetic variants and post-translational modifications of cardiac troponin C. J. Muscle Res. Cell Motil. 2020, 42, 323–342. [Google Scholar] [CrossRef]
- Willott, R. H.; Gomes, A. V.; Chang, A. N.; Parvatiyar, M. S.; Pinto, J. R.; Potter, J. D. , Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J. Mol. Cell. Cardiol. 2010, 48, 882–92. [Google Scholar] [CrossRef] [PubMed]
- Tadros, H.J.; Life, C.S.; Garcia, G.; Pirozzi, E.; Jones, E.G.; Datta, S.; Parvatiyar, M.S.; Chase, P.B.; Allen, H.D.; Kim, J.J.; et al. Meta-analysis of cardiomyopathy-associated variants in troponin genes identifies loci and intragenic hot spots that are associated with worse clinical outcomes. J. Mol. Cell. Cardiol. 2020, 142, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Na, I.; Kong, M. J.; Straight, S.; Pinto, J. R.; Uversky, V. N. , Troponins, intrinsic disorder, and cardiomyopathy. Biol Chem 2016, 397, 731–51. [Google Scholar] [CrossRef] [PubMed]
- Landstrom, A.P.; Parvatiyar, M.S.; Pinto, J.R.; Marquardt, M.L.; Bos, J.M.; Tester, D.J.; Ommen, S.R.; Potter, J.D.; Ackerman, M.J. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin C. J. Mol. Cell. Cardiol. 2008, 45, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.R.; Parvatiyar, M.S.; Jones, M.A.; Liang, J.; Ackerman, M.J.; Potter, J.D. A Functional and Structural Study of Troponin C Mutations Related to Hypertrophic Cardiomyopathy. J. Biol. Chem. 2009, 284, 19090–19100. [Google Scholar] [CrossRef]
- Swindle, N.; Tikunova, S.B. Hypertrophic Cardiomyopathy-Linked Mutation D145E Drastically Alters Calcium Binding by the C-Domain of Cardiac Troponin C. Biochemistry 2010, 49, 4813–4820. [Google Scholar] [CrossRef]
- Pinto, J. R.; Reynaldo, D. P.; Parvatiyar, M. S.; Dweck, D.; Liang, J.; Jones, M. A.; Sorenson, M. M.; Potter, J. D. , Strong cross-bridges potentiate the Ca2+ affinity changes produced by hypertrophic cardiomyopathy cardiac troponin C mutants in myofilaments: a fast kinetic approach. J. Biol. Chem. 2011, 286, 1005–13. [Google Scholar] [CrossRef]
- Albury, A.N.J.; Swindle, N.; Swartz, D.R.; Tikunova, S.B. Effect of Hypertrophic Cardiomyopathy-Linked Troponin C Mutations on the Response of Reconstituted Thin Filaments to Calcium upon Troponin I Phosphorylation. Biochemistry 2012, 51, 3614–3621. [Google Scholar] [CrossRef]
- Martins, A.S.; Parvatiyar, M.S.; Turna, R.; Badger, C.D.; Griffin, B.; Zorio, D.; Vukmirovic, M.; Sanchez-Gonzalez, M.A.; Dweck, D.; Ruiz, E.L.; et al. In Vivo Analysis of Troponin C Knock-In (A8V) Mice: Evidence that TNNC1 is a Hypertrophic Cardiomyopathy Susceptibility Gene. Biophys. J. 2014, 106, 723a–723A. [Google Scholar] [CrossRef]
- Kathuria, S. V.; Chan, Y. H.; Nobrega, R. P.; Özen, A.; Matthews, C. R. , Clusters of isoleucine, leucine, and valine side chains define cores of stability in high-energy states of globular proteins: Sequence determinants of structure and stability. Protein Sci 2016, 25, 662–75. [Google Scholar]
- Zot, H.G.; Hasbun, J.E.; Michell, C.A.; Landim-Vieira, M.; Pinto, J.R. Enhanced troponin I binding explains the functional changes produced by the hypertrophic cardiomyopathy mutation A8V of cardiac troponin C. Arch. Biochem. Biophys. 2016, 601, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Baxley, T.; Johnson, D.; Pinto, J. R.; Chalovich, J. M. , Troponin C Mutations Partially Stabilize the Active State of Regulated Actin and Fully Stabilize the Active State When Paired with Δ14 TnT. Biochemistry 2017, 56, 2928–2937. [Google Scholar] [PubMed]
- Kawai, M.; Johnston, J.R.; Karam, T.; Wang, L.; Singh, R.K.; Pinto, J.R. Myosin Rod Hypophosphorylation and CB Kinetics in Papillary Muscles from a TnC-A8V KI Mouse Model. Biophys. J. 2017, 112, 1726–1736. [Google Scholar] [CrossRef]
- Stevens, C.M.; Rayani, K.; Singh, G.; Lotfalisalmasi, B.; Tieleman, D.; Tibbits, G.F. Changes in the dynamics of the cardiac troponin C molecule explain the effects of Ca2+-sensitizing mutations. J. Biol. Chem. 2017, 292, 11915–11926. [Google Scholar] [CrossRef] [PubMed]
- Veltri, T.; Landim-Vieira, M.; Parvatiyar, M.S.; Gonzalez-Martinez, D.; Jones, K.M.D.; Michell, C.A.; Dweck, D.; Landstrom, A.P.; Chase, P.B.; Pinto, J.R. Hypertrophic Cardiomyopathy Cardiac Troponin C Mutations Differentially Affect Slow Skeletal and Cardiac Muscle Regulation. Front. Physiol. 2017, 8, 221. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, D.; Johnston, J.R.; Landim-Vieira, M.; Ma, W.; Antipova, O.; Awan, O.; Irving, T.C.; Chase, P.B.; Pinto, J.R. Structural and functional impact of troponin C-mediated Ca2+ sensitization on myofilament lattice spacing and cross-bridge mechanics in mouse cardiac muscle. J. Mol. Cell. Cardiol. 2018, 123, 26–37. [Google Scholar] [CrossRef]
- Johnston, J.R.; Landim-Vieira, M.; Marques, M.A.; de Oliveira, G.A.; Gonzalez-Martinez, D.; Moraes, A.H.; He, H.; Iqbal, A.; Wilnai, Y.; Birk, E.; et al. The intrinsically disordered C terminus of troponin T binds to troponin C to modulate myocardial force generation. J. Biol. Chem. 2019, 294, 20054–20069. [Google Scholar] [CrossRef]
- Jones, K.M.D.; Vied, C.; Valera, I.C.; Chase, P.B.; Parvatiyar, M.S.; Pinto, J.R. Sexual dimorphism in cardiac transcriptome associated with a troponin C murine model of hypertrophic cardiomyopathy. Physiol. Rep. 2020, 8, e14396. [Google Scholar] [CrossRef]
- E de Feria, A.; E Kott, A.; Becker, J.R. Sarcomere mutation negative hypertrophic cardiomyopathy is associated with ageing and obesity. Open Hear. 2021, 8, e001560. [Google Scholar] [CrossRef]
- Pinto, J.R.; Siegfried, J.D.; Parvatiyar, M.S.; Li, D.; Norton, N.; Jones, M.A.; Liang, J.; Potter, J.D.; Hershberger, R.E. Functional Characterization of TNNC1 Rare Variants Identified in Dilated Cardiomyopathy. J. Biol. Chem. 2011, 286, 34404–34412. [Google Scholar] [CrossRef]
- Hespe, S.; Waddell, A.; Asatryan, B.; Owens, E.; Thaxton, C.; Adduru, M.-L.; Anderson, K.; Brown, E. E.; Hoffman-Andrews, L.; Jordan, E.; Josephs, K.; Mayers, M.; Peters, S.; Stafford, F.; Bagnall, R. D.; Bronicki, L.; Callewaert, B.; Chahal, C. A. A.; James, C. A.; Jarinova, O.; Landstrom, A. P.; McNally, E. M.; Murray, B.; Muiño-Mosquera, L.; Parikh, V.; Reuter, C.; Walsh, R.; Wayburn, B.; Ware, J. S.; Ingles, J. , Genes Associated with Hypertrophic Cardiomyopathy: A Reappraisal by the ClinGen Hereditary Cardiovascular Disease Gene Curation Expert Panel. J. Am. Coll. Cardiol. 2025, 85, 727–740. [Google Scholar] [PubMed]
- Juárez, C.K.; Sequeira, V.; Boogaard, M.v.D.; Veerman, C.C.; Hoetjes, N.J.; Poel, E.; Tanck, M.W.; Deprez, R.H.L.; Vermeer, A.M.; van der Velden, J.; et al. Tropomyosin–troponin complex in inherited cardiomyopathies. Hear. Rhythm. 2024, 21, 1173–1175. [Google Scholar] [CrossRef]
- Tikunova, S.B.; Thuma, J.; Davis, J.P. Mouse Models of Cardiomyopathies Caused by Mutations in Troponin C. Int. J. Mol. Sci. 2023, 24, 12349. [Google Scholar] [CrossRef]
- Keyt, L.K.; Duran, J.M.; Bui, Q.M.; Chen, C.; Miyamoto, M.I.; Enciso, J.S.; Tardiff, J.C.; Adler, E.D. Thin filament cardiomyopathies: A review of genetics, disease mechanisms, and emerging therapeutics. Front. Cardiovasc. Med. 2022, 9, 972301. [Google Scholar] [CrossRef]
- Hassoun, R.; Budde, H.; Mannherz, H.G.; Lódi, M.; Fujita-Becker, S.; Laser, K.T.; Gärtner, A.; Klingel, K.; Möhner, D.; Stehle, R.; et al. De Novo Missense Mutations in TNNC1 and TNNI3 Causing Severe Infantile Cardiomyopathy Affect Myofilament Structure and Function and Are Modulated by Troponin Targeting Agents. Int. J. Mol. Sci. 2021, 22, 9625. [Google Scholar] [CrossRef]
- Tobacman, L.S.; Cammarato, A. Cardiomyopathic troponin mutations predominantly occur at its interface with actin and tropomyosin. J. Gen. Physiol. 2021, 153. [Google Scholar] [CrossRef]
- Ploski, R.; Rydzanicz, M.; Ksiazczyk, T.M.; Franaszczyk, M.; Pollak, A.; Kosinska, J.; Michalak, E.; Stawinski, P.; Ziolkowska, L.; Bilinska, Z.T.; et al. Evidence for troponin C (TNNC1) as a gene for autosomal recessive restrictive cardiomyopathy with fatal outcome in infancy. Am. J. Med Genet. Part A 2016, 170, 3241–3248. [Google Scholar] [CrossRef]
- Patsalis, C.; Kyriakou, S.; Georgiadou, M.; Ioannou, L.; Constantinou, L.; Soteriou, V.; Jossif, A.; Evangelidou, P.; Sismani, C.; Kypri, E.; et al. Investigating TNNC1 gene inheritance and clinical outcomes through a comprehensive familial study. Am. J. Med Genet. Part A 2024, 197, e63838. [Google Scholar] [CrossRef]
- Veltri, T.; de Oliveira, G.A.P.; Bienkiewicz, E.A.; Palhano, F.L.; Marques, M.d.A.; Moraes, A.H.; Silva, J.L.; Sorenson, M.M.; Pinto, J.R. Amide hydrogens reveal a temperature-dependent structural transition that enhances site-II Ca2+-binding affinity in a C-domain mutant of cardiac troponin C. Sci. Rep. 2017, 7, 691. [Google Scholar] [CrossRef]
- Marques, M.A.; Landim-Vieira, M.; Moraes, A.H.; Sun, B.; Johnston, J.R.; Jones, K.M.D.; Cino, E.A.; Parvatiyar, M.S.; Valera, I.C.; Silva, J.L.; et al. Anomalous structural dynamics of minimally frustrated residues in cardiac troponin C triggers hypertrophic cardiomyopathy. Chem. Sci. 2021, 12, 7308–7323. [Google Scholar] [CrossRef]
- Varughese, J.F.; Baxley, T.; Chalovich, J.M.; Li, Y. A Computational and Experimental Approach To Investigate Bepridil Binding with Cardiac Troponin. J. Phys. Chem. B 2011, 115, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- van de Locht, M.; Donkervoort, S.; de Winter, J.M.; Conijn, S.; Begthel, L.; Kusters, B.; Mohassel, P.; Hu, Y.; Medne, L.; Quinn, C.; et al. Pathogenic variants in TNNC2 cause congenital myopathy due to an impaired force response to calcium. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Garcia, M.R.; Schmeckpeper, J.; Landim-Vieira, M.; Coscarella, I.L.; Fang, X.; Ma, W.; Spran, P.A.; Yuan, S.; Qi, L.; Kahmini, A.R.; et al. Disruption of Z-Disc Function Promotes Mechanical Dysfunction in Human Myocardium: Evidence for a Dual Myofilament Modulatory Role by Alpha-Actinin Int. J. Mol. Sci. 2023, 24, 14572. [Google Scholar] [CrossRef]
- Landim-Vieira, M.; Childers, M.C.; Wacker, A.L.; Garcia, M.R.; He, H.; Singh, R.; A Brundage, E.; Johnston, J.R.; A Whitson, B.; Chase, P.B.; et al. Post-translational modification patterns on β-myosin heavy chain are altered in ischemic and nonischemic human hearts. eLife 2022, 11. [Google Scholar] [CrossRef]
- Sevrieva, I.; Knowles, A.C.; Kampourakis, T.; Sun, Y.-B. Regulatory domain of troponin moves dynamically during activation of cardiac muscle. J. Mol. Cell. Cardiol. 2014, 75, 181–187. [Google Scholar] [CrossRef]
- Sevrieva, I.R.; Kampourakis, T.; Irving, M. Structural changes in troponin during activation of skeletal and heart muscle determined in situ by polarised fluorescence. Biophys. Rev. 2024, 16, 753–772. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




