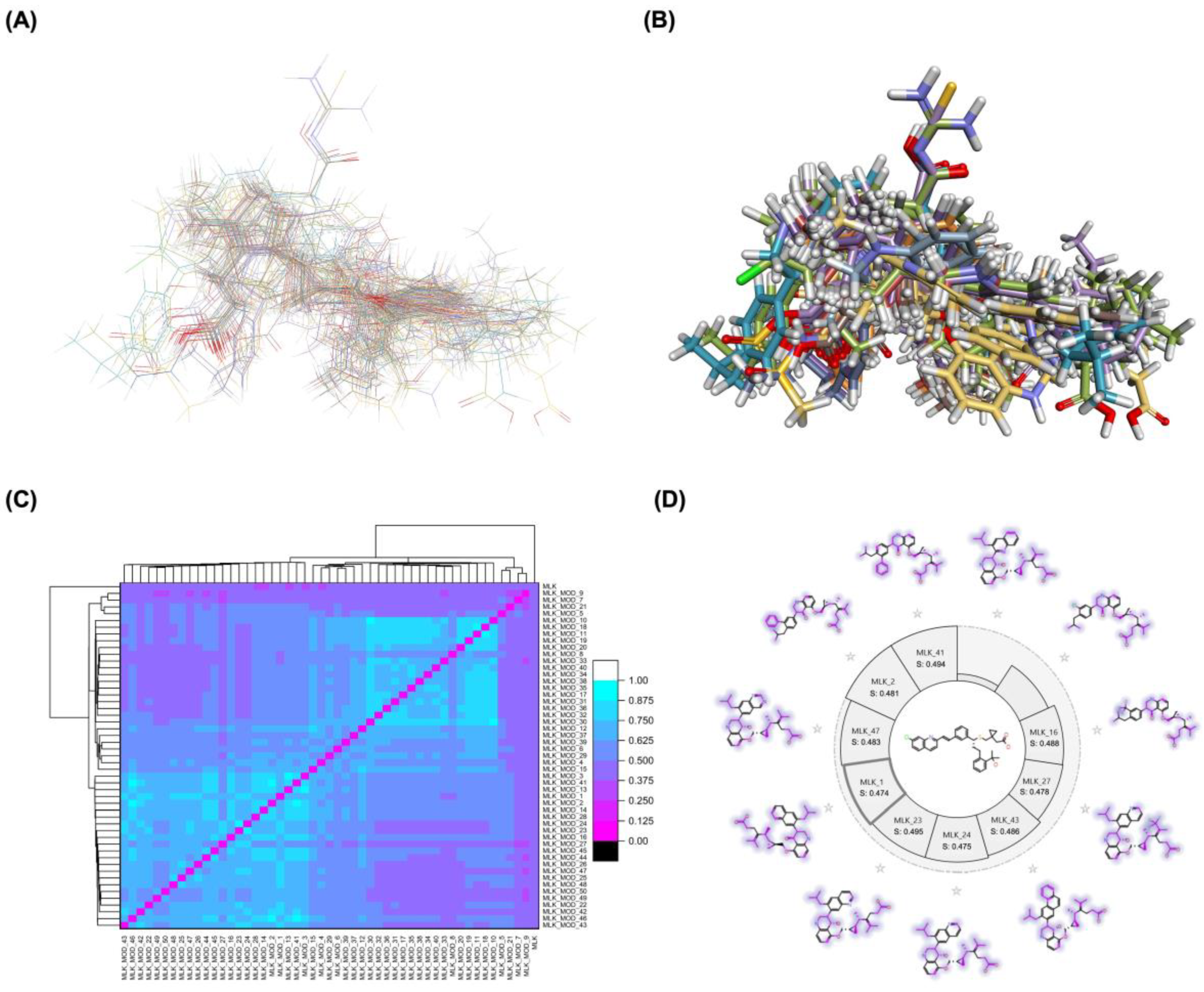
2.1. Modification Strategy and Similarity Analysis
A comprehensive structural modification strategy was employed to explore the structure-activity relationship of Montelukast, aiming to enhance its pharmacokinetic and pharmacodynamic properties. The strategy encompassed a diverse set of chemical modifications, including aromatic substitutions, heterocyclic replacements, amide modifications, halogenation, carboxyl alterations, etherification, and side-chain modifications (
Table 1). These modifications were strategically designed to optimize Montelukast's receptor binding affinity, metabolic stability, solubility, lipophilicity, and bioavailability. Following energy minimization, the modified structures were superimposed onto the native Montelukast backbone to evaluate structural conservation and deviation. The 3D superimposition analysis revealed that despite extensive modifications, the core structural framework of Montelukast remained largely intact, with key pharmacophoric features preserved. Given the broad scope of modifications, a flexible root-mean-square deviation (RMSD) threshold was applied to accommodate structural variations while ensuring alignment accuracy.
Figure 1A and 1B illustrate this superimposition, with
Figure 1A depicting the backbone alignment and
Figure 1B providing a detailed 3D representation. The complete list of Montelukast modifications is available in
Supplementary Data S1.
To further assess the degree of structural similarity, a pairwise similarity correlation heatmap (
Figure 1C) was generated. The similarity scores between the modified structures and the native montelukast ranged from 0.25 to 0.50, indicating moderate structural deviation, whereas inter-modification similarity values ranged from 0.625 to 1.00, suggesting that the modified derivatives retained significant structural resemblance to each other. Notably, MLK_MOD-2 (biphenyl substitution), MLK_MOD-24 (benzimidazole substitution), and MLK_MOD-43 (carbazole substitution) exhibited similarity values of 0.481, 0.475, and 0.486, respectively (
Figure 1D). These results indicate that while the modifications introduce distinct chemical properties, they maintain a reasonable level of structural conservation with the native molecule. The observed similarity trends suggest that certain modification strategies (such as heterocyclic replacements, halogenation, and fused ring systems) tend to introduce greater deviations from the native structure due to significant alterations in electronic properties and steric configurations. On the other hand, modifications involving functional group substitutions (e.g., amide, carboxyl, and hydroxyl modifications) generally exhibited higher similarity scores, reflecting minimal perturbations to the overall molecular framework. This balance between structural conservation and chemical diversification is critical for optimizing Montelukast derivatives while maintaining receptor compatibility.
Aromatic modifications, such as biphenyl (MLK_MOD-2) and fluorophenyl (MLK_MOD-3) substitutions, were introduced to enhance π-π stacking interactions with the target receptor. This strategy is particularly beneficial for improving binding affinity and receptor specificity, as increased aromatic surface area can facilitate stronger hydrophobic and van der Waals interactions. Similarly, heterocyclic replacements (e.g., pyridine in MLK_MOD-4 and thiophene in MLK_MOD-5) were designed to introduce polar functional groups that enhance hydrogen bonding interactions while maintaining a favorable balance of lipophilicity and metabolic stability. Amide modifications (e.g., methylation at amide nitrogen in MLK_MOD-8 and urea substitution in MLK_MOD-9) were specifically incorporated to fine-tune hydrogen bonding potential and receptor selectivity. These modifications can significantly influence protein-ligand interactions by stabilizing key binding interactions while also affecting drug metabolism and excretion profiles. Carboxyl modifications, such as carboxyl-to-amide (MLK_MOD-10) and carboxyl-to-ester (MLK_MOD-11) substitutions, were aimed at improving membrane permeability and lipophilicity, thereby enhancing oral bioavailability. Halogenation strategies (e.g., fluorine addition in MLK_MOD-13 and chlorine substitution in MLK_MOD-14) were explored to improve metabolic stability and enhance hydrophobic interactions. The incorporation of halogens often increases binding affinity by strengthening dipole-induced dipole interactions while also enhancing the compound’s resistance to enzymatic degradation. Similarly, fluorinated modifications (e.g., trifluoromethyl addition in MLK_MOD-16) were designed to improve BBB permeability, which is crucial for CNS-targeting applications. Hydroxylation (MLK_MOD-12), etherification (MLK_MOD-15), and sulfonylation (MLK_MOD-17) were applied to increase water solubility and hydrophilicity. While these modifications enhance aqueous solubility, they also introduce potential challenges related to plasma protein binding and renal clearance, requiring a careful balance between hydrophilic and lipophilic properties. The introduction of fused ring systems (e.g., fluorene in MLK_MOD-42 and carbazole in MLK_MOD-43) aimed to enhance metabolic stability and π-π interactions, ensuring stronger receptor binding and improved pharmacokinetic properties. A key finding from this study is the correlation between structural similarity and drug-likeness. Modifications that significantly deviated from the native structure (e.g., fused ring systems and extensive halogenation) often led to increased lipophilicity and metabolic stability but may have introduced challenges related to solubility and bioavailability. Conversely, modifications that retained high structural similarity (e.g., hydroxylation, carboxyl-to-amide conversion) generally resulted in favorable solubility and permeability profiles, making them promising candidates for further optimization. The observed similarity trends suggest that moderate structural deviation (similarity values between 0.45 and 0.55) is optimal for balancing receptor affinity, metabolic stability, and bioavailability. This highlights the importance of strategically selecting modification strategies that introduce beneficial chemical properties while maintaining a structurally viable scaffold.
2.2. Molecular Docking Results, Binding Pose, and Binding Affinity Analysis
To assess the potential of Montelukast and its structural modifications as DRD2 modulators, molecular docking studies were performed using HADDOCK scoring and free-binding energy calculations. The standard antagonist, Haloperidol, served as a benchmark, exhibiting a HADDOCK score of -41.0 ± 1.1 a.u. and a binding energy of -9.82 kcal/mol. This established a reference for evaluating the binding efficiency of Montelukast and its derivatives. Native Montelukast (MLK_DDR2) displayed a HADDOCK score of -43.7 ± 4.7 a.u., slightly more negative than Haloperidol, indicating strong receptor-ligand interactions. However, its binding energy was -8.26 kcal/mol, lower than Haloperidol, suggesting weaker thermodynamic stability and less favorable receptor binding. This was further supported by its electrostatic energy contribution (-25.1 kcal/mol), which was significantly weaker than that of Haloperidol (-58.8 kcal/mol). Among the modified Montelukast structures, MLK_MOD-43, MLK_MOD-42, and MLK_MOD-22 demonstrated the strongest interactions with DRD2, surpassing native Montelukast and even showing competitive binding with Haloperidol. MLK_MOD-43 exhibited the highest binding energy (-11.22 kcal/mol), suggesting a more stable interaction with DRD2. This modification also showed the most favorable electrostatic energy (-125.0 kcal/mol), indicating a strong charge-based interaction within the ligand-binding domain (LBD). Similarly, MLK_MOD-42 and MLK_MOD-22 displayed binding energies of -10.29 kcal/mol and -10.11 kcal/mol, respectively, with MLK_MOD-42 demonstrating the highest van der Waals energy contribution (-40.5 kcal/mol), suggesting increased hydrophobic contacts within the receptor pocket. In contrast, other modifications such as MLK_MOD-27, MLK_MOD-33, and MLK_MOD-3 exhibited moderate binding affinities but failed to reach the binding efficiency of Haloperidol or the top three performing modifications. While they demonstrated favorable van der Waals and electrostatic contributions, their overall stability and binding pose did not align as closely with Haloperidol as MLK_MOD-43, MLK_MOD-42, and MLK_MOD-22. These results highlight those specific modifications, particularly those enhancing electrostatic interactions and van der Waals forces, contribute significantly to receptor binding efficiency.
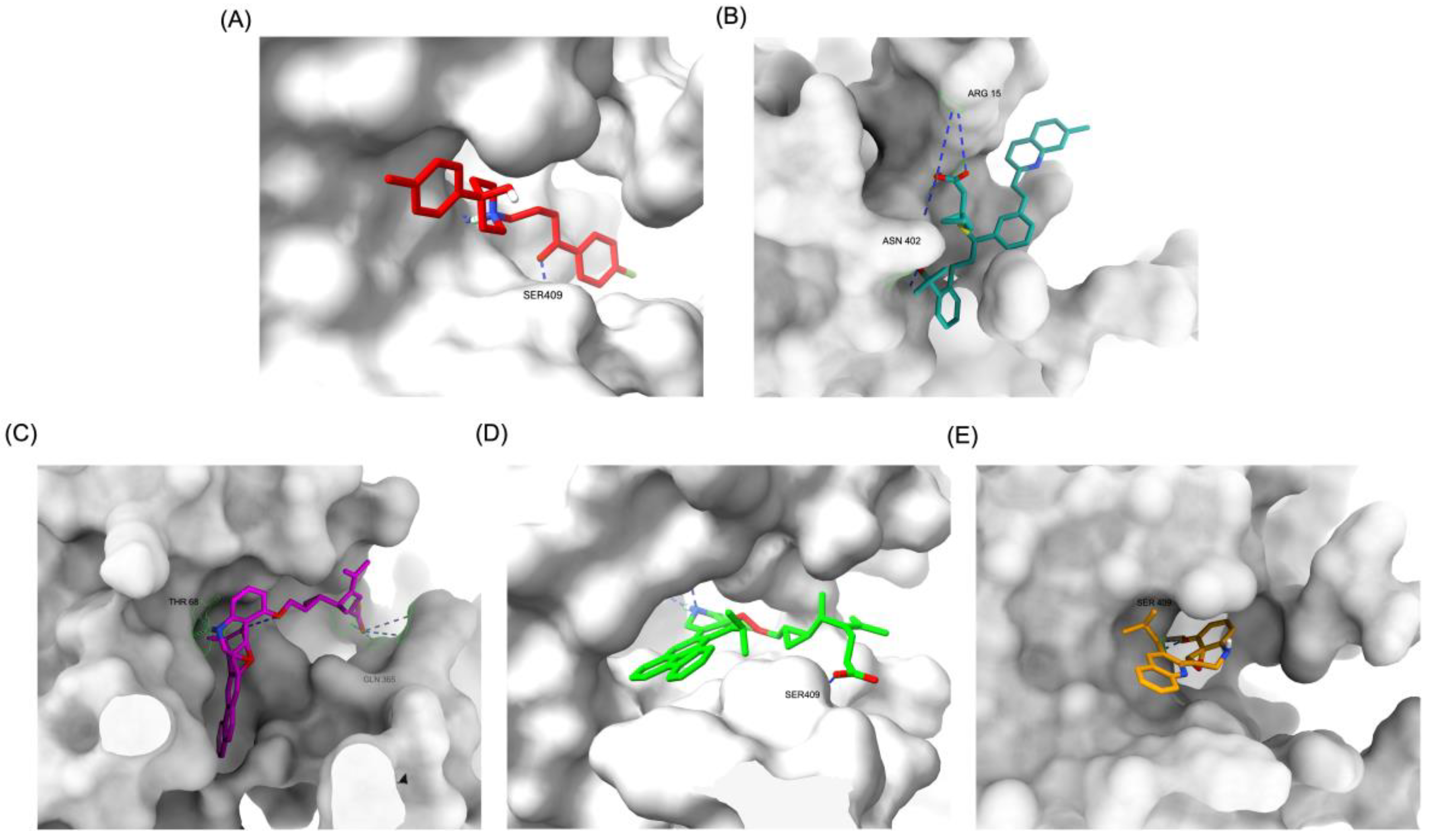
The structural positioning of the ligands within the DRD2 receptor was further examined by analyzing their docking poses in the ligand-binding domain (LBD).
Figure 2A illustrates the binding pose of Haloperidol, which is deeply embedded in the LBD, optimizing its interaction with key receptor residues. This deep binding mode is critical for its antagonistic function, ensuring strong receptor engagement and stable inhibition. In contrast, native Montelukast (MLK_DDR2) was positioned more peripherally within the receptor pocket (
Figure 2B), failing to fully engage with critical binding residues. This suboptimal placement likely contributes to its weaker binding affinity and lower binding energy compared to Haloperidol. The peripheral positioning may reduce receptor-ligand interactions, leading to lower overall stability and functional efficacy. Interestingly, MLK_MOD-43, MLK_MOD-42, and MLK_MOD-22 adopted binding poses that closely resembled Haloperidol’s (
Figure 2C–2E). These modifications were positioned deeper in the LBD, allowing them to establish stronger interactions with the receptor. This suggests that the introduced modifications improved binding affinity and enhanced the ligand’s ability to fit into the receptor pocket in a functionally relevant manner. This deeper binding mode likely contributes to their superior binding energies and interaction profiles.
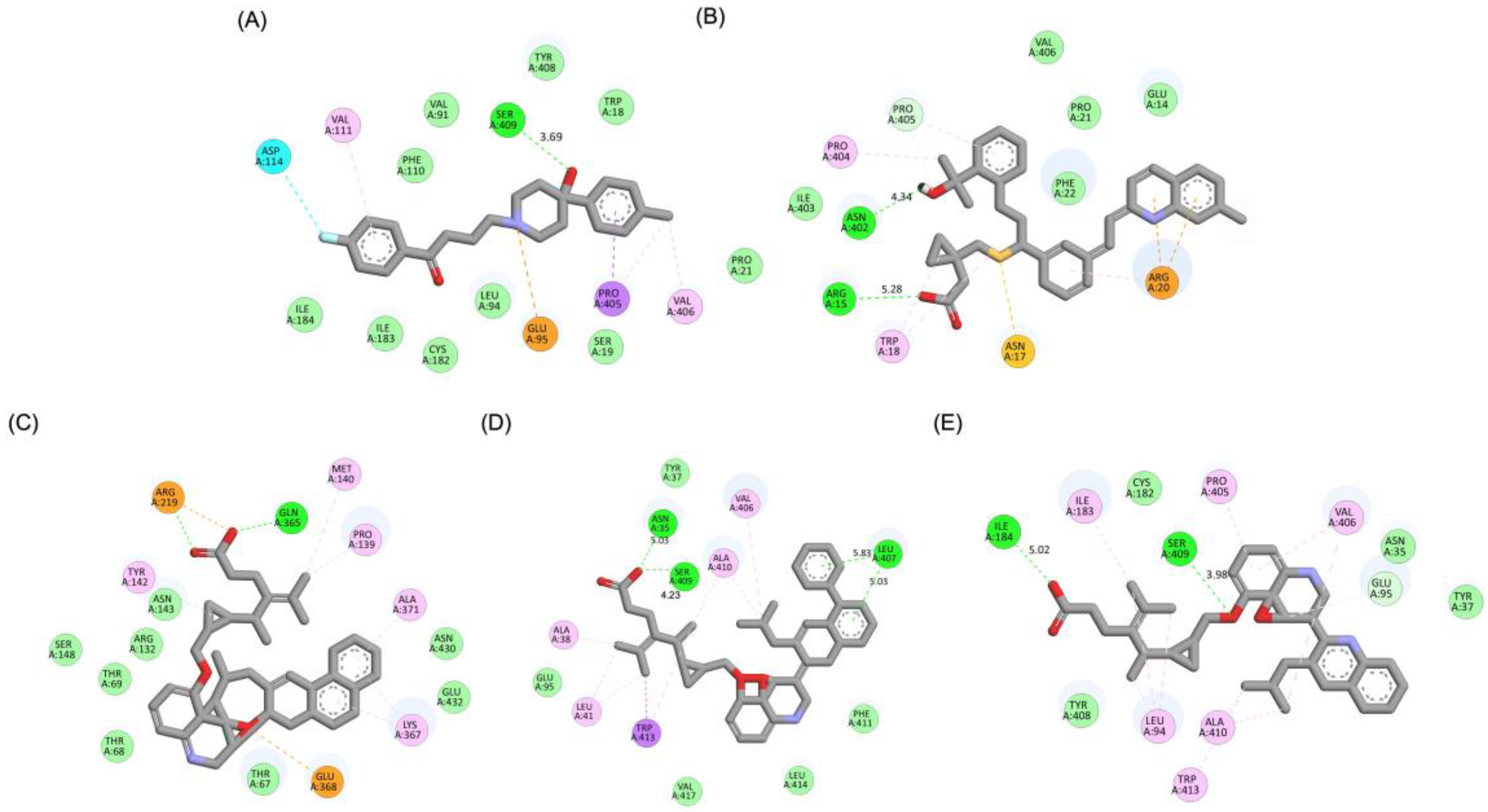
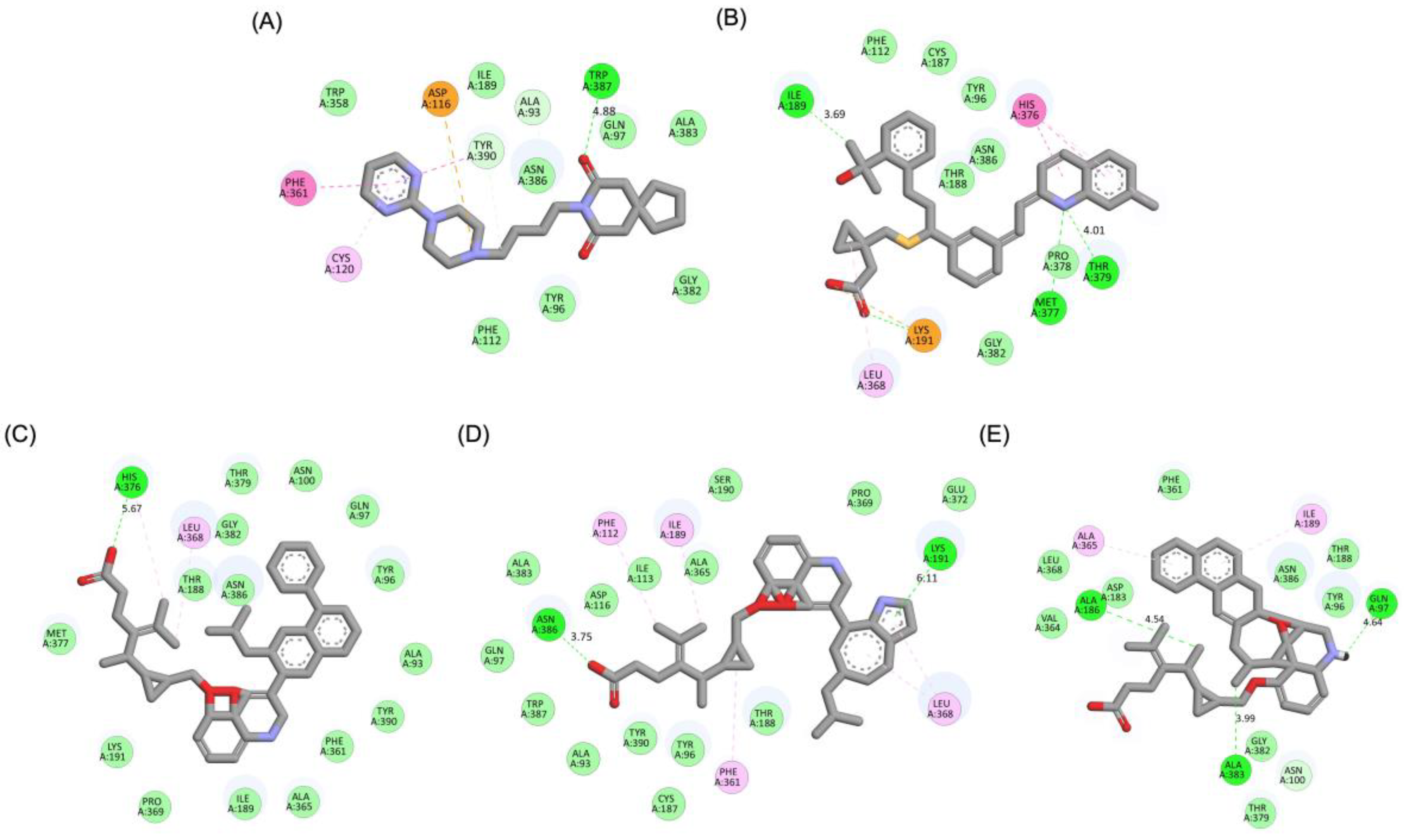
A detailed interaction analysis revealed that one of the most critical factors distinguishing the high-affinity modifications (MLK_MOD-43, MLK_MOD-42, MLK_MOD-22) from native Montelukast was the formation of hydrogen bonds with Ser409, a key residue involved in Haloperidol’s binding mechanism. Native Montelukast failed to form this specific hydrogen bond, potentially explaining its weaker receptor affinity. Beyond hydrogen bonding, 2D interaction mapping (
Figure 3) provided further insights into additional molecular interactions stabilizing the ligand-receptor complex. The analysis revealed that MLK_MOD-42, particularly, exhibited a Pi-Sigma interaction pattern similar to Haloperidol, further reinforcing its ability to mimic the standard antagonist's binding properties. Other significant interactions observed included π-π stacking interactions, contributing to increased receptor-ligand stability. Van der Waals interactions enhance hydrophobic contacts and promote ligand retention in the receptor pocket. Pi-Alkyl and Pi-Sigma interactions, both observed in MLK_MOD-42, mimic Haloperidol’s stabilizing interactions. Halogen and attractive charge interactions, particularly in MLK_MOD-43, further support its high binding affinity. These additional interactions indicate that the modified Montelukast derivatives exhibit improved binding energies and interact with DRD2 in a manner similar to Haloperidol, increasing their potential as alternative DRD2 modulators.
The results strongly suggest that specific chemical modifications to Montelukast’s scaffold can significantly enhance its affinity for DRD2. The introduction of fused aromatic systems, such as the carbazole group in MLK_MOD-43, increased the ligand’s ability to engage in π-π stacking interactions, a critical factor in stabilizing DRD2 binding. Similarly, the fluorene modification in MLK_MOD-42 enhanced hydrophobic interactions, while MLK_MOD-22’s quinoline substitution optimized electrostatic complementarity. These findings demonstrate that structural modifications targeting key interaction mechanisms can successfully repurpose Montelukast as a potential DRD2 modulator. Additionally, the significant electrostatic contributions observed for MLK_MOD-43 (electrostatic energy of -125.0 kcal/mol) suggest that charge-based interactions may play an essential role in ligand stabilization within the receptor pocket. This highlights the importance of strategically introducing functional groups that enhance electrostatic and hydrogen bonding interactions, thereby optimizing receptor binding and increasing ligand potency.
The docking results reveal that Montelukast and its modifications exhibit improved binding affinities toward the 5-HT1A receptor compared to Buspirone, the standard agonist. The HADDOCK score and binding energy (kcal/mol) values indicate that several MLK modifications outperform both native MLK and Buspirone in receptor binding (
Table 3). Notably, MLK_MOD-42, MLK_MOD-24, and MLK_MOD-21 demonstrate the strongest binding affinity, with HADDOCK scores of −34.9 ± 1.6, −34.2 ± 2.8, and −34.1 ± 1.7, respectively, and binding energies of −10.97 kcal/mol, −11.04 kcal/mol, and −10.76 kcal/mol. These values suggest that these modifications form stronger interactions with the receptor compared to Buspirone (HADDOCK score: −23.1 ± 0.8, binding energy: −8.3 kcal/mol) and native MLK (HADDOCK score: −27.0 ± 1.3, binding energy: −9.71 kcal/mol). A detailed energy decomposition analysis further supports these findings. Van der Waals interactions, which play a crucial role in stabilizing ligand binding, are highest for MLK_MOD-42 (−38.2 ± 0.8), followed by MLK_MOD-24 (−31.2 ± 0.6) and MLK_MOD-21 (−33.8 ± 0.6). In contrast, Buspirone shows a slightly lower van der Waals contribution of −34.3 ± 0.3, while native MLK exhibits −28.7 ± 2.2. Additionally, electrostatic interactions vary significantly among the modifications, with MLK_MOD-24 exhibiting the strongest electrostatic stabilization (−117.5 ± 6.1 kcal/mol), which is notably higher than Buspirone (−3.8 ± 2.4 kcal/mol) and native MLK (−49.5 ± 10.1 kcal/mol). This suggests that MLK_MOD-24 may form stronger ionic or hydrogen-bond interactions with charged residues within the receptor. The complete docking and binding energy results of Montelukast and its modifications against the DRD2 and 5-HT1A receptors are available in
Supplementary Data S2.
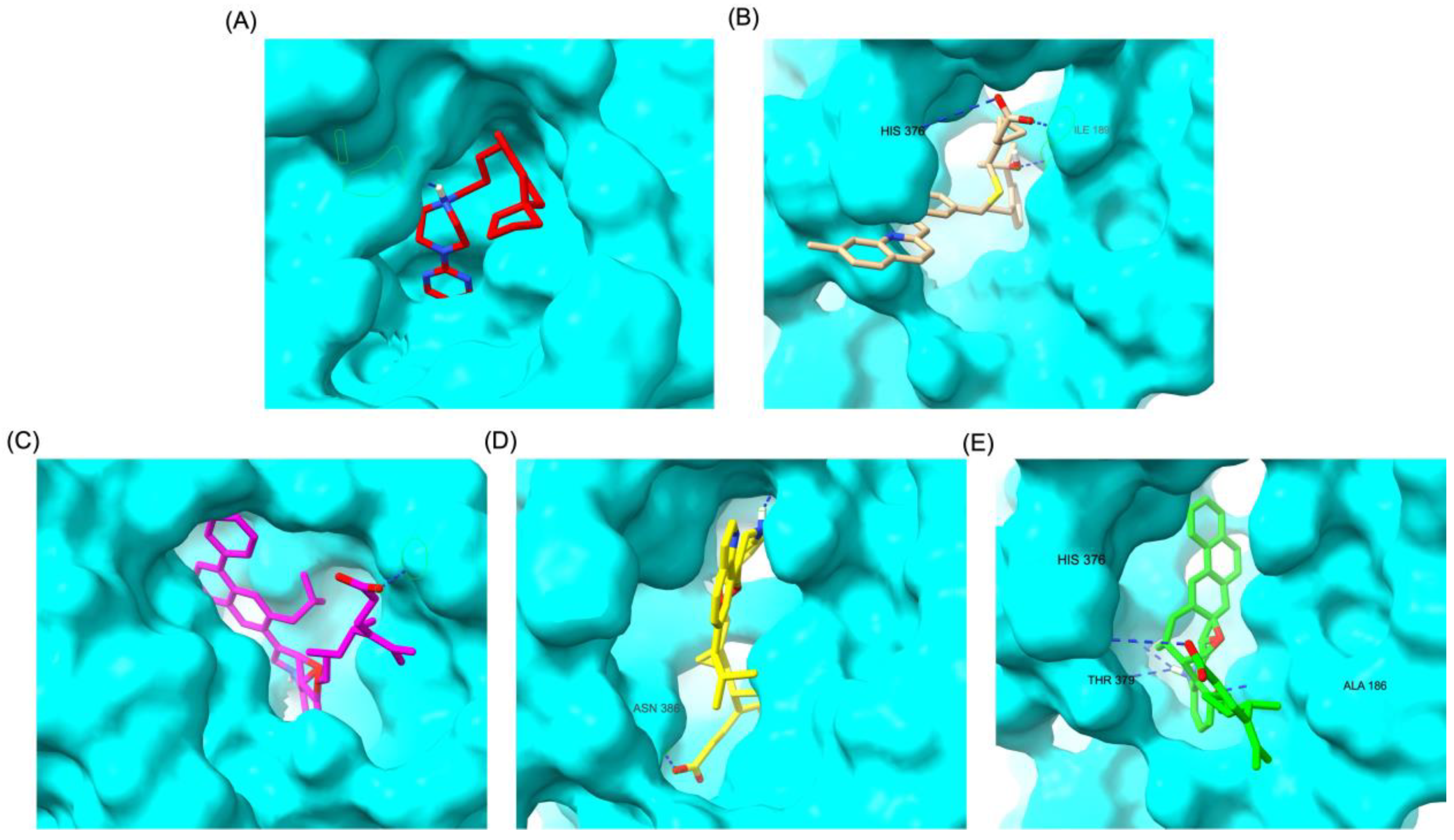
Similar to the DRD2 receptor findings, Montelukast modifications exhibit binding poses more closely aligned with Buspirone in the 5-HT1A receptor ligand-binding domain (LBD).
Figure 4A illustrates the deep positioning of Buspirone within the receptor pocket, indicating strong anchoring within the LBD. However, native Montelukast (
Figure 4B) binds more peripherally, suggesting weaker interactions and a less optimal binding conformation. Interestingly, the top-performing MLK modifications (MLK_MOD-42, MLK_MOD-21, and MLK_MOD-43) exhibit binding poses highly similar to Buspirone (
Figure 4C-4E). These modifications penetrate deeper into the LBD, mimicking the interaction profile of the standard agonist. This adaptation likely contributes to their enhanced binding affinities, with MLK_MOD-42, MLK_MOD-21, and MLK_MOD-43 exhibiting binding energies of −10.97 kcal/mol, −10.76 kcal/mol, and −10.42 kcal/mol, respectively. The 2D interaction maps (
Figure 5) further validate these findings, highlighting key molecular interactions between the MLK modifications and the 5-HT1A receptor. Compared to native MLK, which forms fewer hydrogen bonds and hydrophobic interactions, MLK_MOD-42, MLK_MOD-21, and MLK_MOD-43 establish stronger hydrogen bonding, Pi-stacking, and van der Waals contacts with key receptor residues. These modifications likely introduce structural refinements that enhance complementarity with the receptor’s binding site, resulting in greater binding stability and agonist-like behavior. Overall, these findings suggest that specific Montelukast modifications could serve as potential 5-HT1A receptor modulators. Their enhanced binding affinity, favorable energetic contributions, and structural mimicry of Buspirone highlight their potential in serotonin receptor targeting.
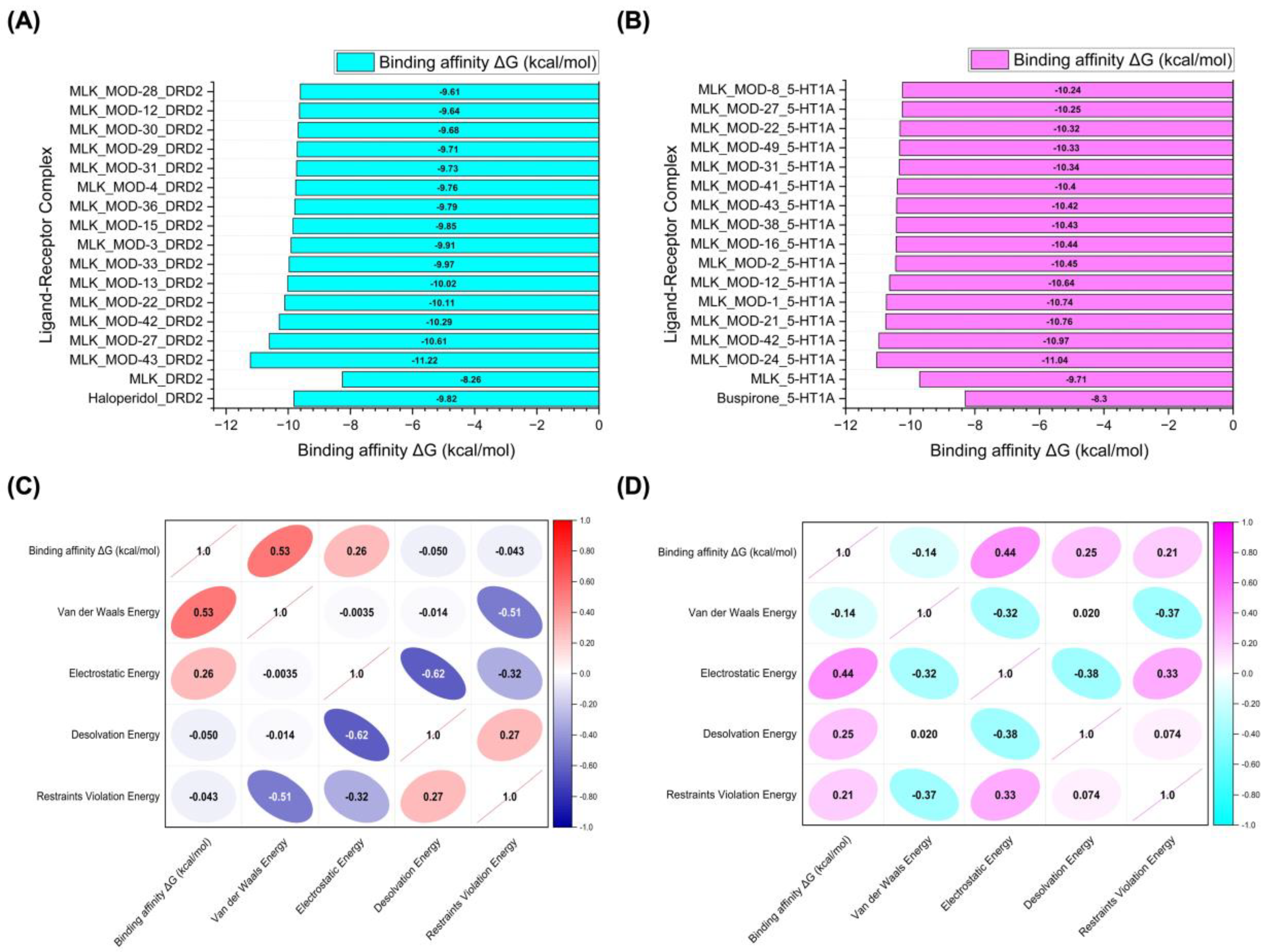
Figure 6A and 6B illustrate the top 15 Montelukast modifications that exhibited the highest binding affinities against the DRD2 and serotonin 5-HT1A receptors, respectively. The docking results reveal that specific structural modifications significantly enhance the interaction of MLK with both receptors, leading to lower binding energies compared to the native MLK structure. In the DRD2 dataset (
Figure 6A), MLK_MOD-43, MLK_MOD-27, and MLK_MOD-42 exhibited the most favorable binding scores, characterized by significantly improved HADDOCK scores and binding energies. These modifications demonstrated stronger molecular interactions within the receptor’s binding pocket, likely due to enhanced hydrophobic interactions, improved steric complementarity, and optimized electrostatic forces, which contribute to greater complex stability. A similar trend was observed in the 5-HT1A docking results (
Figure 6B), where MLK_MOD-42, MLK_MOD-21, and MLK_MOD-43 emerged as the top-performing modifications. Notably, these modifications aligned closely with the binding pose of Buspirone, a well-established 5-HT1A agonist, suggesting that they may mimic the natural binding mode of known serotonergic ligands. The overlap in top-performing modifications across both DRD2 and 5-HT1A suggests that certain structural features contribute to dual receptor targeting. This dual interaction profile implies that specific MLK derivatives could be designed to selectively modulate both dopaminergic and serotonergic pathways, potentially reducing MLK’s neuropsychiatric risks. By strategically modifying MLK’s molecular structure, it may be possible to enhance its interaction with 5-HT1A while acting as an antagonist to DRD2, thereby mitigating adverse neuropsychiatric outcomes while preserving or even enhancing its therapeutic efficacy.
To further investigate the molecular forces governing MLK modifications' binding to DRD2,
Figure 6C presents a correlation analysis between binding energy (kcal/mol) and its key energy components: van der Waals energy, electrostatic energy, desolvation energy, and restraints violation energy. Understanding these correlations is critical for identifying which interactions should be minimized to reduce MLK's neuropsychiatric effects. The strongest correlation was observed between van der Waals interactions and binding energy (correlation coefficient: 0.53), indicating that hydrophobic interactions significantly contribute to MLK's binding to DRD2. Since the DRD2 binding pocket is largely hydrophobic, ligands with highly lipophilic features tend to have stronger affinities. This suggests that reducing the hydrophobicity of MLK modifications may weaken its interaction with DRD2, which could be an effective strategy for reducing its neuropsychiatric risks. Electrostatic interactions also contributed to DRD2 binding, but to a lesser extent (correlation coefficient: 0.26). This suggests that while polar interactions can enhance binding, they are not the primary driver of MLK’s affinity for DRD2. Interestingly, desolvation energy (correlation coefficient: -0.050) and restraints violation energy (correlation coefficient: -0.043) had minimal influence, suggesting that solvent effects and structural constraints are not major factors in MLK’s interaction with DRD2. From a drug design perspective, these findings imply that introducing polar or hydrophilic substitutions to MLK’s structure may help reduce DRD2 binding by disrupting its dominant hydrophobic interactions, thereby lowering its risk of neuropsychiatric side effects.
A similar correlation analysis for MLK modifications docked to 5-HT1A (
Figure 6D) revealed distinct interaction patterns compared to DRD2, highlighting the potential for selective optimization to enhance 5-HT1A binding while reducing DRD2 affinity. Unlike DRD2, electrostatic interactions were the dominant factor in 5-HT1A binding (correlation coefficient: 0.44). This suggests that MLK modifications with polar functional groups capable of forming hydrogen bonds and charge interactions may have enhanced serotonergic activity. Given that 5-HT1A activation is associated with anxiolytic and antidepressant effects, modifications that increase electrostatic interactions with this receptor may provide a protective effect against MLK-induced psychiatric symptoms. Desolvation energy (correlation coefficient: 0.25) and restraints violation energy (correlation coefficient: 0.21) also exhibited moderate correlations, indicating that ligand solvation effects and structural flexibility contribute to 5-HT1A binding. This suggests that MLK modifications should be designed to efficiently displace bound water molecules and adopt favorable conformations within the receptor pocket. Interestingly, van der Waals interactions showed a weak negative correlation with 5-HT1A binding affinity (-0.14). This contrasts with DRD2, where van der Waals interactions were the strongest contributor to binding. The negative correlation suggests that excessive hydrophobic interactions may be detrimental to 5-HT1A binding, possibly due to steric clashes or suboptimal orientation within the receptor site. Therefore, modifications that increase polarity and reduce hydrophobicity may help reduce DRD2 affinity while enhancing 5-HT1A binding, which is the ideal balance for lowering neuropsychiatric risks.
The residue interaction analysis provides critical insights into the molecular interactions between Montelukast and its modifications with DRD2 and serotonin 5-HT1A receptors (
Table 4). The standard antagonist Haloperidol and standard agonist Buspirone serve as benchmarks for evaluating the effectiveness of MLK modifications in targeting these receptors. Key interaction types analyzed include carbon-carbon (CC), carbon-oxygen (CO), carbon-nitrogen (CN), carbon-halogen (CX), oxygen-oxygen (OO), oxygen-halogen (OX), nitrogen-oxygen (NO), nitrogen-nitrogen (NN), nitrogen-halogen (NX), and halogen-halogen (XX) interactions. For DRD2, MLK_MOD-27 demonstrated the highest number of interactions across multiple categories, with 3813 CC interactions, 1400 CO interactions, and 1074 CN interactions. These values surpass those of Haloperidol (2782 CC, 945 CO, and 700 CN), indicating that MLK_MOD-27 forms a more extensive interaction network with DRD2. Notably, MLK_MOD-42 exhibited the lowest CX and NX interactions (3 and 2, respectively), suggesting a selective interaction pattern that may contribute to its role as a potential DRD2 antagonist. MLK_MOD-43 and MLK_MOD-22 also showed strong interactions, particularly in CC, CO, and CN bonding, which are essential for stabilizing the ligand-receptor complex. For 5-HT1A, MLK_MOD-42 exhibited the highest number of CC interactions (4104), along with a strong presence of CO (1407) and CN (1090) interactions. Compared to Buspirone (2650 CC, 888 CO, and 1268 CN), this suggests that MLK_MOD-42 may engage more effectively with the receptor’s binding pocket, potentially enhancing its agonistic properties. Similarly, MLK_MOD-21 and MLK_MOD-1 showed high interaction counts, reinforcing their potential as strong 5-HT1A modulators. Interestingly, MLK_MOD-43 also exhibited a robust interaction profile with 3524 CC interactions, comparable to the other top-performing modifications. The full molecular interaction profiles of Montelukast modifications with DRD2 and 5-HT1A receptors, compared to standard ligands, are available in
Supplementary Data S3.
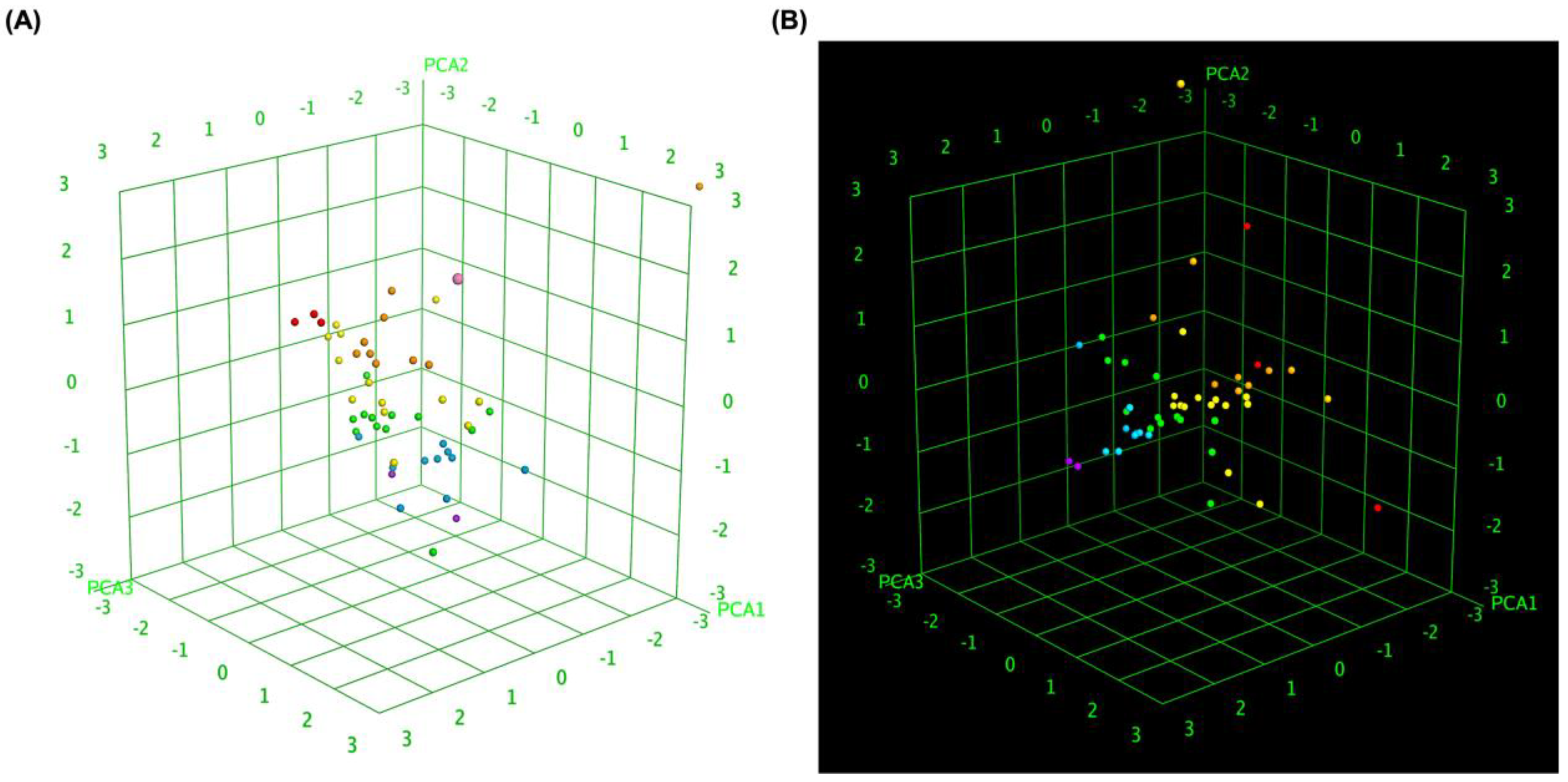
To further elucidate the relationship between molecular similarity and docking performance, 3D box plot analyses were conducted. These visualizations provided insights into how different MLK modifications interact with DRD2 and 5-HT1A, particularly in terms of their predicted activity, binding affinity, and statistical significance of docking scores. By normalizing the axes, the focus was primarily on the binding energy variations among the MLK derivatives, allowing for a comparative evaluation of their molecular modifications and docking efficiency.
Figure 7A illustrates the 3D box plot for MLK modification-DRD2 complexes, while
Figure 7B presents the same analysis for MLK modification-5-HT1A complexes. The distribution of data points in these plots reflects the degree of clustering and dispersion of molecular modifications based on their docking affinities. Notably, the MLK modification-5-HT1A complexes exhibited a more concentrated distribution of data points, suggesting a relatively consistent interaction profile across different modifications. This pattern implies that modifications of MLK targeting 5-HT1A share a more uniform binding conformation and energy landscape, potentially leading to more predictable pharmacological effects.
In contrast, the MLK modification-DRD2 complexes displayed a more dispersed distribution of data points, indicating greater variability in docking scores and binding interactions. This suggests that certain MLK modifications may exhibit stronger or weaker affinities toward DRD2, potentially influencing their role as antagonists in the dopaminergic pathway. To enhance interpretability, principal component analysis (PCA) was employed to reduce the dimensionality of the dataset while retaining the most critical descriptors influencing molecular interactions. PCA enables the projection of high-dimensional structure similarity descriptors into a 3D Euclidean space, capturing essential variations in molecular docking performance. The results revealed that MLK modification-5-HT1A complexes formed a more focused cluster, reinforcing their consistent binding properties. In contrast, MLK modification-DRD2 complexes showed a wider spread, indicating more diverse binding affinities and interaction patterns. These findings align with previous docking analyses, where specific MLK modifications exhibited dual activity at both DRD2 and 5-HT1A. The greater variability observed in the DRD2-targeted MLK modifications suggests that structural alterations significantly impact binding efficiency, which may be crucial for optimizing antagonist properties against DRD2 while preserving 5-HT1A agonistic activity.
2.3. Molecular Dynamics (MD) Simulations: Structural Stability and Interaction Evaluation
To assess the structural stability and molecular interactions of the MLK modifications with DRD2 and 5-HT1A receptors, MD simulations were performed. Key stability parameters, including the RMSD, root mean square fluctuation (RMSF), radius of gyration (RoG), and hydrogen bonding interactions, were analyzed to determine the binding stability and functional relevance of the modified ligands compared to standard reference compounds (
Table 5). The RMSD values provide insights into the overall stability of the ligand-receptor complexes throughout the simulation period. For DRD2-bound complexes, the apo-protein displayed the lowest average RMSD (1.806 Å), indicating its intrinsic stability without any ligand interference. The standard antagonist, Haloperidol, exhibited a higher RMSD (2.776 Å), signifying moderate conformational adjustments upon ligand binding. Among the MLK modifications, MLK_MOD-42 showed the lowest RMSD (2.145 Å), indicating greater structural stability in comparison to other modifications. MLK_MOD-43, MLK_MOD-27, and MLK_MOD-22 demonstrated slightly higher RMSD values (ranging from 2.262 Å to 2.674 Å), suggesting moderate flexibility but stable interactions within the receptor binding pocket. These results indicate that MLK_MOD-42 maintains the most stable interaction with DRD2, potentially acting as a strong antagonist similar to Haloperidol. For 5-HT1A-bound complexes, the apo-protein exhibited an average RMSD of 2.011 Å, indicating a relatively stable unbound structure. Buspirone, the standard agonist, had a slightly higher RMSD (2.487 Å), reflecting minor conformational adjustments upon binding. MLK_MOD-42 again showed the lowest RMSD (2.189 Å) among the modifications, indicating strong receptor stability and favorable binding. Other modifications, including MLK_MOD-24, MLK_MOD-21, and MLK_MOD-43, displayed RMSD values between 2.221 Å and 2.446 Å, suggesting moderate receptor stability. These results highlight that MLK_MOD-42 effectively stabilizes the 5-HT1A receptor, reinforcing its role as a potential 5-HT1A agonist.
The RoG values provide insights into the compactness of the ligand-receptor complexes, where lower RoG values indicate tighter packing and increased structural stability. In DRD2-bound complexes, the apo-protein exhibited an RoG of 2.105 Å, whereas Haloperidol increased the receptor's compactness with an RoG of 2.643 Å. Among the MLK modifications, MLK_MOD-42, MLK_MOD-22, and MLK_MOD-43 had RoG values ranging from 2.524 Å to 2.831 Å, suggesting stable binding-induced conformational changes. This supports the notion that MLK modifications efficiently engage with the DRD2 receptor, altering its structure in a manner similar to Haloperidol. For 5-HT1A-bound complexes, the RoG of the apo-protein was 2.345 Å, while Buspirone induced a slightly higher RoG (2.712 Å), suggesting receptor conformational changes upon binding. MLK_MOD-42, MLK_MOD-24, and MLK_MOD-21 demonstrated RoG values between 2.803 Å and 2.847 Å, indicating a similar level of receptor compaction and stabilization as Buspirone. These findings suggest that MLK modifications effectively modulate the 5-HT1A receptor in a manner consistent with its known agonists. Hydrogen bonding interactions are critical for ligand-receptor stabilization. In DRD2-bound complexes, Haloperidol formed two hydrogen bonds with the receptor. MLK_MOD-42 exhibited the highest number of hydrogen bonds (5), suggesting strong binding stability. MLK_MOD-13, MLK_MOD-43, and MLK_MOD-27 also formed multiple hydrogen bonds (ranging from 2 to 4), reinforcing their potential as DRD2 antagonists. For 5-HT1A-bound complexes, Buspirone formed only one hydrogen bond, whereas MLK_MOD-42, MLK_MOD-24, and MLK_MOD-1 established 2 to 3 hydrogen bonds, suggesting that these modifications enhance receptor stabilization. MLK_MOD-21 and MLK_MOD-43 formed fewer hydrogen bonds (1–2), but their overall docking stability and RMSD/RMSF profiles indicate effective receptor modulation.
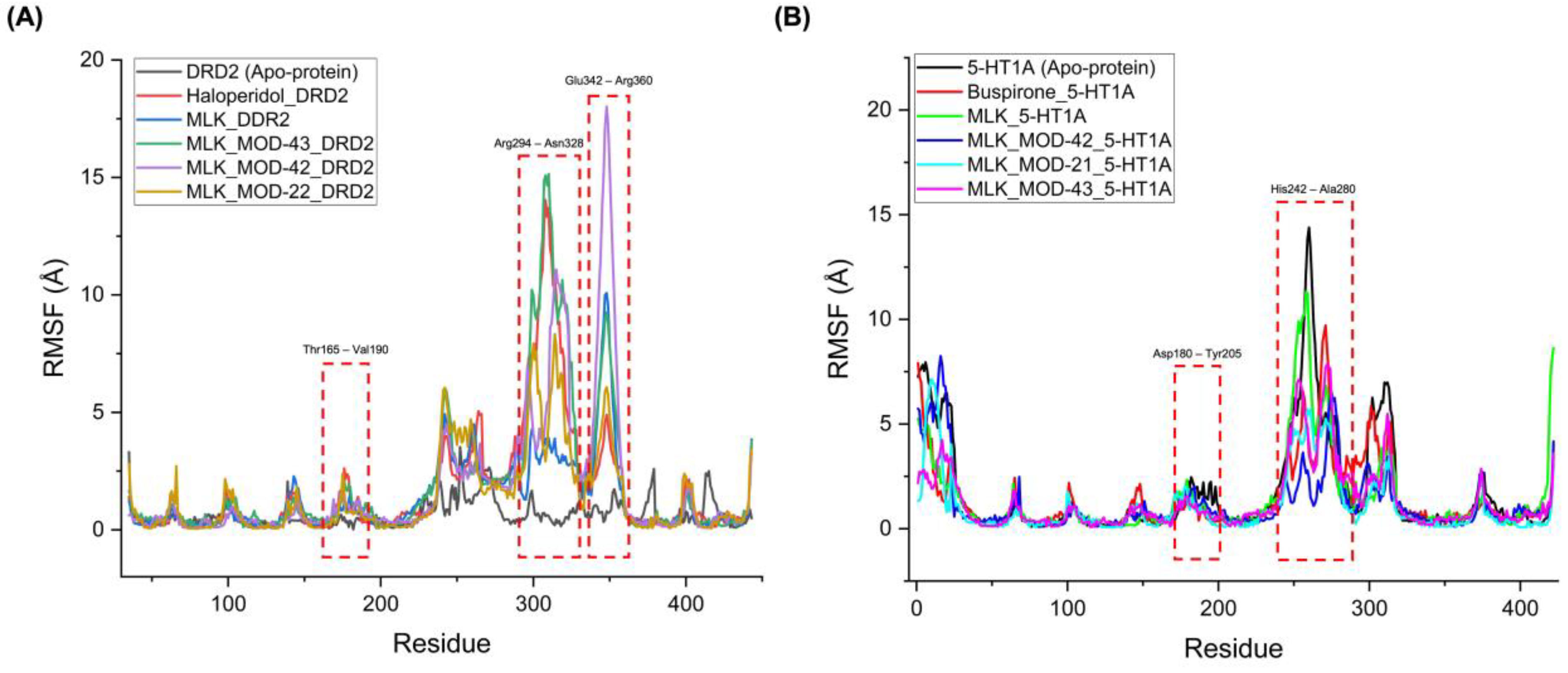
The RMSF analysis provides insights into the flexibility and dynamic behavior of specific amino acid residues upon ligand binding. Lower RMSF values generally indicate greater structural rigidity, while higher fluctuations suggest increased flexibility, which may correspond to conformational changes in the receptor-ligand complex. By comparing the RMSF profiles of different complexes, we can assess the impact of MLK modifications on receptor stability and functional interactions. For DRD2-bound complexes, the top-performing MLK modifications (MLK_MOD-43, MLK_MOD-42, and MLK_MOD-22) exhibited similar fluctuation patterns to Haloperidol in key interaction regions, particularly within the Thr165-Val190, Arg294-Asn328, and Glu342-Arg360 residues (
Figure 8A). These fluctuations suggest that MLK modifications can effectively disrupt native hydrogen bonding interactions within DRD2, mimicking the antagonist behavior of Haloperidol. Additionally, the MLK modifications demonstrated lower overall flexibility than the native MLK structure, reinforcing their enhanced stability and binding efficiency. In contrast, for 5-HT1A-bound complexes, the MLK modifications MLK_MOD-43, MLK_MOD-42, and MLK_MOD-21 displayed RMSF patterns comparable to Buspirone (
Figure 8B). The stabilization of critical interacting residues suggests that these modifications maintain hydrogen bonding networks necessary for receptor activation. The improved stability over the native MLK structure indicates that these modifications can effectively act as agonists, similar to Buspirone, thereby modulating the receptor's function.
The Molecular Mechanics/Poisson–Boltzmann Surface Area (MM/PBSA) approach was employed to calculate the free binding energy (ΔG_binding) of MLK modifications in complex with DRD2 and 5-HT1A receptors. The binding free energy of Haloperidol-DRD2 was calculated as −23.41 ± 3.24 kcal/mol, serving as the benchmark for antagonist binding. The unmodified MLK ligand (MLK_DDR2) displayed a less favorable binding energy of −19.32 ± 4.18 kcal/mol, suggesting weaker interaction with DRD2. However, MLK modifications significantly improved binding affinity. Among them, MLK_MOD-42_DRD2 exhibited the strongest binding with −31.92 ± 2.54 kcal/mol, outperforming Haloperidol. Other modifications, such as MLK_MOD-43_DRD2 (−27.37 ± 2.22 kcal/mol) and MLK_MOD-22_DRD2 (−26.81 ± 3.32 kcal/mol), also demonstrated enhanced binding affinities relative to Haloperidol, indicating their potential as effective DRD2 antagonists. These improvements in binding energy suggest that the MLK modifications enhance receptor interactions by optimizing hydrogen bonding, hydrophobic interactions, or conformational stability. The lower binding energy of MLK_MOD-42_DRD2, in particular, may be attributed to its ability to establish stronger intermolecular interactions within the DRD2 binding pocket. The results align with the MD findings, where these modifications exhibited structural stability and consistent interactions with key DRD2 residues. For the 5-HT1A receptor, Buspirone demonstrated a ΔG_binding of −27.92 ± 1.34 kcal/mol, serving as the reference agonist. The unmodified MLK ligand (MLK_5-HT1A) showed a weaker binding affinity (−20.14 ± 3.67 kcal/mol), indicating suboptimal interaction. However, MLK modifications substantially improved binding strength, with MLK_MOD-42_5-HT1A (−30.22 ± 2.29 kcal/mol) exhibiting the strongest binding affinity, surpassing Buspirone. Other modifications, such as MLK_MOD-43_5-HT1A (−28.19 ± 2.14 kcal/mol) and MLK_MOD-21_5-HT1A (−27.87 ± 3.38 kcal/mol), also displayed comparable or superior binding affinities relative to the standard agonist. These results indicate that specific MLK modifications can effectively stabilize the 5-HT1A receptor in an agonist-bound state, enhancing receptor activation. The stronger binding affinities may result from increased hydrogen bonding and π-π stacking interactions with key residues in the receptor binding pocket. These findings corroborate the MD results, which revealed that MLK_MOD-42_5-HT1A maintained stable hydrogen bonding patterns and RMSF values similar to Buspirone.
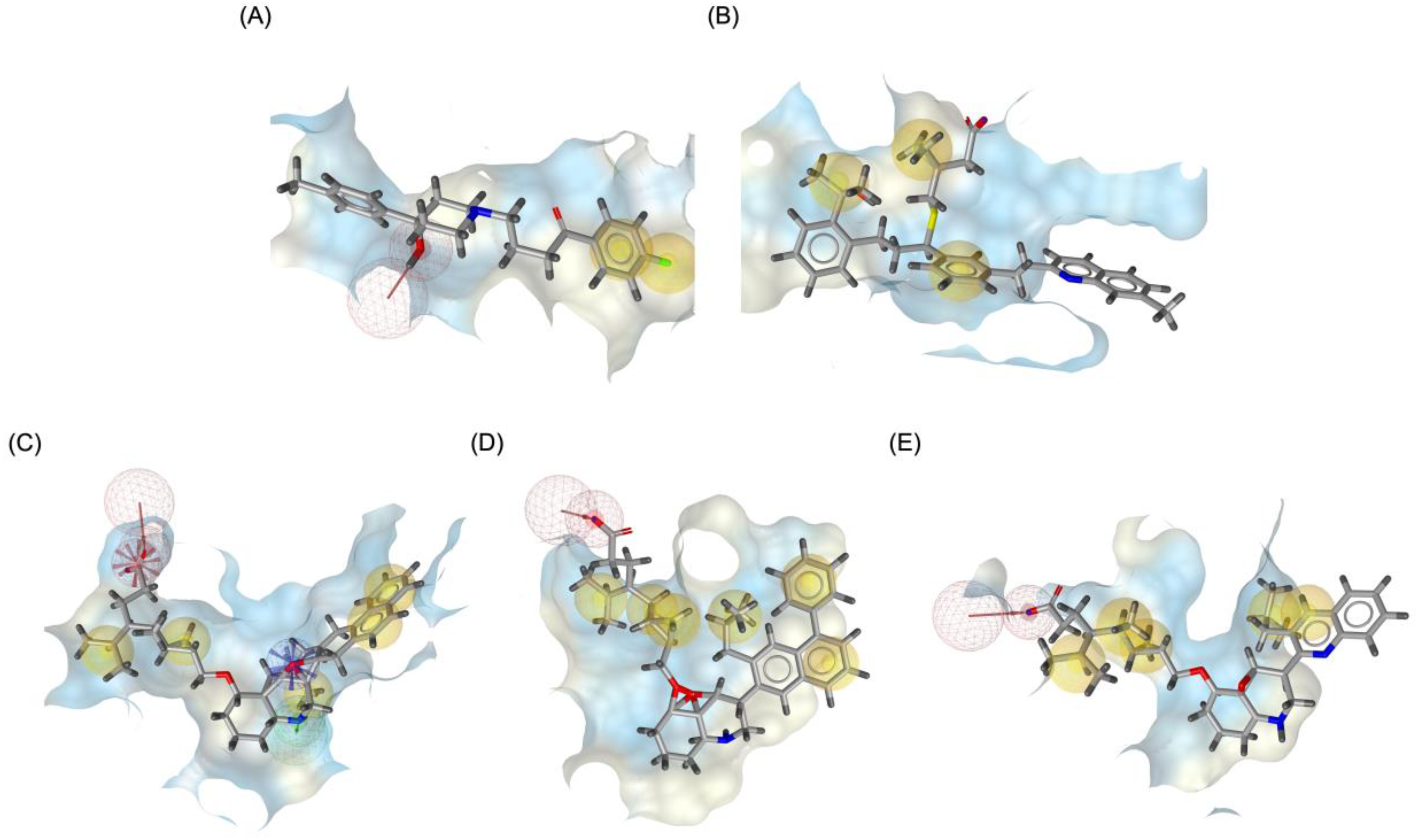
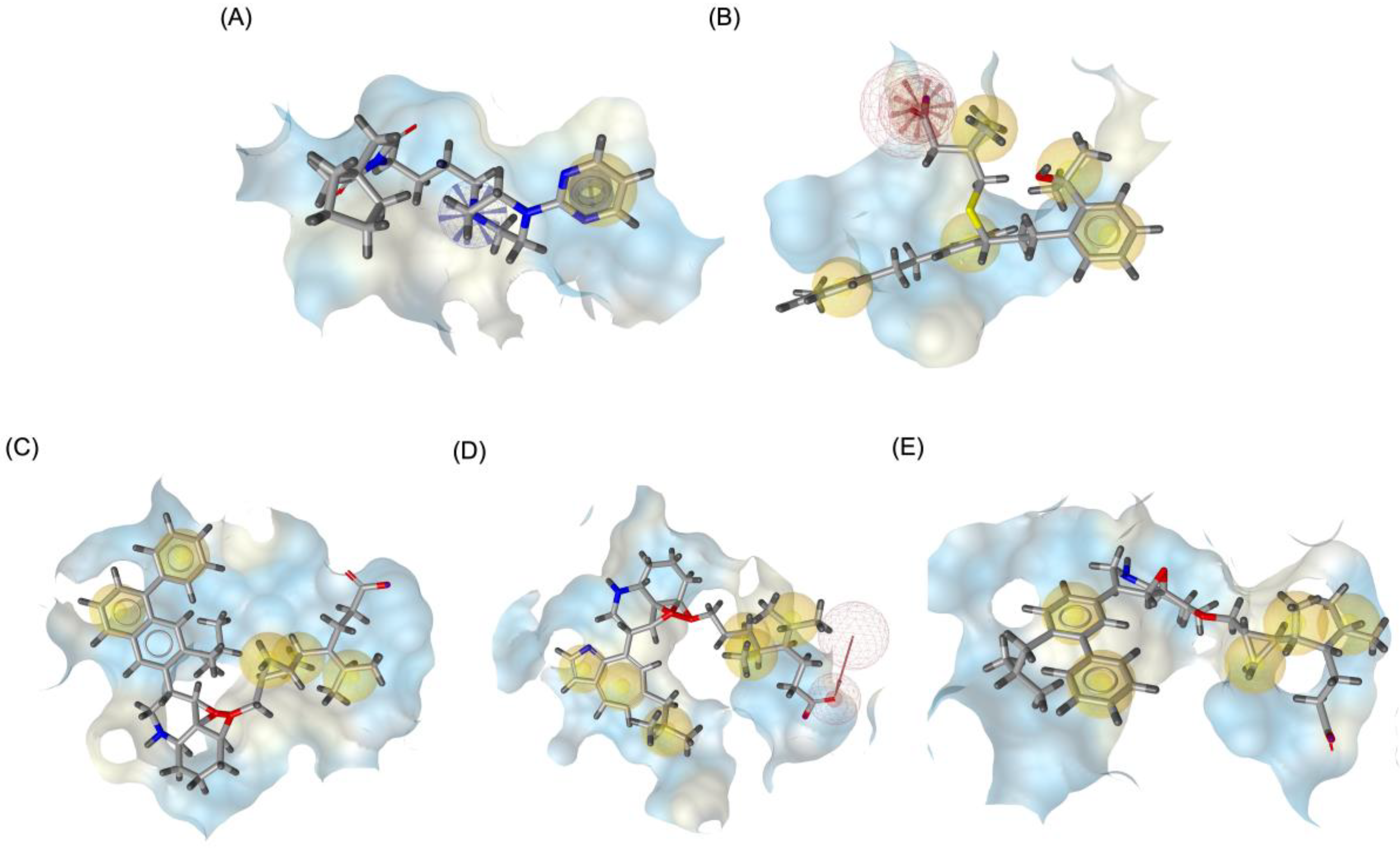
2.4. Pharmacophore Modeling and In-Silico ADMET Evaluation
Figure 9 illustrates the pharmacophore modeling of various ligand-receptor complexes, including the Haloperidol_DRD2, MLK_DDR2, and several modified MLK complexes with DRD2 and 5-HT1A receptors. The key pharmacophoric features observed in the molecular interactions include hydrophobic interactions (yellow spheres), hydrogen bond donors (green arrows), and hydrogen bond acceptors (red arrows). These features provide insights into the binding characteristics of the ligands and their ability to mimic the interaction profile of known standard drugs. In the case of DRD2 binding, the reference antagonist Haloperidol displayed a strong hydrogen bond acceptor (HBA) interaction through its hydroxyl groups with the active residues of the DRD2 receptor. This interaction is crucial for its antagonistic activity. Similarly, the modified MLK derivatives—MLK_MOD-43_DRD2, MLK_MOD-42_DRD2, and MLK_MOD-22_DRD2—exhibited comparable HBA interactions, predominantly through hydroxyl or carbonyl functional groups. These modifications significantly improved binding affinity and mimicked the pharmacophore features of Haloperidol. In contrast, the native MLK did not form any hydrogen bonds and interacted only via hydrophobic forces, indicating a weaker or different mode of interaction. The increased number of hydrophobic interactions in MLK modifications, particularly due to the presence of benzene rings and additional functional groups, suggests that these derivatives successfully replicated the binding characteristics of Haloperidol and could potentially function as DRD2 antagonists.
For the 5-HT1A receptor interactions, the standard agonist Buspirone did not exhibit any hydrogen bonding interactions and relied solely on hydrophobic interactions for binding. Interestingly, the native MLK ligand, in contrast to Buspirone, displayed hydrogen bond interactions, which might suggest a deviation from the ideal pharmacophore model of a 5-HT1A agonist (
Figure 10). However, the modified MLK derivatives, specifically MLK_MOD-42 and MLK_MOD-43, successfully adopted a pharmacophore profile similar to that of Buspirone, as they primarily exhibited hydrophobic interactions without forming hydrogen bonds. This structural adaptation indicates that these MLK modifications could effectively mimic the agonistic properties of Buspirone and enhance receptor binding.
The ADME analysis was performed to evaluate the pharmacokinetic properties, drug-likeness, and metabolic stability of Montelukast and its derivatives. The native compound, MLK, exhibited two violations of Lipinski’s Rule of Five (MW >500 and MlogP >4.15), which were retained in most modified derivatives, except for MLK_MOD-6, which adhered completely to Lipinski’s criteria. The reduction in molecular weight for MLK_MOD-6 suggests potential improvements in oral bioavailability and permeability. MlogP, a measure of lipophilicity, varied across the derivatives, with MLK_MOD-6 exhibiting the lowest value (3.94), while MLK_MOD-42 had the highest (6.49). High lipophilicity is associated with better membrane permeability but may lead to poor aqueous solubility and increased risk of non-specific interactions. Notably, MLK_MOD-6 remained within Lipinski’s recommended threshold, which might enhance its pharmacokinetic profile. The total polar surface area (TPSA) ranged from 71.63 Ų (MLK_MOD-35) to 99.62 Ų (MLK_MOD-12), with values below 140 Ų suggesting good cell permeability.
Table 7.
ADME properties and CYP inhibition profiles of Montelukast (MLK) and its derivatives.
Table 7.
ADME properties and CYP inhibition profiles of Montelukast (MLK) and its derivatives.
| Molecule |
MW (g/mol) |
MlogP |
HBA |
HBD |
TPSA (Å2) |
Lipinski Violation |
CYP Inhibitor |
| Montelukast (MLK) |
586.18 |
5.70 |
4 |
2 |
95.72 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2D6, CYP3A4 |
| MLK_MOD-1 |
551.72 |
5.65 |
4 |
2 |
79.39 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2D6, CYP3A4 |
| MLK_MOD-6 |
491.62 |
3.94 |
5 |
2 |
92.53 |
0 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-12 |
517.66 |
4.55 |
5 |
3 |
99.62 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-13 |
519.65 |
5.46 |
5 |
2 |
79.39 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-21 |
552.70 |
4.69 |
5 |
2 |
92.28 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-22 |
552.70 |
4.93 |
5 |
2 |
92.28 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-35 |
570.76 |
4.61 |
4 |
1 |
71.63 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2D6, CYP3A4 |
| MLK_MOD-36 |
527.70 |
5.21 |
4 |
2 |
97.54 |
2 violations: MW >500, MLOGP >4.15 |
CYP2C19, CYP2C9, CYP2D6, CYP3A4 |
| MLK_MOD-42 |
627.81 |
6.49 |
4 |
2 |
79.39 |
2 violations: MW >500, MLOGP >4.15 |
CYP2D6 |
| MLK_MOD-43 |
601.77 |
6.18 |
4 |
2 |
79.39 |
2 violations: MW >500, MLOGP >4.15 |
CYP2D6 |
The number of hydrogen bond acceptors (HBA) and donors (HBD) varied across the derivatives. MLK_MOD-6, MLK_MOD-12, MLK_MOD-13, MLK_MOD-21, and MLK_MOD-22 exhibited a slight increase in HBA compared to the native MLK. Hydrogen bonding influences solubility, receptor interactions, and bioavailability, with modifications in HBA and HBD impacting molecular interactions. The presence of functional groups influencing hydrogen bonding was particularly evident in MLK_MOD-12, which had the highest TPSA, suggesting a balance between permeability and solubility. MLK was predicted to inhibit CYP2C19, CYP2D6, and CYP3A4, indicating potential drug-drug interaction (DDI) risks due to interference with metabolic enzymes. Interestingly, most derivatives exhibited a similar CYP inhibition profile, except MLK_MOD-6, which also inhibited CYP2C9, broadening its potential metabolic liabilities. MLK_MOD-42 and MLK_MOD-43 exclusively inhibited CYP2D6, potentially reducing metabolic interactions with CYP3A4 and CYP2C19 substrates. The inhibition of multiple CYP isoforms suggests that most MLK derivatives retain potential metabolic concerns, possibly leading to altered drug clearance and pharmacokinetics. However, MLK_MOD-6 emerges as a promising modification due to its balanced physicochemical properties, improved compliance with Lipinski’s rule, and distinct CYP inhibition profile. The pharmacokinetic properties of MLK derivatives indicate that structural modifications significantly impact drug-likeness, metabolism, and potential bioavailability. MLK_MOD-6 stands out as the most promising modification due to its optimal molecular weight, acceptable lipophilicity, favorable hydrogen bonding profile, and lack of Lipinski violations. This suggests a potential improvement in oral bioavailability and metabolic stability. Conversely, MLK_MOD-42 and MLK_MOD-43, despite high lipophilicity, may offer advantages in receptor interactions, as indicated by pharmacophore modeling. Their selective inhibition of CYP2D6 reduces interactions with CYP3A4 and CYP2C19, potentially mitigating some metabolic concerns. The full ADME properties and CYP inhibition profiles of Montelukast and its derivatives are available in Supplementary Data S4.
Table 8.
Predicted drug-likeness and toxicity profiles of Montelukast (MLK) derivatives.
Table 8.
Predicted drug-likeness and toxicity profiles of Montelukast (MLK) derivatives.
| Molecule |
Drug-likeness |
Mutagenic |
Tumorigenic |
Reproductive Effective |
Irritant |
| MLK_MOD-1 |
1.433 |
None |
None |
High |
None |
| MLK_MOD-6 |
2.669 |
High |
None |
None |
None |
| MLK_MOD-12 |
1.433 |
None |
None |
High |
None |
| MLK_MOD-13 |
0.093 |
None |
None |
High |
None |
| MLK_MOD-21 |
2.031 |
None |
None |
None |
None |
| MLK_MOD-22 |
1.433 |
None |
None |
None |
None |
| MLK_MOD-35 |
3.371 |
None |
None |
High |
High |
| MLK_MOD-36 |
1.504 |
None |
None |
High |
None |
| MLK_MOD-42 |
4.862 |
None |
None |
High |
None |
| MLK_MOD-43 |
1.433 |
None |
None |
High |
None |
The ADME-based toxicity predictions provide critical insights into the safety profiles of Montelukast derivatives. A higher drug-likeness score generally suggests a better potential for oral bioavailability, but extreme values may indicate deviations from optimal pharmacokinetic properties. Among all modifications, MLK_MOD-42 and MLK_MOD-43 emerged as the most favorable and consistent modifications. MLK_MOD-42 exhibited the highest drug-likeness score (4.862), indicating excellent pharmacokinetic potential. Similarly, MLK_MOD-43 maintained a favorable drug-likeness score of 1.433 while avoiding mutagenic, tumorigenic, or irritant risks. Both derivatives displayed no mutagenicity or tumorigenicity concerns, reinforcing their suitability for drug development. The primary concern with these compounds is their potential for reproductive toxicity, which should be further investigated to ensure long-term safety. In terms of toxicity, mutagenic and tumorigenic risks were minimal across all MLK derivatives. Only MLK_MOD-6 was flagged as having a high mutagenic risk, which may indicate potential concerns regarding its genomic stability and necessitate further in vitro and in vivo validation. Notably, none of the derivatives exhibited tumorigenic properties, reinforcing their suitability for drug development. However, the reproductive toxicity risk remains a concern for several derivatives, particularly MLK_MOD-1, MLK_MOD-12, MLK_MOD-13, MLK_MOD-35, MLK_MOD-36, MLK_MOD-42, and MLK_MOD-43, all of which showed a high likelihood of reproductive effects. This suggests that careful evaluation is needed to assess potential endocrine disruption or teratogenicity. The irritation potential of the MLK derivatives was generally low, with only MLK_MOD-35 displaying both high reproductive toxicity and irritation risk. This could limit its therapeutic applicability unless formulation strategies can mitigate these effects. Overall, while most MLK derivatives exhibit favorable drug-likeness and low mutagenicity or tumorigenicity, the reproductive effects in some candidates warrant further investigation. The full predicted drug-likeness and toxicity profiles of Montelukast derivatives are available in Supplementary Data S5.