Submitted:
06 March 2025
Posted:
07 March 2025
You are already at the latest version
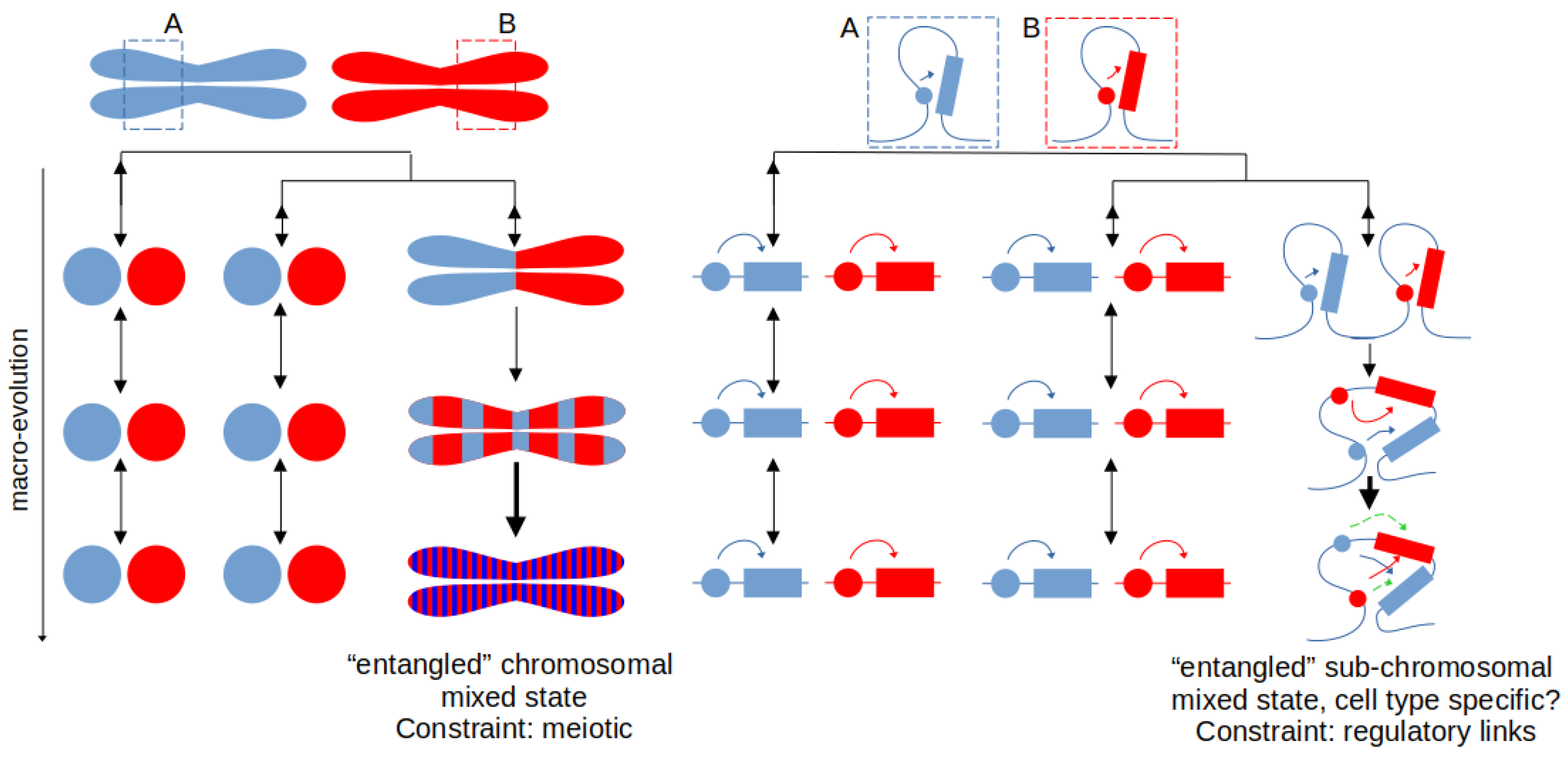
Abstract
Keywords:
Funding
Acknowledgments
References
- Baer KE von. Über Entwicklungsgeschichte der Thiere. 1. Auflage. Königsberg: Bornträger; 1828.
- Hall, B.K. Phylotypic stage or phantom: is there a highly conserved embryonic stage in vertebrates? Trends Ecol. Evol. 1997, 12, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Valentine JW. Cell Types, Numbers, and Body Plan Complexity. In: Hall BK, editor. Keywords and Concepts in Evolutionary Developmental Biology [Internet]. Harvard University Press; 2006 [cited 2024 Dec 2]. p. 35–43. Available from: https://www.degruyter.com/document/doi/10.4159/9780674273320-008/html.
- Arendt, D.; Musser, J.M.; Baker, C.V.H.; Bergman, A.; Cepko, C.; Erwin, D.H.; Pavlicev, M.; Schlosser, G.; Widder, S.; Laubichler, M.D.; et al. The origin and evolution of cell types. Nat. Rev. Genet. 2016, 17, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Wagner GP. Homology, Genes, and Evolutionary Innovation. 1st edition. Princeton: Princeton University Press; 2014.
- Gould SJ. Ontogeny and phylogeny. Harvard University Press; 1985.
- Sebé-Pedrós, A.; Chomsky, E.; Pang, K.; Lara-Astiaso, D.; Gaiti, F.; Mukamel, Z.; Amit, I.; Hejnol, A.; Degnan, B.M.; Tanay, A. Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat. Ecol. Evol. 2018, 2, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Sebé-Pedrós, A.; Saudemont, B.; Chomsky, E.; Plessier, F.; Mailhé, M.-P.; Renno, J.; Loe-Mie, Y.; Lifshitz, A.; Mukamel, Z.; Schmutz, S.; et al. Cnidarian Cell Type Diversity and Regulation Revealed by Whole-Organism Single-Cell RNA-Seq. Cell 2018, 173, 1520–1534.e20. [Google Scholar] [CrossRef]
- Martín-Durán, J.M.; Hejnol, A. A developmental perspective on the evolution of the nervous system. Dev. Biol. 2021, 475, 181–192. [Google Scholar] [CrossRef]
- Kalinka, A.T.; Varga, K.M.; Gerrard, D.T.; Preibisch, S.; Corcoran, D.L.; Jarrells, J.; Ohler, U.; Bergman, C.M.; Tomancak, P. Gene expression divergence recapitulates the developmental hourglass model. Nature 2010, 468, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Paganos P, Voronov D, Musser J, Arendt D, Arnone MI. Single cell RNA sequencing of the Strongylocentrotus purpuratus larva reveals the blueprint of major cell types and nervous system of a non-chordate deuterostome [Internet]. bioRxiv; 2021 [cited 2022 Dec 5]. p. 2021.03.16.435574. Available from: https://www.biorxiv.org/content/10.1101/2021.03.16.435574v3.
- Davidson, E.H.; Erwin, D.H. Gene regulatory networks and the evolution of animal body plans. Science 2006, 311, 796–797. [Google Scholar] [CrossRef]
- Touceda-Suárez, M.; Kita, E.M.; Acemel, R.D.; Firbas, P.N.; Magri, M.S.; Naranjo, S.; Tena, J.J.; Gómez-Skarmeta, J.L.; Maeso, I.; Irimia, M. Ancient Genomic Regulatory Blocks Are a Source for Regulatory Gene Deserts in Vertebrates after Whole-Genome Duplications. Mol. Biol. Evol. 2020, 37, 2857–2864. [Google Scholar] [CrossRef]
- Sommer-Trembo, C.; Santos, M.E.; Clark, B.; Werner, M.; Fages, A.; Matschiner, M.; Hornung, S.; Ronco, F.; Oliver, C.; Garcia, C.; et al. The genetics of niche-specific behavioral tendencies in an adaptive radiation of cichlid fishes. Science 2024, 384, 470–475. [Google Scholar] [CrossRef]
- Villar, D.; Berthelot, C.; Aldridge, S.; Rayner, T.F.; Lukk, M.; Pignatelli, M.; Park, T.J.; Deaville, R.; Erichsen, J.T.; Jasinska, A.J.; et al. Enhancer Evolution across 20 Mammalian Species. Cell 2015, 160, 554–566. [Google Scholar] [CrossRef]
- Babonis, L.S.; Enjolras, C.; Reft, A.J.; Foster, B.M.; Hugosson, F.; Ryan, J.F.; Daly, M.; Martindale, M.Q. Single-cell atavism reveals an ancient mechanism of cell type diversification in a sea anemone. Nat. Commun. 2023, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McColgan, Á.; DiFrisco, J. Understanding developmental system drift. Development 2024, 151. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.P. The developmental genetics of homology. Nat. Rev. Genet. 2007, 8, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Haeckel E, Haeckel E. Generelle morphologie der organismen. Allgemeine grundzüge der organischen formen-wissenschaft, mechanisch begründet durch die von Charles Darwin reformirte descendenztheorie [Internet]. Berlin: G. Reimer; 1866. Available from: https://www.biodiversitylibrary.org/bibliography/3953.
- Levit, G.S.; Hoßfeld, U.; Naumann, B.; Lukas, P.; Olsson, L. The biogenetic law and the Gastraea theory: From Ernst Haeckel's discoveries to contemporary views. J. Exp. Zoöl. Part B: Mol. Dev. Evol. 2021, 338, 13–27. [Google Scholar] [CrossRef]
- Uesaka, M.; Kuratani, S.; Irie, N. The developmental hourglass model and recapitulation: An attempt to integrate the two models. J. Exp. Zoöl. Part B: Mol. Dev. Evol. 2021, 338, 76–86. [Google Scholar] [CrossRef]
- Schultz DT, Blümel A, Destanović D, Sarigol F, Simakov O. Topological mixing and irreversibility in animal chromosome evolution. bioRxiv. 2024;2024–07.
- Simakov, O.; Marlétaz, F.; Yue, J.-X.; O’connell, B.; Jenkins, J.; Brandt, A.; Calef, R.; Tung, C.-H.; Huang, T.-K.; Schmutz, J.; et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef]
- Simakov, O.; Bredeson, J.; Berkoff, K.; Marletaz, F.; Mitros, T.; Schultz, D.T.; O’connell, B.L.; Dear, P.; Martinez, D.E.; Steele, R.E.; et al. Deeply conserved synteny and the evolution of metazoan chromosomes. Sci. Adv. 2022, 8, eabi5884. [Google Scholar] [CrossRef]
- Schultz, D.T.; Haddock, S.H.D.; Bredeson, J.V.; Green, R.E.; Simakov, O.; Rokhsar, D.S. Ancient gene linkages support ctenophores as sister to other animals. Nature 2023, 618, 110–117. [Google Scholar] [CrossRef]
- Muller HJ. Bearing of the “Drosophila” work on systematics. The New Systematics [Internet]. 1940 [cited 2021 Feb 8]. p. 185–268. Available from: https://ci.nii.ac.jp/naid/10004957361/.
- Wright, S. On the Probability of Fixation of Reciprocal Translocations. Am. Nat. 1941, 75, 513–522. [Google Scholar] [CrossRef]
- Lv, J.; Havlak, P.; Putnam, N.H. Constraints on genes shape long-term conservation of macro-synteny in metazoan genomes. BMC Bioinform. 2011, 12, S11–S11. [Google Scholar] [CrossRef]
- Clarence, T.; Robert, N.S.; Sarigol, F.; Fu, X.; Bates, P.A.; Simakov, O. Robust 3D modeling reveals spatiosyntenic properties of animal genomes. iScience 2023, 26, 106136. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Kawaguchi, A.; Clarence, T.; Fu, X.; Hoang, O.P.; Zimmermann, B.; Ritschard, E.A.; Weissenbacher, A.; Foster, J.S.; Nyholm, S.V.; et al. Emergence of novel cephalopod gene regulation and expression through large-scale genome reorganization. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Szalay, M.-F.; Majchrzycka, B.; Jerković, I.; Cavalli, G.; Ibrahim, D.M. Evolution and function of chromatin domains across the tree of life. Nat. Struct. Mol. Biol. 2024, 31, 1824–1837. [Google Scholar] [CrossRef]
- Rogers, T.F.; Simakov, O. Emerging questions on the mechanisms and dynamics of 3D genome evolution in spiralians. Briefings Funct. Genom. 2023, 22, 533–542. [Google Scholar] [CrossRef]
- Irimia, M.; Tena, J.J.; Alexis, M.S.; Fernandez-Miñan, A.; Maeso, I.; Bogdanović, O.; de la Calle-Mustienes, E.; Roy, S.W.; Gómez-Skarmeta, J.L.; Fraser, H.B. Extensive conservation of ancient microsynteny across metazoans due to cis-regulatory constraints. Genome Res. 2012, 22, 2356–2367. [Google Scholar] [CrossRef]
- Simakov, O.; Marletaz, F.; Cho, S.-J.; Edsinger-Gonzales, E.; Havlak, P.; Hellsten, U.; Kuo, D.-H.; Larsson, T.; Lv, J.; Arendt, D.; et al. Insights into bilaterian evolution from three spiralian genomes. Nature 2012, 493, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Engström, P.G.; Sui, S.J.H.; Drivenes, Ø.; Becker, T.S.; Lenhard, B. Genomic regulatory blocks underlie extensive microsynteny conservation in insects. Genome Res. 2007, 17, 1898–1908. [Google Scholar] [CrossRef]
- Kikuta, H.; Laplante, M.; Navratilova, P.; Komisarczuk, A.Z.; Engström, P.G.; Fredman, D.; Akalin, A.; Caccamo, M.; Sealy, I.; Howe, K.; et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 2007, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.; Robert, N.S.M.; Technau, U.; Simakov, O. Ancient animal genome architecture reflects cell type identities. Nat. Ecol. Evol. 2019, 3, 1289–1293. [Google Scholar] [CrossRef]
- Robert, N.S.M.; Sarigol, F.; Zimmermann, B.; Meyer, A.; Voolstra, C.R.; Simakov, O. Emergence of distinct syntenic density regimes is associated with early metazoan genomic transitions. BMC Genom. 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Álvarez-González, L.; Burden, F.; Doddamani, D.; Malinverni, R.; Leach, E.; Marín-García, C.; Marín-Gual, L.; Gubern, A.; Vara, C.; Paytuví-Gallart, A.; et al. 3D chromatin remodelling in the germ line modulates genome evolutionary plasticity. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Erwin, D.H. The topology of evolutionary novelty and innovation in macroevolution. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160422. [Google Scholar] [CrossRef]
- Choi, J.; Lysakovskaia, K.; Stik, G.; Demel, C.; Söding, J.; Tian, T.V.; Graf, T.; Cramer, P. Evidence for additive and synergistic action of mammalian enhancers during cell fate determination. eLife 2021, 10. [Google Scholar] [CrossRef]
- Rickels, R.; Shilatifard, A. Enhancer Logic and Mechanics in Development and Disease. Trends Cell Biol. 2018, 28, 608–630. [Google Scholar] [CrossRef] [PubMed]
- Montavon, T.; Soshnikova, N.; Mascrez, B.; Joye, E.; Thevenet, L.; Splinter, E.; de Laat, W.; Spitz, F.; Duboule, D. A Regulatory Archipelago Controls Hox Genes Transcription in Digits. Cell 2011, 147, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carballo, E.; Lopez-Delisle, L.; Willemin, A.; Beccari, L.; Gitto, S.; Mascrez, B.; Duboule, D. Chromatin topology and the timing of enhancer function at the HoxD locus. Proc. Natl. Acad. Sci. 2020, 117, 31231–31241. [Google Scholar] [CrossRef]
- Acemel, R.D.; Tena, J.J.; Irastorza-Azcarate, I.; Marlétaz, F.; Gómez-Marín, C.; de la Calle-Mustienes, E.; Bertrand, S.; Diaz, S.G.; Aldea, D.; Aury, J.-M.; et al. A single three-dimensional chromatin compartment in amphioxus indicates a stepwise evolution of vertebrate Hox bimodal regulation. Nat. Genet. 2016, 48, 336–341. [Google Scholar] [CrossRef]
- Batut, P.J.; Bing, X.Y.; Sisco, Z.; Raimundo, J.; Levo, M.; Levine, M.S. Genome organization controls transcriptional dynamics during development. Science 2022, 375, 566–570. [Google Scholar] [CrossRef]
- Albertin, C.B.; Medina-Ruiz, S.; Mitros, T.; Schmidbaur, H.; Sanchez, G.; Wang, Z.Y.; Grimwood, J.; Rosenthal, J.J.C.; Ragsdale, C.W.; Simakov, O.; et al. Genome and transcriptome mechanisms driving cephalopod evolution. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Ikuta, T.; Yoshida, N.; Satoh, N.; Saiga, H. Ciona intestinalis Hox gene cluster: Its dispersed structure and residual colinear expression in development. Proc. Natl. Acad. Sci. 2004, 101, 15118–15123. [Google Scholar] [CrossRef]
- Seo, H.-C.; Edvardsen, R.B.; Maeland, A.D.; Bjordal, M.; Jensen, M.F.; Hansen, A.; Flaat, M.; Weissenbach, J.; Lehrach, H.; Wincker, P.; et al. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature 2004, 431, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Butts, T.; Holland, P.W.; Ferrier, D.E. The Urbilaterian Super-Hox cluster. Trends Genet. 2008, 24, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T.; Brajković, J.; Tautz, D. A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 2007, 23, 533–539. [Google Scholar] [CrossRef]
- Bleidorn, C. Rare Genomic Changes. In Phylogenomics: An Introduction [Internet]; Bleidorn, C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 195–211. [Google Scholar] [CrossRef]
- Park, Y.; Nnamani, M.C.; Maziarz, J.; Wagner, G.P. Cis-Regulatory Evolution of Forkhead Box O1 (FOXO1), a Terminal Selector Gene for Decidual Stromal Cell Identity. Mol. Biol. Evol. 2016, 33, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.J.; Leclerc, R.D.; May, G.; Wagner, G.P. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat. Genet. 2011, 43, 1154–1159. [Google Scholar] [CrossRef]
- Kon-Nanjo K, Kon T, Yu TC-TK, Rodriguez-Terrones D, Falcon F, Martínez DE, et al. The dynamic genomes of Hydra and the anciently active repeat complement of animal chromosomes [Internet]. bioRxiv; 2024 [cited 2024 Nov 12]. p. 2024.03.13.584568. Available from: https://www.biorxiv.org/content/10.1101/2024.03.13.584568v1.
- Meyer, A.; Schloissnig, S.; Franchini, P.; Du, K.; Woltering, J.M.; Irisarri, I.; Wong, W.Y.; Nowoshilow, S.; Kneitz, S.; Kawaguchi, A.; et al. Giant lungfish genome elucidates the conquest of land by vertebrates. Nature 2021, 590, 284–289. [Google Scholar] [CrossRef]
- Schartl, M.; Woltering, J.M.; Irisarri, I.; Du, K.; Kneitz, S.; Pippel, M.; Brown, T.; Franchini, P.; Li, J.; Li, M.; et al. The genomes of all lungfish inform on genome expansion and tetrapod evolution. Nature 2024, 634, 96–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



