Submitted:
11 March 2025
Posted:
12 March 2025
You are already at the latest version
Abstract
The yeast Saccharomyces cerevisiae is the paradigm of a eukaryotic model organism. In virtue of a substantial degree of functional conservation, it has been extensively exploited to understand multiple aspects of the genetics, molecular and cellular biology of human disease. Many aspects of cell signaling in cancer, aging or metabolic diseases, have been tackled in yeast. Here, we review the strategies undertaken through the years for the development of humanized yeast models to study regulated cell death (RCD) pathways in general, and specifically those related to innate immunity and inflammation, with emphasis in pyroptosis and necroptosis. Such pathways involve the assembly of distinct modular signaling complexes such as the inflammasome and the necrosome. Like other supramolecular organizing centers (SMOCs), such intricate molecular arrangements trigger the activity of enzymes, like caspases or protein kinases, culminating in the activation of lytic pore-forming final effectors, respectively gasdermin D (GSDMD) in pyroptosis and MLKL in necroptosis. Even though pathways related to those governing innate immunity and inflammation in mammals are missing in fungi, heterologous expression of their components in the S. cerevisiae model provides a “cellular test tube” to readily study their properties and interactions, thus constituting a valuable tool for finding novel therapies.
Keywords:
1. Introduction: SMOC Assembly, Innate Immunity and Cell Death
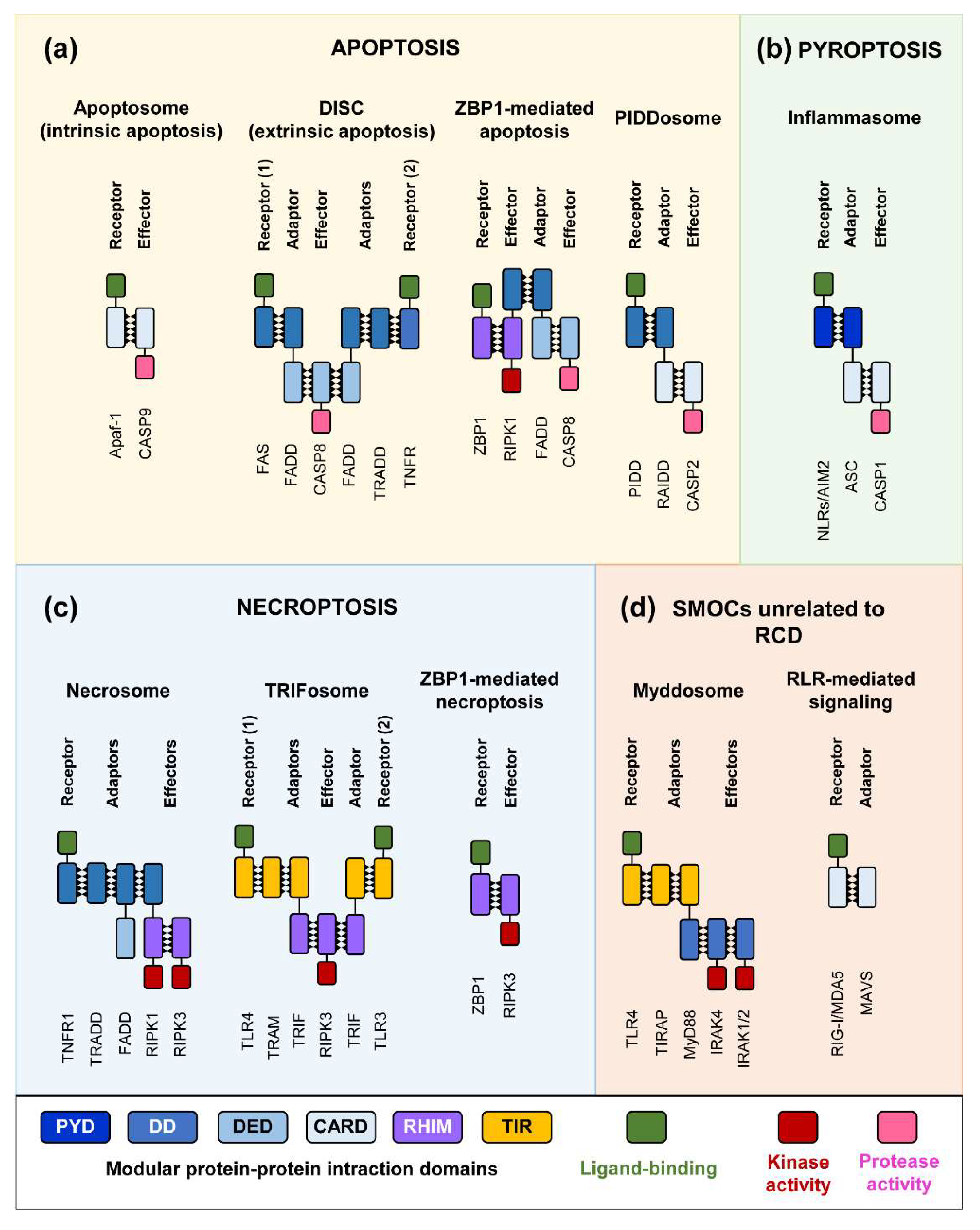
2. RCD Pathways and SMOC Assembly
3. Pore-Forming Executors of Cell Death: Pyroptosis vs. Necroptosis
4. Modeling SMOC Assembly, Necroptosis and Pyroptosis in Yeast
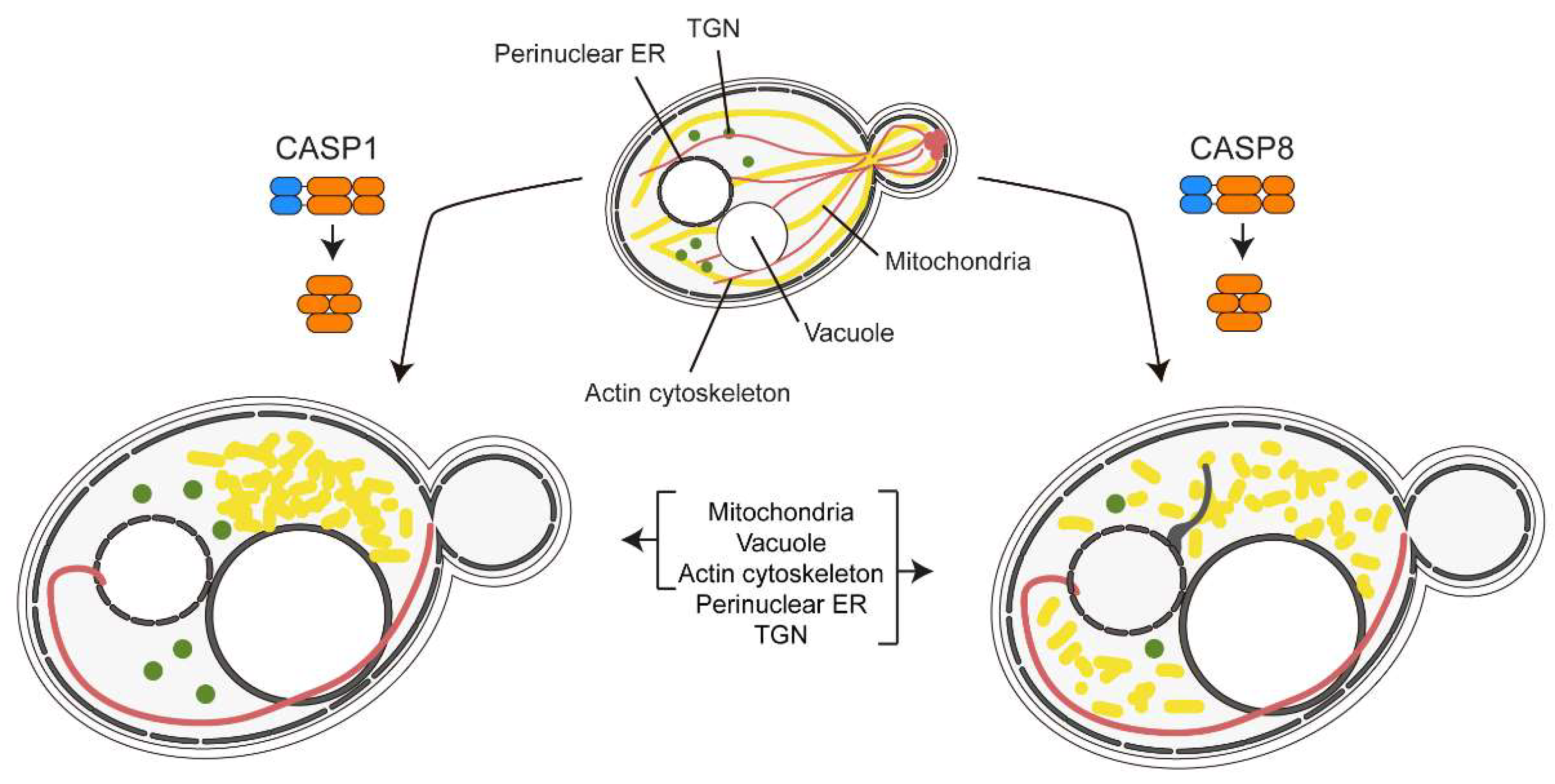
4.1. Heterologous Expression of Caspases in S. cerevisae
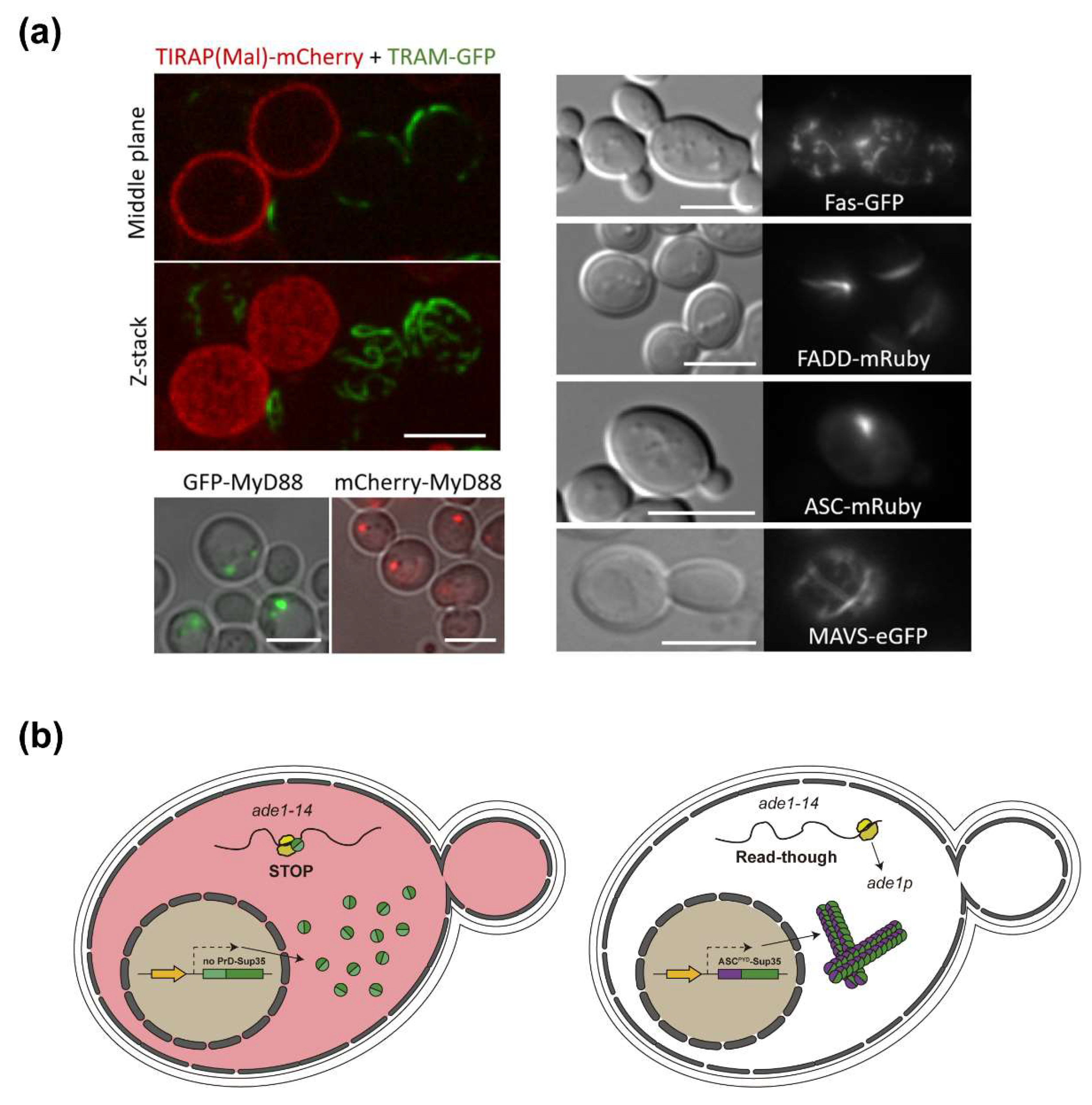
4.2. Yeast-Based Models for Inflammasome and Necrosome Assembly
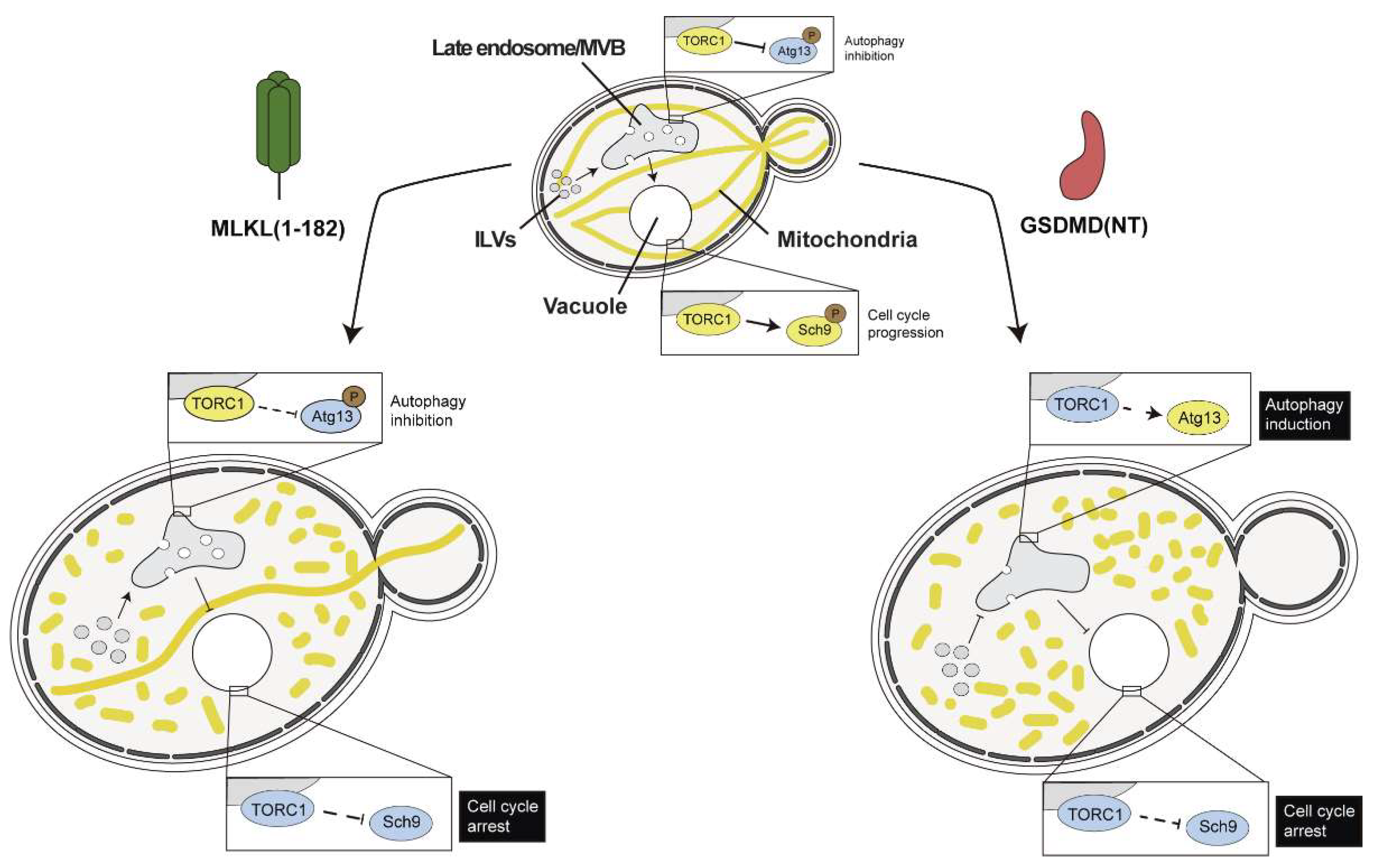
4.3. Necroptotic and Pyroptotic Pore-Forming Cell Death Executors Kill Yeast Cells by Means Other than Cell Lysis
4.4. ASC and Other SMOC Adaptor Proteins in Yeast: A Prion-Like Model
5. Caveats and Challenges of Signaling by Cooperative Assembly Formation (SCAF) Yeast-Based Models
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riera Romo, M. Cell Death as Part of Innate Immunity: Cause or Consequence? Immunology 2021, 163, 399–415. [Google Scholar] [CrossRef]
- Lee, E.; Song, C.-H.; Bae, S.-J.; Ha, K.-T.; Karki, R. Regulated Cell Death Pathways and Their Roles in Homeostasis, Infection, Inflammation, and Tumorigenesis. Exp Mol Med 2023, 55, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Magupalli, V.G.; Wu, H. SMOCs: Supramolecular Organizing Centres That Control Innate Immunity. Nat Rev Immunol 2014, 14, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Sušjan-Leite, P.; Ramuta, T.Ž.; Boršić, E.; Orehek, S.; Hafner-Bratkovič, I. Supramolecular Organizing Centers at the Interface of Inflammation and Neurodegeneration. Front. Immunol. 2022, 13, 940969. [Google Scholar] [CrossRef]
- Vajjhala, P.R.; Ve, T.; Bentham, A.; Stacey, K.J.; Kobe, B. The Molecular Mechanisms of Signaling by Cooperative Assembly Formation in Innate Immunity Pathways. Molecular Immunology 2017, 86, 23–37. [Google Scholar] [CrossRef]
- Nanson, J.D.; Rahaman, Md.H.; Ve, T.; Kobe, B. Regulation of Signaling by Cooperative Assembly Formation in Mammalian Innate Immunity Signalosomes by Molecular Mimics. Seminars in Cell & Developmental Biology 2020, 99, 96–114. [Google Scholar] [CrossRef]
- Nanson, J.D.; Kobe, B.; Ve, T. Death, TIR, and RHIM: Self-Assembling Domains Involved in Innate Immunity and Cell-Death Signaling. J Leukoc Biol 2019, 105, 363–375. [Google Scholar] [CrossRef]
- Ha, H.J. Assembly of Platforms for Signal Transduction in the New Era: Dimerization, Helical Filament Assembly, and Beyond. Molecular Medicine 2020. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, S.; Li, Y.; Zhang, D.; Wang, B.; Xie, J.; Wang, J. Regulated Cell Death: Discovery, Features and Implications for Neurodegenerative Diseases. Cell Commun Signal 2021, 19, 120. [Google Scholar] [CrossRef]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Sun, G. Death and Survival from Executioner Caspase Activation. Seminars in Cell & Developmental Biology 2024, 156, 66–73. [Google Scholar] [CrossRef]
- Park, H.H. Structural Features of Caspase-Activating Complexes. IJMS 2012, 13, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.S.; Szabo, T.G.; Garcia-Carpio, I.; Villunger, A. PIDD1 in Cell Cycle Control, Sterile Inflammation and Cell Death. Biochemical Society Transactions 2022, 50, 813–824. [Google Scholar] [CrossRef]
- Brown-Suedel, A.N.; Bouchier-Hayes, L. Caspase-2 Substrates: To Apoptosis, Cell Cycle Control, and Beyond. Front. Cell Dev. Biol. 2020, 8, 610022. [Google Scholar] [CrossRef]
- De Torre-Minguela, C.; Mesa Del Castillo, P.; Pelegrín, P. The NLRP3 and Pyrin Inflammasomes: Implications in the Pathophysiology of Autoinflammatory Diseases. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, R.; Ouyang, Y.; Gu, W.; Xiao, T.; Yang, H.; Tang, L.; Wang, H.; Xiang, B.; Chen, P. Pyroptosis in Health and Disease: Mechanisms, Regulation and Clinical Perspective. Sig Transduct Target Ther 2024, 9, 245. [Google Scholar] [CrossRef]
- Horne, C.R.; Samson, A.L.; Murphy, J.M. The Web of Death: The Expanding Complexity of Necroptotic Signaling. Trends Cell Biol 2023, 33, 162–174. [Google Scholar] [CrossRef]
- Petrie, E.J.; Czabotar, P.E.; Murphy, J.M. The Structural Basis of Necroptotic Cell Death Signaling. Trends in Biochemical Sciences 2019, 44, 53–63. [Google Scholar] [CrossRef]
- Orning, P.; Lien, E. Multiple Roles of Caspase-8 in Cell Death, Inflammation, and Innate Immunity. Journal of Leukocyte Biology 2021, 109, 121–141. [Google Scholar] [CrossRef]
- Li, M.; Beg, A.A. Induction of Necrotic-Like Cell Death by Tumor Necrosis Factor Alpha and Caspase Inhibitors: Novel Mechanism for Killing Virus-Infected Cells. J Virol 2000, 74, 7470–7477. [Google Scholar] [CrossRef]
- Upton, J.W.; Chan, F.K.-M. Staying Alive: Cell Death in Antiviral Immunity. Molecular Cell 2014, 54, 273–280. [Google Scholar] [CrossRef]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.-S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Devin, A.; Rodriguez, Y.; Liu, Z. -g. Cleavage of the Death Domain Kinase RIP by Caspase-8 Prompts TNF-Induced Apoptosis. Genes & Development 1999, 13, 2514–2526. [Google Scholar] [CrossRef]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.-G. Mixed Lineage Kinase Domain-like Is a Key Receptor Interacting Protein 3 Downstream Component of TNF-Induced Necrosis. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 5322–5327. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dai, Y.; Wan, X.; Hu, X.; Zhao, W.; Ban, X.; Wan, H.; Huang, K.; Zhang, Q.; Xiong, K. ZBP1-Mediated Necroptosis: Mechanisms and Therapeutic Implications. Molecules 2022, 28, 52. [Google Scholar] [CrossRef]
- Bryant, C.E. Rethinking Toll-like Receptor Signalling. Current Opinion in Immunology 2024, 91, 102460. [Google Scholar] [CrossRef]
- Pereira, M.; Gazzinelli, R.T. Regulation of Innate Immune Signaling by IRAK Proteins. Front. Immunol. 2023, 14, 1133354. [Google Scholar] [CrossRef]
- Lin, K.-M.; Hu, W.; Troutman, T.D.; Jennings, M.; Brewer, T.; Li, X.; Nanda, S.; Cohen, P.; Thomas, J.A.; Pasare, C. IRAK-1 Bypasses Priming and Directly Links TLRs to Rapid NLRP3 Inflammasome Activation. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 775–780. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-Dependent TLR Signaling, Its Functions in Host Defense and Inflammation, and Its Potential as a Therapeutic Target. Journal of Leukocyte Biology 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, A.; Liu, J.; Li, X.; Wang, J.; Zhang, L.; Liu, Y.; Xiong, Y.; Li, G.; He, X. PANoptosis: Bridging Apoptosis, Pyroptosis, and Necroptosis in Cancer Progression and Treatment. Cancer Gene Ther 2024, 31, 970–983. [Google Scholar] [CrossRef]
- Chen, W.; Gullett, J.M.; Tweedell, R.E.; Kanneganti, T. Innate Immune Inflammatory Cell Death: PANoptosis and PANoptosomes in Host Defense and Disease. Eur J Immunol 2023, 53, 2250235. [Google Scholar] [CrossRef]
- Pandian, N.; Kanneganti, T.-D. PANoptosis: A Unique Innate Immune Inflammatory Cell Death Modality. J Immunol 2022, 209, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef]
- Zhu, P.; Ke, Z.-R.; Chen, J.-X.; Li, S.-J.; Ma, T.-L.; Fan, X.-L. Advances in Mechanism and Regulation of PANoptosis: Prospects in Disease Treatment. Front. Immunol. 2023, 14, 1120034. [Google Scholar] [CrossRef]
- Nadella, V.; Kanneganti, T.-D. Inflammasomes and Their Role in PANoptosomes. Current Opinion in Immunology 2024, 91, 102489. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.-C.; Shao, F. Pore-Forming Activity and Structural Autoinhibition of the Gasdermin Family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrín, P.; Shao, F. The Gasdermins, a Protein Family Executing Cell Death and Inflammation. Nat Rev Immunol 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Liu, Z.; Busscher, B.M.; Storl-Desmond, M.; Xiao, T.S. Mechanisms of Gasdermin Recognition by Proteases. Journal of Molecular Biology 2022, 434, 167274. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat Rev Immunol 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-Canonical Inflammasome Activation Targets Caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed Cell Death as a Defence against Infection. Nat Rev Immunol 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Chai, Q.; Yu, S.; Zhong, Y.; Lu, Z.; Qiu, C.; Yu, Y.; Zhang, X.; Zhang, Y.; Lei, Z.; Qiang, L.; et al. A Bacterial Phospholipid Phosphatase Inhibits Host Pyroptosis by Hijacking Ubiquitin. Science 2022, 378, eabq0132. [Google Scholar] [CrossRef]
- Wang, C.; Ruan, J. Mechanistic Insights into Gasdermin Pore Formation and Regulation in Pyroptosis. Journal of Molecular Biology 2022, 434, 167297. [Google Scholar] [CrossRef]
- Chen, X.; He, W.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis Is Driven by Non-Selective Gasdermin-D Pore and Its Morphology Is Different from MLKL Channel-Mediated Necroptosis. Cell Res 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Du, G.; Healy, L.B.; David, L.; Walker, C.; Fontana, P.; Dong, Y.; Devant, P.; Puthenveetil, R.; Ficarro, S.B.; Banerjee, A.; et al. ROS-Dependent Palmitoylation Is an Obligate Licensing Modification for GSDMD Pore Formation; Immunology, 2023;
- Balasubramanian, A.; Ghimire, L.; Hsu, A.Y.; Kambara, H.; Liu, X.; Hasegawa, T.; Xu, R.; Tahir, M.; Yu, H.; Lieberman, J.; et al. Palmitoylation of Gasdermin D Directs Its Membrane Translocation and Pore Formation in Pyroptosis; Immunology, 2023;
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-Approved Disulfiram Inhibits Pyroptosis by Blocking Gasdermin D Pore Formation. Nat Immunol 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Borges, J.P.; Sætra, R.S.; Volchuk, A.; Bugge, M.; Devant, P.; Sporsheim, B.; Kilburn, B.R.; Evavold, C.L.; Kagan, J.C.; Goldenberg, N.M.; et al. Glycine Inhibits NINJ1 Membrane Clustering to Suppress Plasma Membrane Rupture in Cell Death. eLife 2022, 11, e78609. [Google Scholar] [CrossRef]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 Mediates Plasma Membrane Rupture during Lytic Cell Death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Degen, M.; Santos, J.C.; Pluhackova, K.; Cebrero, G.; Ramos, S.; Jankevicius, G.; Hartenian, E.; Guillerm, U.; Mari, S.A.; Kohl, B.; et al. Structural Basis of NINJ1-Mediated Plasma Membrane Rupture in Cell Death. Nature 2023, 618, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Bridelance, J.; Roelandt, R.; Vandenabeele, P.; Takahashi, N. MLKL in Cancer: More than a Necroptosis Regulator. Cell Death Differ 2021, 28, 1757–1772. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.-F.; Wang, F.-S.; Wang, X. Mixed Lineage Kinase Domain-like Protein MLKL Causes Necrotic Membrane Disruption upon Phosphorylation by RIP3. Molecular Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Garnish, S.E.; Davies, K.A.; Black, K.A.; Leis, A.P.; Horne, C.R.; Hildebrand, J.M.; Hoblos, H.; Fitzgibbon, C.; Young, S.N.; et al. Phosphorylation-Dependent Pseudokinase Domain Dimerization Drives Full-Length MLKL Oligomerization. Nat Commun 2023, 14, 6804. [Google Scholar] [CrossRef]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.-C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.; Liu, Z.-G. Plasma Membrane Translocation of Trimerized MLKL Protein Is Required for TNF-Induced Necroptosis. Nat Cell Biol 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zheng, X.; Wang, Z.; Chen, X.; He, W.; Zhang, Y.; Xu, J.-G.; Zhao, H.; Shi, W.; Wang, X.; et al. The MLKL Channel in Necroptosis Is an Octamer Formed by Tetramers in a Dyadic Process. Molecular and Cellular Biology 2017, 37, e00497–16. [Google Scholar] [CrossRef]
- Davies, K.A.; Tanzer, M.C.; Griffin, M.D.W.; Mok, Y.F.; Young, S.N.; Qin, R.; Petrie, E.J.; Czabotar, P.E.; Silke, J.; Murphy, J.M. The Brace Helices of MLKL Mediate Interdomain Communication and Oligomerisation to Regulate Cell Death by Necroptosis. Cell Death Differ 2018, 25, 1567–1580. [Google Scholar] [CrossRef]
- Samson, A.L.; Zhang, Y.; Geoghegan, N.D.; Gavin, X.J.; Davies, K.A.; Mlodzianoski, M.J.; Whitehead, L.W.; Frank, D.; Garnish, S.E.; Fitzgibbon, C.; et al. MLKL Trafficking and Accumulation at the Plasma Membrane Control the Kinetics and Threshold for Necroptosis. Nat Commun 2020, 11, 3151. [Google Scholar] [CrossRef]
- Ramirez, R.X.; Campbell, O.; Pradhan, A.J.; Atilla-Gokcumen, G.E.; Monje-Galvan, V. Modeling the Molecular Fingerprint of Protein-Lipid Interactions of MLKL on Complex Bilayers. Front. Chem. 2023, 10, 1088058. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Ros, U.; Garcia-Saez, A.J. Pore Formation in Regulated Cell Death. The EMBO Journal 2020, 39, e105753. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Johnston, A.; Hanna-Addams, S.; Reynoso, E.; Xiang, Y.; Wang, Z. MLKL Forms Disulfide Bond-Dependent Amyloid-like Polymers to Induce Necroptosis. Proc. Natl. Acad. Sci. U.S.A. 2017, 114. [Google Scholar] [CrossRef]
- Ruan, J.; Xia, S.; Liu, X.; Lieberman, J.; Wu, H. Cryo-EM Structure of the Gasdermin A3 Membrane Pore. Nature 2018, 557, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Kornfeld, O.S.; Lee, B.L.; Stowe, I.B.; O’Rourke, K.; Li, Q.; Sandoval, W.; Yan, D.; Kang, J.; Xu, M.; et al. NINJ1 Mediates Plasma Membrane Rupture during Lytic Cell Death. Nature 2021, 591, 131–136. [Google Scholar] [CrossRef]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein That Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65.e7. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Riquet, F.; Cappe, B. Necroptosis: (Last) Message in a Bubble. Immunity 2017, 47, 1–3. [Google Scholar] [CrossRef]
- Fan, W.; Guo, J.; Gao, B.; Zhang, W.; Ling, L.; Xu, T.; Pan, C.; Li, L.; Chen, S.; Wang, H.; et al. Flotillin-Mediated Endocytosis and ALIX–Syntenin-1–Mediated Exocytosis Protect the Cell Membrane from Damage Caused by Necroptosis. Sci. Signal. 2019, 12, eaaw3423. [Google Scholar] [CrossRef]
- Gong, Y.-N.; Guy, C.; Olauson, H.; Becker, J.U.; Yang, M.; Fitzgerald, P.; Linkermann, A.; Green, D.R. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 2017, 169, 286–300.e16. [Google Scholar] [CrossRef]
- Conos, S.A.; Chen, K.W.; De Nardo, D.; Hara, H.; Whitehead, L.; Núñez, G.; Masters, S.L.; Murphy, J.M.; Schroder, K.; Vaux, D.L.; et al. Active MLKL Triggers the NLRP3 Inflammasome in a Cell-Intrinsic Manner. Proc. Natl. Acad. Sci. U.S.A. 2017, 114. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, C.; Guo, L.; He, H.; Jiang, K.; Huang, Y.; Zhang, X.; Zhang, H.; Wei, W.; Zhang, Y.; et al. A Necroptotic-Independent Function of MLKL in Regulating Endothelial Cell Adhesion Molecule Expression. Cell Death Dis 2020, 11, 282. [Google Scholar] [CrossRef]
- Yoon, S.; Bogdanov, K.; Kovalenko, A.; Wallach, D. Necroptosis Is Preceded by Nuclear Translocation of the Signaling Proteins That Induce It. Cell Death Differ 2016, 23, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.E.; Murphy, J.M.; Vince, J.E. Gasdermin and MLKL Necrotic Cell Death Effectors: Signaling and Diseases. Immunity 2024, 57, 429–445. [Google Scholar] [CrossRef]
- Madeo, F.; Herker, E.; Maldener, C.; Wissing, S.; Lächelt, S.; Herlan, M.; Fehr, M.; Lauber, K.; Sigrist, S.J.; Wesselborg, S.; et al. A Caspase-Related Protease Regulates Apoptosis in Yeast. Mol Cell 2002, 9, 911–917. [Google Scholar] [CrossRef]
- Lam, D.K.; Sherlock, G. Yca1 Metacaspase: Diverse Functions Determine How Yeast Live and Let Die. FEMS Yeast Res 2023, 23, foad022. [Google Scholar] [CrossRef]
- Wilkinson, D.; Ramsdale, M. Proteases and Caspase-like Activity in the Yeast Saccharomyces Cerevisiae. Biochem Soc Trans 2011, 39, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Meitzler, J.L.; Gray, J.J.; Hendrickson, T.L. Truncation of the Caspase-Related Subunit (Gpi8p) of Saccharomyces Cerevisiae GPI Transamidase: Dimerization Revealed. Arch Biochem Biophys 2007, 462, 83–93. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Q.; Zhang, Z. Cleavage of Mcd1 by Caspase-like Protease Esp1 Promotes Apoptosis in Budding Yeast. Mol Biol Cell 2008, 19, 2127–2134. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Wang, S.L.; Hay, B.A. Monitoring Activity of Caspases and Their Regulators in Yeast Saccharomyces Cerevisiae. In Methods in Enzymology; Elsevier, 2000; Vol. 322, pp. 162–174 ISBN 978-0-12-182223-1.
- Puryer, M.A.; Hawkins, C.J. Human, Insect and Nematode Caspases Kill Saccharomyces Cerevisiae Independently of YCA1 and Aif1p. Apoptosis 2006, 11, 509–517. [Google Scholar] [CrossRef]
- Kang, J.J.; Schaber, M.D.; Srinivasula, S.M.; Alnemri, E.S.; Litwack, G.; Hall, D.J.; Bjornsti, M.A. Cascades of Mammalian Caspase Activation in the Yeast Saccharomyces Cerevisiae. J Biol Chem 1999, 274, 3189–3198. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Wang, S.L.; Hay, B.A. A Cloning Method to Identify Caspases and Their Regulators in Yeast: Identification of Drosophila IAP1 as an Inhibitor of the Drosophila Caspase DCP-1. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 2885–2890. [Google Scholar] [CrossRef]
- Wright, M.E.; Han, D.K.; Carter, L.; Fields, S.; Schwartz, S.M.; Hockenbery, D.M. Caspase-3 Inhibits Growth in Saccharomyces Cerevisiae without Causing Cell Death 1. FEBS Letters 1999, 446, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.; Jabbour, A.M.; Ekert, P.G.; Hawkins, C.J. Caspase-2 Is Resistant to Inhibition by Inhibitor of Apoptosis Proteins (IAPs) and Can Activate Caspase-7. The FEBS Journal 2005, 272, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.J.; Silke, J.; Verhagen, A.M.; Foster, R.; Ekert, P.G.; Ashley, D.M. Analysis of Candidate Antagonists of IAP-Mediated Caspase Inhibition Using Yeast Reconstituted with the Mammalian Apaf-1-Activated Apoptosis Mechanism. Apoptosis 2001, 6, 331–338. [Google Scholar] [CrossRef]
- Pereira, C.; Lopes-Rodrigues, V.; Coutinho, I.; Neves, M.P.; Lima, R.T.; Pinto, M.; Cidade, H.; Vasconcelos, M.H.; Saraiva, L. Potential Small-Molecule Activators of Caspase-7 Identified Using Yeast-Based Caspase-3 and -7 Screening Assays. Eur J Pharm Sci 2014, 54, 8–16. [Google Scholar] [CrossRef]
- Kitevska, T.; Roberts, S.J.; Pantaki-Eimany, D.; Boyd, S.E.; Scott, F.L.; Hawkins, C.J. Analysis of the Minimal Specificity of Caspase-2 and Identification of Ac-VDTTD-AFC as a Caspase-2-Selective Peptide Substrate. Bioscience Reports 2014, 34, e00100. [Google Scholar] [CrossRef]
- Jabbour, A.M.; Ekert, P.G.; Coulson, E.J.; Knight, M.J.; Ashley, D.M.; Hawkins, C.J. The P35 Relative, P49, Inhibits Mammalian and Drosophila Caspases Including DRONC and Protects against Apoptosis. Cell Death Differ 2002, 9, 1311–1320. [Google Scholar] [CrossRef]
- Hawkins, C.J.; Yoo, S.J.; Peterson, E.P.; Wang, S.L.; Vernooy, S.Y.; Hay, B.A. The Drosophila Caspase DRONC Cleaves Following Glutamate or Aspartate and Is Regulated by DIAP1, HID, and GRIM. J Biol Chem 2000, 275, 27084–27093. [Google Scholar] [CrossRef]
- Jabbour, A.M.; Ho, P. -k; Puryer, M.A.; Ashley, D.M.; Ekert, P.G.; Hawkins, C.J. The Caenorhabditis Elegans CED-9 Protein Does Not Directly Inhibit the Caspase CED-3, in Vitro nor in Yeast. Cell Death Differ 2004, 11, 1309–1316. [Google Scholar] [CrossRef]
- Beaumont, T.E.; Shekhar, T.M.; Kaur, L.; Pantaki-Eimany, D.; Kvansakul, M.; Hawkins, C.J. Yeast Techniques for Modeling Drugs Targeting Bcl-2 and Caspase Family Members. Cell Death Dis 2013, 4, e619. [Google Scholar] [CrossRef]
- Ekert, P.G.; Silke, J.; Hawkins, C.J.; Verhagen, A.M.; Vaux, D.L. DIABLO Promotes Apoptosis by Removing MIHA/XIAP from Processed Caspase 9. J Cell Biol 2001, 152, 483–490. [Google Scholar] [CrossRef]
- Wright, M.E.; Han, D.K.; Hockenbery, D.M. Caspase-3 and Inhibitor of Apoptosis Protein(s) Interactions in Saccharomyces Cerevisiae and Mammalian Cells. FEBS Lett 2000, 481, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Silke, J.; Ekert, P.G.; Day, C.L.; Hawkins, C.J.; Baca, M.; Chew, J.; Pakusch, M.; Verhagen, A.M.; Vaux, D.L. Direct Inhibition of Caspase 3 Is Dispensable for the Anti-Apoptotic Activity of XIAP. EMBO J 2001, 20, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Glória, P.M.C.; Coutinho, I.; Gonçalves, L.M.; Baptista, C.; Soares, J.; Newton, A.S.; Moreira, R.; Saraiva, L.; Santos, M.M.M. Aspartic Vinyl Sulfones: Inhibitors of a Caspase-3-Dependent Pathway. Eur J Med Chem 2011, 46, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Lisa-Santamaría, P.; Neiman, A.M.; Cuesta-Marbán, Á.; Mollinedo, F.; Revuelta, J.L.; Jiménez, A. Human Initiator Caspases Trigger Apoptotic and Autophagic Phenotypes in Saccharomyces Cerevisiae. Biochimica et Biophysica Acta (BBA)—Molecular Cell Research 2009, 1793, 561–571. [Google Scholar] [CrossRef]
- Brunette, S.; Sharma, A.; Bell, R.; Puente, L.; Megeney, L.A. Caspase 3 Exhibits a Yeast Metacaspase Proteostasis Function That Protects Mitochondria from Toxic TDP43 Aggregates. Microb Cell 2023, 10, 157–169. [Google Scholar] [CrossRef]
- Lisa-Santamaría, P.; Neiman, A.M.; Cuesta-Marbán, A.; Mollinedo, F.; Revuelta, J.L.; Jiménez, A. Human Initiator Caspases Trigger Apoptotic and Autophagic Phenotypes in Saccharomyces Cerevisiae. Biochim Biophys Acta 2009, 1793, 561–571. [Google Scholar] [CrossRef]
- Lisa-Santamaría, P.; Jiménez, A.; Revuelta, J.L. The Protein Factor-Arrest 11 (Far11) Is Essential for the Toxicity of Human Caspase-10 in Yeast and Participates in the Regulation of Autophagy and the DNA Damage Signaling. J Biol Chem 2012, 287, 29636–29647. [Google Scholar] [CrossRef]
- Valenti, M.; Molina, M.; Cid, V.J. Heterologous Expression and Auto-Activation of Human Pro-Inflammatory Caspase-1 in Saccharomyces Cerevisiae and Comparison to Caspase-8. Front Immunol 2021, 12, 668602. [Google Scholar] [CrossRef]
- Manon, S.; Chaudhuri, B.; Guérin, M. Release of Cytochrome c and Decrease of Cytochrome c Oxidase in Bax-expressing Yeast Cells, and Prevention of These Effects by Coexpression of Bcl-x L. FEBS Letters 1997, 415, 29–32. [Google Scholar] [CrossRef]
- Ligr, M.; Madeo, F.; Fröhlich, E.; Hilt, W.; Fröhlich, K.-U.; Wolf, D.H. Mammalian Bax Triggers Apoptotic Changes in Yeast. FEBS Letters 1998, 438, 61–65. [Google Scholar] [CrossRef]
- Zha, H.; Fisk, H.A.; Yaffe, M.P.; Mahajan, N.; Herman, B.; Reed, J.C. Structure-Function Comparisons of the Proapoptotic Protein Bax in Yeast and Mammalian Cells. Molecular and Cellular Biology 1996, 16, 6494–6508. [Google Scholar] [CrossRef] [PubMed]
- Polčic, P.; Jaká, P.; Mentel, M. Yeast as a Tool for Studying Proteins of the Bcl-2 Family. Microb Cell 2015, 2, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Srinivasula, S.M.; Ahmad, M.; Fernandes-Alnemri, T.; Alnemri, E.S. Autoactivation of Procaspase-9 by Apaf-1-Mediated Oligomerization. Molecular Cell 1998, 1, 949–957. [Google Scholar] [CrossRef]
- Hayashi, H.; Cuddy, M.; Shu, V.C.-W.; Yip, K.W.; Madiraju, C.; Diaz, P.; Matsuyama, T.; Kaibara, M.; Taniyama, K.; Vasile, S.; et al. Versatile Assays for High Throughput Screening for Activators or Inhibitors of Intracellular Proteases and Their Cellular Regulators. PLoS ONE 2009, 4, e7655. [Google Scholar] [CrossRef]
- Valenti, M.; Molina, M.; Cid, V.J. Human Gasdermin D and MLKL Disrupt Mitochondria, Endocytic Traffic and TORC1 Signalling in Budding Yeast. Open Biol. 2023, 13, 220366. [Google Scholar] [CrossRef]
- Ji, Y.; Hawkins, C.J. Reconstitution of Human Pyroptotic Cell Death in Saccharomyces Cerevisiae. Sci Rep 2023, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Du, G.; Zhang, Y.; Zhang, H.; Vora, S.M.; Hu, J.J.; Shi, M.; Tufan, A.B.; Healy, L.B.; Xia, S.; et al. Small-Molecule GSDMD Agonism in Tumors Stimulates Antitumor Immunity without Toxicity. Cell 2024, 187, 6165–6181.e22. [Google Scholar] [CrossRef]
- Ji, Y.; Ward, L.A.; Hawkins, C.J. Reconstitution of Human Necrosome Interactions in Saccharomyces Cerevisiae. Biomolecules 2021, 11, 153. [Google Scholar] [CrossRef]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein That Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65.e7. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, X.; Miller-Little, W.; Wang, H.; Willard, B.; Bulek, K.; Zhao, J.; Li, X. The Ras GTPase -activating-like Protein IQGAP1 Bridges Gasdermin D to the ESCRT System to Promote IL -1β Release via Exosomes. The EMBO Journal 2023, 42, e110780. [Google Scholar] [CrossRef]
- Evavold, C.L.; Hafner-Bratkovič, I.; Devant, P.; D’Andrea, J.M.; Ngwa, E.M.; Boršić, E.; Doench, J.G.; LaFleur, M.W.; Sharpe, A.H.; Thiagarajah, J.R.; et al. Control of Gasdermin D Oligomerization and Pyroptosis by the Ragulator-Rag-mTORC1 Pathway. Cell 2021, 184, 4495–4511.e19. [Google Scholar] [CrossRef] [PubMed]
- Coronas-Serna, J.M.; Del Val, E.; Kagan, J.C.; Molina, M.; Cid, V.J. Heterologous Expression and Assembly of Human TLR Signaling Components in Saccharomyces Cerevisiae. Biomolecules 2021, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, I.; Jha, S. Comprehensive Review of ASC Structure and Function in Immune Homeostasis and Disease. Mol Biol Rep 2020, 47, 3077–3096. [Google Scholar] [CrossRef]
- Walker, L.C.; Jucker, M. Neurodegenerative Diseases: Expanding the Prion Concept. Annu. Rev. Neurosci. 2015, 38, 87–103. [Google Scholar] [CrossRef]
- Alberti, S.; Halfmann, R.; King, O.; Kapila, A.; Lindquist, S. A Systematic Survey Identifies Prions and Illuminates Sequence Features of Prionogenic Proteins. Cell 2009, 137, 146–158. [Google Scholar] [CrossRef]
- Cai, X.; Chen, J.; Xu, H.; Liu, S.; Jiang, Q.-X.; Halfmann, R.; Chen, Z.J. Prion-like Polymerization Underlies Signal Transduction in Antiviral Immune Defense and Inflammasome Activation. Cell 2014, 156, 1207–1222. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Poyet, J.-L.; Razmara, M.; Datta, P.; Zhang, Z.; Alnemri, E.S. The PYRIN-CARD Protein ASC Is an Activating Adaptor for Caspase-1. Journal of Biological Chemistry 2002, 277, 21119–21122. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, Q.; Zhou, X.; Wang, C.; Guan, Y.; Tao, J.; Xi, J.; Feng, J.-M.; Jiang, Z. MAVS Activates TBK1 and IKKε through TRAFs in NEMO Dependent and Independent Manner. PLoS Pathog 2017, 13, e1006720. [Google Scholar] [CrossRef]
- Alberti, S.; Halfmann, R.; Lindquist, S. Biochemical, Cell Biological, and Genetic Assays to Analyze Amyloid and Prion Aggregation in Yeast. In Methods in Enzymology; Elsevier, 2010; Vol. 470, pp. 709–734 ISBN 978-0-12-375172-0.
- Riek, R.; Saupe, S.J. The HET-S/s Prion Motif in the Control of Programmed Cell Death. Cold Spring Harb Perspect Biol 2016, 8, a023515. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Y.; Jia, H.; Han, Y.; Zheng, X.; Wang, M.; Feng, W. From Plant to Yeast—Advances in Biosynthesis of Artemisinin. Molecules 2022, 27, 6888. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Balschmidt, P.; Diers, I.; Hach, M.; Kaarsholm, N.C.; Ludvigsen, S. Expression of Insulin in Yeast: The Importance of Molecular Adaptation for Secretion and Conversion. Biotechnology and Genetic Engineering Reviews 2001, 18, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Mager, W.H.; Winderickx, J. Yeast as a Model for Medical and Medicinal Research. Trends in Pharmacological Sciences 2005, 26, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, J.; Yang, Y.; Shan, H.; Hou, S.; Fang, H.; Ma, M.; Chen, Z.; Tan, L.; Xu, D. A Palmitoylation–Depalmitoylation Relay Spatiotemporally Controls GSDMD Activation in Pyroptosis. Nat Cell Biol 2024, 26, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.; John, S.W.; Liccardi, G.; Tenev, T.; Jaco, I.; Chen, C.-H.; Choi, J.; Kasperkiewicz, P.; Fernandes-Alnemri, T.; Alnemri, E.; et al. SUMO-Mediated Regulation of NLRP3 Modulates Inflammasome Activity. Nat Commun 2018, 9, 3001. [Google Scholar] [CrossRef]
- Dong, D.; Du, Y.; Fei, X.; Yang, H.; Li, X.; Yang, X.; Ma, J.; Huang, S.; Ma, Z.; Zheng, J.; et al. Inflammasome Activity Is Controlled by ZBTB16-Dependent SUMOylation of ASC. Nat Commun 2023, 14, 8465. [Google Scholar] [CrossRef]
- Madiraju, C.; Novack, J.P.; Reed, J.C.; Matsuzawa, S. K63 Ubiquitination in Immune Signaling. Trends in Immunology 2022, 43, 148–162. [Google Scholar] [CrossRef]
- Vanhelmont, T.; Vandebroek, T.; De Vos, A.; Terwel, D.; Lemaire, K.; Anandhakumar, J.; Franssens, V.; Swinnen, E.; Van Leuven, F.; Winderickx, J. Serine-409 Phosphorylation and Oxidative Damage Define Aggregation of Human Protein Tau in Yeast: Determinants of Tau Aggregation in Yeast. FEMS Yeast Research 2010, 10, 992–1005. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Brandt, R. Signaling Pathways and Posttranslational Modifications of Tau in Alzheimer’s Disease: The Humanization of Yeast Cells. MIC 2016, 3, 135–146. [Google Scholar] [CrossRef]
- Lehle, L.; Strahl, S.; Tanner, W. Protein Glycosylation, Conserved from Yeast to Man: A Model Organism Helps Elucidate Congenital Human Diseases. Angew Chem Int Ed 2006, 45, 6802–6818. [Google Scholar] [CrossRef]
- Arico, C.; Bonnet, C.; Javaud, C. N-Glycosylation Humanization for Production of Therapeutic Recombinant Glycoproteins in Saccharomyces Cerevisiae. In Glycosylation Engineering of Biopharmaceuticals; Beck, A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2013; ISBN 978-1-62703-326-8. [Google Scholar]

| Caspase1 | Inhibitor | Reference |
|---|---|---|
| Dm DCP-1 | DIAP1 | [83] |
| Baculovirus p35 protein | [89] | |
| Baculovirus p49 protein | [89] | |
| Dm DRONC | DIAP1 | [90] |
| Baculovirus p49 protein | [89] | |
| Dm drICE | Baculovirus p35 protein | [89] |
| Baculovirus p49 protein | [89] | |
| DIAP1 | [89] | |
| Ce CED-4 | Ce CED-9 | [91] |
| Hs CASP-8 | Baculovirus p35 protein | [82,89] |
| Cowpox virus CrmA | [92] | |
| Hs CASP-9 | XIAP | [93] |
| Hs CASP-3 | Cowpox virus CrmA-mut | [84] |
| XIAP, c-IAP1, c-IAP2 | [94,95] | |
| Baculovirus p35 protein | [89] | |
| Baculovirus p49 protein | [89] | |
| ZVAD-fluoromethyl ketone | [84] | |
| Q-VD-OPh | [92] | |
| Ac-DEVD-chloromethyl ketone | [96] | |
| Aspartic vinyl sulphones | [96] | |
| Hs CASP-7 | Baculovirus p35 protein | [89] |
| Baculovirus p49 protein | [89] | |
| XIAP | [89] | |
| Hs CASP-2 | Baculovirus p35 protein | [89] |
| Baculovirus p49 protein | [89] | |
| Hs CASP-4 | Baculovirus p35 protein | [89] |
| Hs CASP-5 | Baculovirus p35 protein | [89] |
| Hs CASP-1 | Q-VD-OPh | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





