Submitted:
15 March 2025
Posted:
17 March 2025
You are already at the latest version
Abstract
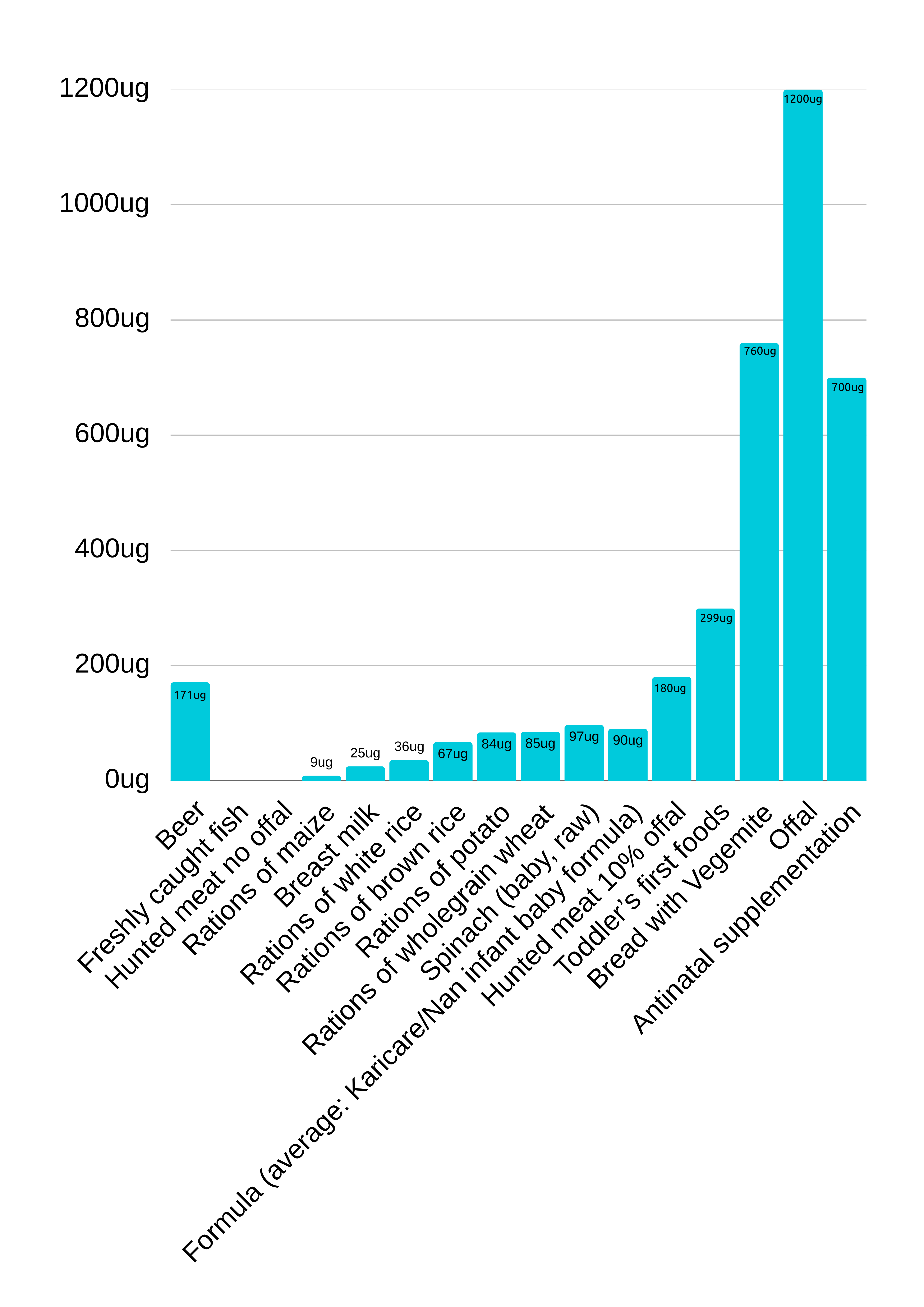
Keywords:
1. Introduction
1.1. Folate
2. Methods
2.1. Limitations
2.2. Estimated Servicing Sizes and Justification
2.2.1. Potato
2.2.2. Milk
2.2.3. Whole Grain and Hulled Wheat, Rice and Maize
2.2.4. Animal Protein Estimates
2.2.5. Breads and Fortified Foods
2.2.6. Cereal
2.2.7. Beer
3. Analysis
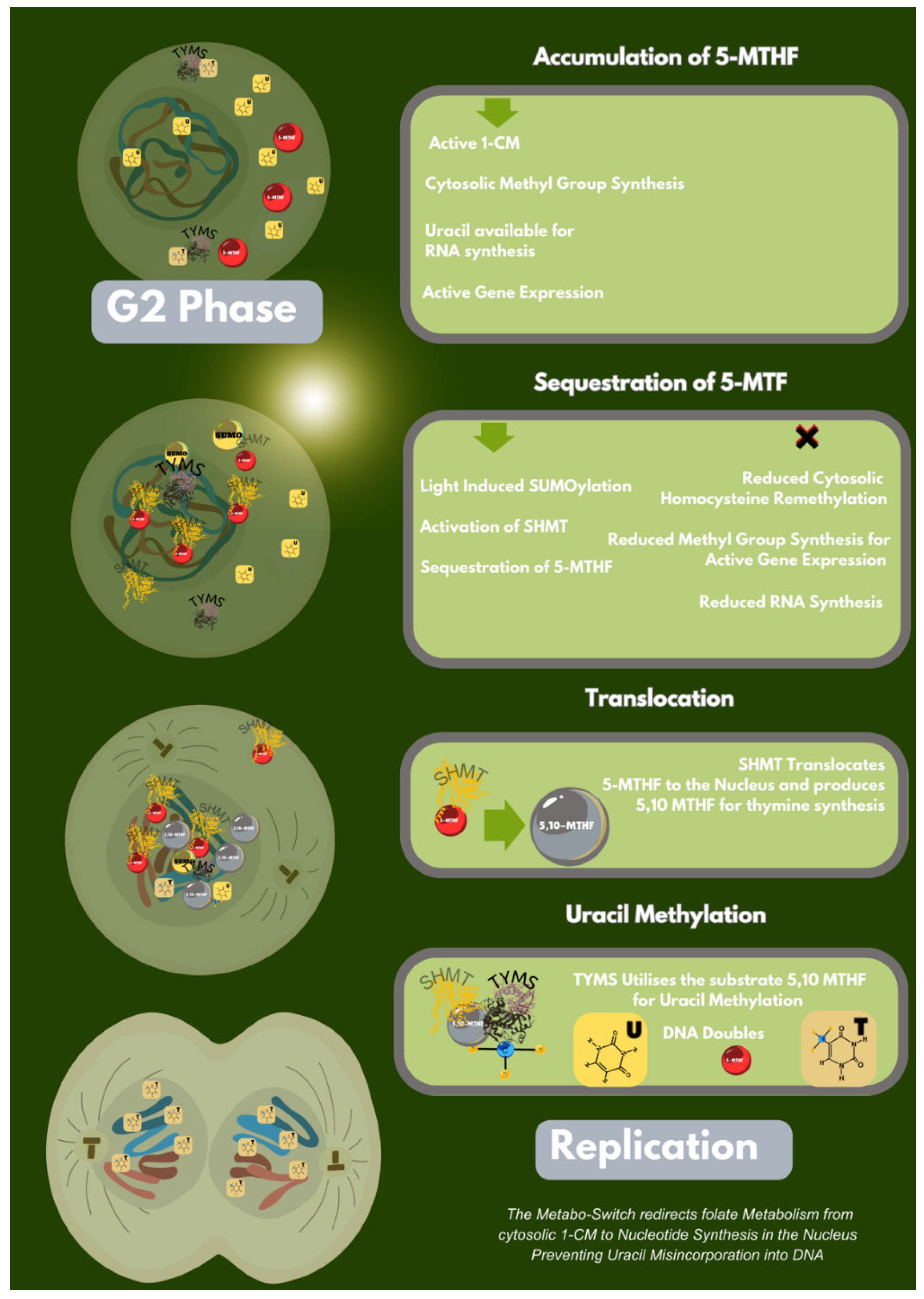
3.1. Compartmentalisation
3.2. Uracil
3.3. Thymine
3.4. Purine and Pyrimidine Synthesis
3.4. Folate Analogues, Compartmentalisation and Nucleotide Synthesis for DNA Replication
3.5. Thymine Synthesis from One Carbon Metabolism
3.6. Compartmentalisation of Thymine Synthesis
3.7. A Metabo-Switch Between Nucleotide Synthesis, Cell Cycle and Methylation.
3.8. Light Dependant Epigenetics and the Metabo-Switch
3.9. Early Folate Quantification
3.9.1. In-Vitro
3.9.2. In-Vivo
4. Results
5. Discussion
5.1. Public Health Implications and Future Directions
6. Conclusion
References
- Albala, K. Eating right in the renaissance; University of California Press; Los Angeles, 2002. [Google Scholar]
- Anderson, D. D.; Woeller, C. F.; et al. Serine hydroxymethyltransferase anchors the de novo thymidylate synthesis pathway to the nuclear lamina for DNA synthesis. Journal of Biological Chemistry 2012, 287(10), 7051–7062. [Google Scholar] [CrossRef] [PubMed]
- Armelagos, G. J. Brain evolution, the determinants of food choice, and the omnivore’s dilemma. Critical Reviews in Food Science and Nutrition 2014, 54(10), 1330–1341. [Google Scholar] [CrossRef]
- Carnivores, Australian. Carnivore diet: Meals and portion sizes. n.d. Available online: https://www.australiancarnivores.com.au/meals-portion-sizes.
- Australian Institute of Health and Welfare. Mandatory folic acid and iodine fortification in Australia and New Zealand: Baseline report for monitoring. AIHW. 2011. Available online: https://www.aihw.gov.au/getmedia/1a4bb10d-dba2-479e-b99f-abefcfaf62d1/10787.pdf.
- Avagliano, L.; Massa, V.; George, T. M.; Qureshy, S.; Bulfamante, G. P.; Finnell, R. H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Research 2019, 111(19), 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Baggott, JE; Vaughn, WH. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase adenosine deaminase and 5’-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J 1986, 236. [Google Scholar]
- Blount, B. C.; Mack, M. M.; et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Medical Sciences 1997, 94. [Google Scholar]
- Capasso, C; Supuran, TC. Bonev, BB, Brownnm, Eds.; Bacterial Resistance to Antibiotics–From Molecules to Man, Dihydropteroate synthase (sulfonamides) and dihydrofolate reductase inhibitors; Wiley Online Library, 2019. [Google Scholar]
- Clapham, N. Seventh-day Adventists in the South Pacific 1885-1985: Australia New Zealand South Sea Islands, SignsdoPub C, 1985.
- Cooper, LF. The new cookery: A book of recipes most of which are in use at the Battle Creek Sanitarium; The Good Health Pub Co, 1913. [Google Scholar]
- Do, H.; Takemoto, S.; Tomonaga, S. Folic acid supplementation during the fattening period affects growth and nutritional metabolism in Japanese Black beef cattle. Scientific Reports 2024, 14, 24653. [Google Scholar] [CrossRef]
- Ducker, G. S.; Rabinowitz, J. D. ZMP: A master regulator of 1-CM. Molecular Cell 2015, 57(2), 203–204. [Google Scholar] [CrossRef]
- Elevit. Elevit products. Elevit. 2025. Available online: https://www.elevit.com.au/products/elevit.
- Engelbrecht, HR; Merrill, SM. (2022) Sex differences in epigenetic age in Mediterranean high longevity regions. Front Aging 3. [CrossRef]
- Editors of Council on Foreign Relations Abortion law: Global comparisons;Council on Foreign Relations, 2023.
- Australian Dietary Guidelines. In Editors at Nutrition Australia; Nutrition Australia, 2013.
- Editors of Food Standards Australia and New Zealand Australian Food Composition Database–Release, 2020.
- FSANZ. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd.
- Fehling, C.; Jägerstad, M.; et al. Reduction of folate levels in the rat: Difference in depletion between the central and peripheral nervous systems. Zeitschrift für Ernährungswissenschaft 1976, 15(1), 1–8. [Google Scholar] [CrossRef]
- Fox, J. T.; Shin, W. K.; et al. A UV-responsive internal ribosome entry site enhances serine hydroxymethyltransferase 1 expression for DNA damage repair. Journal of Biological Chemistry 2009, 284(45), 31097–31108. [Google Scholar] [CrossRef]
- George, L. Plasma folate levels and the risk of spontaneous abortion. JAMA Network Open 2002, 288(15), 1867. [Google Scholar] [CrossRef]
- Genome Reference Consortium. Human Build 38 patch release 14 (GRCh38.p14): Mat2A, 2022.
- Graham, S. Lectures on the science of human life. In The Office of the Health reformer. Battle Creek SR Wells and Company Publishers; New Yor.
- Haas, VH; Stewart, SE. ) Folic acid deficiency and the sparing of mice infected with the virus of lymphocytic choriomeningitis. Virology 1957, 3. [Google Scholar]
- Haas, M.; Schreiber, M.; Mascher, M. Domestication and crop evolution of wheat and barley: Genes, genomics, and future directions. Journal of Integrative Plant Biology 2018, 60(12), 1067–1080. [Google Scholar] [CrossRef]
- Hancock, A. M.; Witonsky, D. B.; et al. Human adaptations to diet, subsistence, and ecoregion are due to subtle shifts in allele frequency. Proceedings of the National Academy of Sciences 2010, 107(2), 8924–8930. [Google Scholar] [CrossRef]
- Hecht, S.; Shlaer, S.; et al. Energy quanta and vision. Journal of General Physiology 1942, 25(6), 819–840. [Google Scholar]
- Hedrick, P. W. Population genetics of malaria resistance in humans. Heredity 2011, 107(4), 283–304. [Google Scholar] [CrossRef]
- Hooton, E. A. The evolution and devolution of the human face. American Journal of Orthodontics and Oral Surgery 1946, 32(41). [Google Scholar]
- Hühner, J; Ingles-Prieto, Á. Quantification of riboflavin flavin mononucleotide and flavin adenine dinucleotide in mammalian model cells by CE with LED-induced fluorescence detection: CE and CEC. Electrophoresis 2015, 36(4), 518–525. [Google Scholar] [CrossRef]
- Igari, S; Ohtaki, A. Properties and crystal structure of methylenetetrahydrofolate reductase from thermus thermophilus HB8. PLos One 2011, 6(8), e23716. [Google Scholar] [CrossRef]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 2000, 2(1), 27–30. [Google Scholar] [CrossRef]
- Kellogg, JH. Are we to be a toothless race? Dent Regist 53(3), 135–144. [PubMed]
- Kellogg, JH. The New Dietetics, A guide to scientific feeding in Health and Disease; The Modern Medical Publishing Company; Revised Edition, 1927. [Google Scholar]
- Kiefer, CM; Hou, C. Epigenetics of β-globin gene regulation. Mutat Res 2008, 647(1-2), 68–76. [Google Scholar] [CrossRef]
- Kim, Y.; Bertagna, F.; et al. Quantum biology: An update and perspective. Quantum Reports 2021, 3(1), 80–126. [Google Scholar]
- Kisliuk, RL; Gaumont, Y. ) Polyglutamyl derivatives of folate as substrates and inhibitors of thymidylate synthetase. J Biol Chem 1974, 249(13), 4100–4103. [Google Scholar] [CrossRef]
- Kommissionen for Ledelsen af de Geologiske og Geografiske Undersøgelser i Grønland, Meddelelser om Grønland (1915) København C, A, Reitzels Forlag 49.
- Koren, D; Orbán, C; Galló, N; Kun, S; Vecseri-Hegyes, B; Kun-Farkas, G. Folic acid content and antioxidant activity of different types of beers available in Hungarian retail. J Food Sci Technol 2017, 54(5), 1158–1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A. Extraction and determination of folates in foods and the fortification of rice with folic acid; University of New South Wales, 2003. [Google Scholar] [CrossRef]
- Laiño, JE; LeBlanc, JG. Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Can J Microbiol 2012, 5(5), 581–588. [Google Scholar] [CrossRef]
- Li, J.; Fan, W.; Wang, X.; Hou, X.; Chen, Z.; Lv, M. Mental health in early pregnancy and spontaneous abortion risk: A prospective cohort study. Alpha Psychiatry 2024, 25(5), 648–655. [Google Scholar] [CrossRef]
- Lynn, R.; Harvey, J. The decline of the world’s IQ. Intelligence 2008, 36(2), 112–120. [Google Scholar] [CrossRef]
- MacFarlane, A. J.; Anderson, D. D.; et al. Nuclear localization of de novo thymidylate biosynthesis pathway is required to prevent uracil accumulation in DNA. Journal of Biological Chemistry 2011, 286(51), 44015–44022. [Google Scholar]
- Noonan, S. C.; Savage, G. P. Oxalate content of foods and its effect on humans. Asia Pacific Journal of Clinical Nutrition 1999, 8(1), 64–74. [Google Scholar] [PubMed]
- New South Wales Ministry of Health. Starting family foods: Guidelines for introducing solid foods to infants (Publication No. GL2020_012). NSW Health. 2020. Available online: https://www.health.nsw.gov.au/heal/Publications/starting-family-foods.pdf.
- Offer, T; Ames, BN. ) 5-Methyltetrahydrofolate inhibits photosensitization reactions and strand breaks in DNA. FASEB J 2007, 21(9), 2101–2107. [Google Scholar] [CrossRef]
- Organization of Teratology Information Specialists (OTIS)Folic acid Folate. Mother To Baby Fact Sheets. January 2024. Retrieved from. Available online: https://www.ncbi.nlm.nih.gov/books/NBK582717/.
- Paone, A; Marani, M. SHMT1 knockdown induces apoptosis in lung cancer cells by causing uracil misincorporation. Cell Death Dis 2014, 5(11). [Google Scholar]
- Pensary, LA. 1903) A retrospect of the Irish poor law dispensary lunatic asylum and workhouse systems, hospitals for paying patients. Br Med J 1(2192), 27–28.
- Ribera, A.; van Treuren, R.; Kik, C.; et al. On the origin and dispersal of cultivated spinach (Spinacia oleraceaL) Genetic Resources and Crop Evolution. 2021, 68, 1023–1032. [Google Scholar] [CrossRef]
- Rios-Leyvraz, M.; Yao, Q. The volume of breast milk intake in infants and young children: A systematic review and meta-analysis. Breastfeeding Medicine 2023, 18(3), 188–197. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, DS; Erbe, RW. Methylenetetrahydrofolate reductase in cultured human cells, II, Genetic and biochemical studies of methylenetetrahydrofolate reductase deficiency. Pediatr Res 1977, 11(11), 1141–1143. [Google Scholar] [CrossRef]
- Shannon, C. Acculturation: Aboriginal and Torres Strait Islander nutrition. Asia Pac J Clin Nutr 2002, 11. [Google Scholar]
- Sharma, C; Gulati, S. Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples. 3 Biotech 2017, 7(1), 53. [Google Scholar] [CrossRef]
- Sedley, L. Advances in nutritional epigenetic a fresh perspective for an old idea; lessons learned limitations and future directions. Epigenet Insights 2020, 13. [Google Scholar] [CrossRef]
- Sedley, L. Dinan T, Ed.; Epigenetics. In Nutritional psychiatry: a primer for clinicians; Cambridge University Press; Cambridge, 2023. [Google Scholar]
- Sedley, L. Illuminating the Connection: Breastfeeding, Lactose Intolerance. and Early Life Disease Prevention 2025. [Google Scholar] [CrossRef]
- Sedley, L. The Epigenetics and Biological Clock of Skin Cancer. 2025. [Google Scholar] [CrossRef]
- Shane, B. Folate status assessment history: Implications for measurement of biomarkers in NHANES. Am J Clin Nutr 2011, 94(1), 337S–342S. [Google Scholar] [PubMed]
- Sherriff, S; Kalucy, D; et al. Murradambirra Dhangaang (make food secure) Aboriginal community and stakeholder perspectives on food insecurity in urban and regional Australia. BMC Public Health 2022, 22(1). [Google Scholar]
- Shurtleff, W.; Aoyagi, A. History of Seventh-day Adventist Work with Soyfoods Vegetarianism Meat (1863-2013); Soyinfo Centre; Lafayette, 2014. [Google Scholar]
- Editors of the Online Etymology Dictionary (2023) Online Etymology Dictionary, Humble. Available online: https://www.etymonline.com/word/humble.
- Valeggia, CR; Snodgrass, JJ. Health of indigenous peoples. Annu Rev Anthropol 2015, 44(1), 117–135. [Google Scholar] [CrossRef]
- Visentin, M; Zhao, R. The Antifolates. Hematol Oncol Clin North Am 2012, 26(3), 629–648. [Google Scholar] [CrossRef]
- Ward, G. J.; Nixon, P. F. Modulation of pteroylpolyglutamate concentration and length in response to altered folate nutrition in a comprehensive range of rat tissues. J Nutr 1990, 120(5), 476–484. [Google Scholar]
- Webster, L. T.; Pritchett, I. W. Microbic virulence and host susceptibility in paratyphoid-enteritidis infection of white mice. J Exp Med 1924, 40(3), 397–404. [Google Scholar]
- World Health Organization. Autism. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/autism.
- Williams, J.; Mai, C. T.; et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morbidity and Mortality Weekly Report 2015, 64(1), 1–5. [Google Scholar] [CrossRef]
- Wilson, B. C. Dr, John Harvey Kellogg and the Religion of Biologic living; Indiana University Press, Bloomington, 2014. [Google Scholar]
- Wyndham, C. H.; McPherson, R. K.; Munro, A. Reactions to heat of Aborigines and Caucasians. J Appl Physiol 1964, 19(6), 1055–1058. [Google Scholar] [CrossRef]
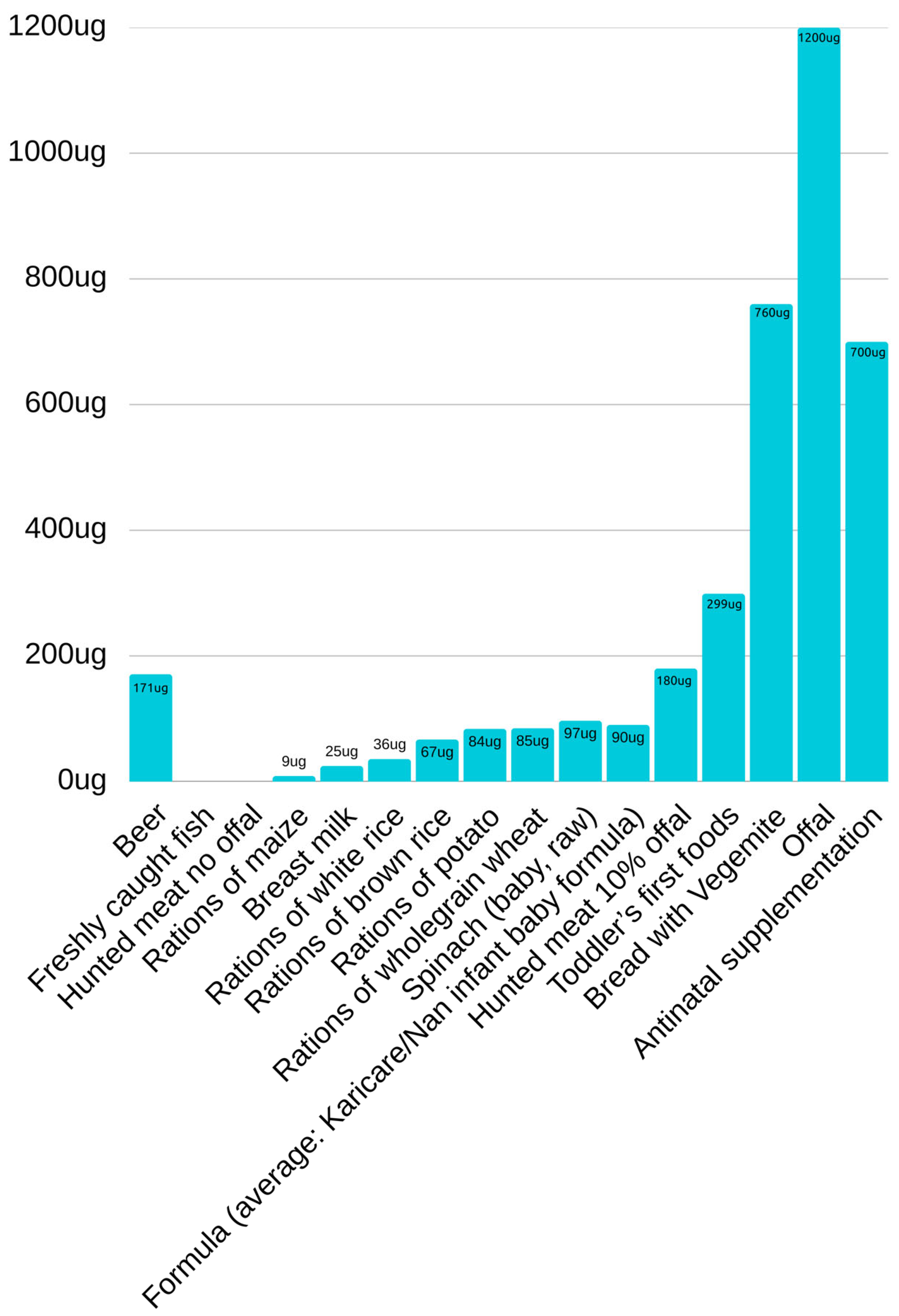
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




