1. Introduction
Cognitive decline is a major concern in the aging population, affecting independence and quality of life and increasing the risk of dementia and associated comorbidities [
1,
2]. The mechanisms underlying cognitive impairment are multifaceted, involving neurodegenerative processes, vascular dysfunction, and metabolic alterations [
3]. Emerging evidence suggests that autonomic nervous system (ANS) dysfunction – particularly changes in heart rate variability (HRV) – may play a significant role in cognitive aging [
4,
5]. HRV, a well-established marker of cardiac autonomic control, reflects the dynamic balance between sympathetic and parasympathetic activity and has been associated with brain health and neurodegeneration [
6].
Previous research indicates that reduced HRV is linked to cognitive impairment, including mild cognitive impairment (MCI) and Alzheimer’s disease [
7,
8]. Lower parasympathetic activity is often reflected in a diminished root mean square of successive R–R interval differences (rMSSD) and the percentage of successive R–R intervals differing by more than 50 milliseconds (pNN50), alterations that have been documented in individuals with cognitive dysfunction [
9]. However, the relationship between HRV and specific cognitive domains, such as information processing speed and executive function, remains insufficiently explored, particularly among older adults [
10].
Information processing speed and executive function are critical cognitive domains that typically decline with age and are frequently early indicators of neurodegenerative processes [
11]. Processing speed underpins the efficiency of cognitive operations and is integral to higher-order cognitive functions, while executive function governs decision-making, cognitive flexibility, and goal-directed behaviors [
12]. Slower processing speed and deficits in executive function have been associated with an elevated risk of cognitive impairment and a reduced quality of life in older adults [
13]. The physiological mechanisms underlying these declines remain poorly understood despite their clinical relevance. Examining the role of autonomic control, as reflected by HRV, may shed light on early dysfunction that precedes more severe cognitive impairment. Investigating these relationships in aging populations can also inform preventive strategies and interventions aimed at preserving cognitive function.
In this context, understanding the link between HRV and cognitive decline could reveal early markers of neurodegeneration, potentially guiding preventive and therapeutic approaches [
14]. Given the growing burden of cognitive disorders and their profound impact on global health, studying modifiable physiological correlates – such as HRV – is crucial. Accordingly, this study aims to examine the relationship between HRV and cognitive performance in older adults, with particular attention to information processing speed and executive function. Specifically, it will compare HRV parameters between older adults with and without cognitive impairment, investigate the association between HRV indices and cognitive performance in these domains, and determine whether alterations in HRV might serve as an early indicator of cognitive decline in aging populations [
15]. By addressing these objectives, this research contributes to the growing body of literature linking cardiovascular autonomic function and neurocognitive aging, potentially paving the way for novel interventions to mitigate cognitive decline.
2. Materials and Methods
2.1. Study Design and Participants
This cross-sectional, descriptive, and analytical study was conducted using the translated [
16] and updated [
17] version of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [
18] and the STROBE Statement Guidelines for reporting observational studies [
19].
The study was carried out at the outpatient clinic of the Dr. Washington Antônio de Barros Teaching Hospital (HU) from Brazilian Hospital Services Company (EBSERH), affiliated with the Federal University of Vale do São Francisco (UNIVASF), in Petrolina, Brazil. The research adhered to the ethical principles of the Declaration of Helsinki (1964, revised in 2013) and complied with all regulatory requirements outlined in Resolutions 466/2012 and 510/2016 of the Brazilian National Health Council. The study was approved by the Research Ethics Committee of the School of Medical Sciences of Pernambuco (CEP-FCM/PE) under Evaluation Report Number 4.389.686 and Certificate of Presentation for Ethical Appraisal (CAAE) Number 38942320.4.0000.5192. Furthermore, all participants provided written informed consent by signing the Informed Consent Form (ICF) before study enrollment.
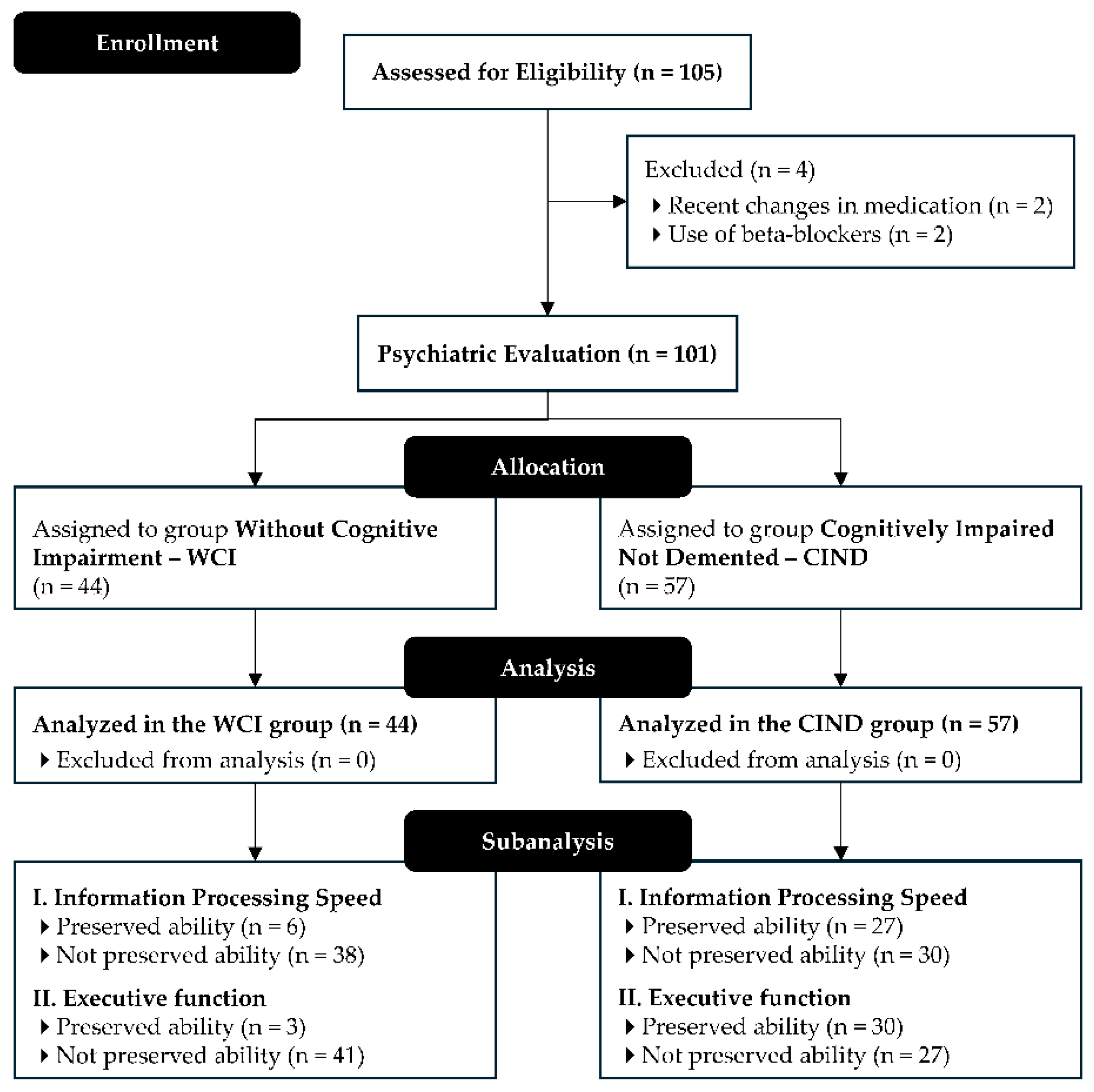
A total of 105 participants aged 60 years and older were recruited. The sample size was estimated based on the total elderly population of Petrolina (20,733 individuals), considering a maximum expected proportion of 50%, a sampling error of 5%, an expected loss of 20%, and a confidence level of 95% [
20].
Participants were included if they were ≥ 60 years old, of either sex, with at least four years of education, regardless of income level, and residing in Petrolina, Brazil. Exclusion criteria included: a) Beck Depression Inventory (BDI) score > 18 (Upton, 2013); b) uncorrected motor or sensory deficits preventing neuropsychological testing; c) recent changes in medication regimen (within four weeks); d) use of psychotropic medications or beta-blockers; e) use of four or more antihypertensive medications; f) systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 110 mmHg; g) history of angina pectoris, myocardial infarction, invasive cardiovascular procedures, heart transplant, or pacemaker presence; h) diagnosis of Parkinson's disease; i) history of stroke or transient ischemic attack; j) untreated hypothyroidism.
Eligible participants were screened using the Mini-Mental State Examination (MMSE) adjusted for education level [
21]. Those identified with potential cognitive impairment were referred for psychiatric evaluation and subsequently categorized into two groups: without cognitive impairment (WCI) or cognitively impaired not demented (CIND).
Figure 1 shows a flow diagram summarizing participant eligibility.
2.2. Procedures
The research project was initially presented at the outpatient clinic of HU-UNIVASF and disseminated through social media platforms to inform and recruit potential participants. Following this initial dissemination, informational letters and ICF were distributed to older adults expressing interest in the study. Those who consented to participate were scheduled for assessment sessions at the HU-UNIVASF outpatient clinic.
The data collection process was conducted in a quiet, reserved room at HU-UNIVASF, exclusively in the morning hours, to minimize the influence of circadian variations. On the day of the initial assessment, participants completed a structured, interview-based questionnaire designed to collect data on general health perception, comorbidities, medication use, and socioeconomic characteristics. Subsequently, cognitive function was assessed using MMSE. Participants exhibiting potential cognitive impairment were referred to a psychiatric evaluation before attending the second assessment day.
On the second day of the study, participants were observed for 10 minutes while at rest before undergoing a series of hemodynamic and autonomic cardiac control assessments. Following this initial phase, their anthropometric measurements were recorded following the International Society for the Advancement of Kinanthropometry (ISAK) guidelines [
22]. Finally, on the third and final day, participants underwent a comprehensive functional capacity assessment.
To ensure methodological rigor and the reliability of findings, all assessments strictly adhered to standardized protocols. Training professionals conducted data collection procedures under control conditions, ensuring consistency and minimizing potential biases.
2.3. Hemodynamic Parameters and Cardiac Autonomic Function Assessments
The measurement of arterial blood pressure (BP) was conducted following the recommendations outlined in the Brazilian guidelines of hypertension [
23] and the updated Brazilian guidelines for in-office and out-of-office BP measurement [
24]. Three consecutive measures were obtained using an automated device (HEM-7130, OMRON Healthcare, Inc., Lake Forest, CA, United States of America [USA]) following a 10-minute rest period to ensure adherence to the most recent standards for accurate BP assessment. The non-dominant arm was utilized for measurement, and the appropriate cuffs were employed based on the arm circumference. The mean of the final two measurements was then calculated to derive a conclusive result.
The HRV assessment was based on R–R interval records collected with a free smartphone application (Elite HRV LLC, Asheville, NC, USA, release 4.0.2, 2018) for Android via Bluetooth 4.0, and a wireless transmitter Polar H10 (Polar Electro Oy, Kempele, Finland) positioned on the patient’s chest [
25]. The signals were subsequently transmitted to a computer for subsequent analysis.
Before data collection, volunteers were instructed to abstain from the consumption of alcoholic and stimulant beverages (sodas, coffee, chocolate milk, green tea, etc.) and the performance of physically demanding activities on the day of data collection and the day before [
26]. They were also instructed to refrain from talking, moving, coughing, and sleeping during data collection. All subjects rested for a minimum of 10 minutes in the supine position. Subsequently, the HRV was recorded for 10 minutes while the volunteers were at rest in the supine position, breathing spontaneously. It has been demonstrated that HRV values obtained from short-term recordings (limited to 10 minutes) reflect the data obtained from long-term recordings. Moreover, the utilization of short-term recordings is more practical and has been employed with greater frequency.
All HRV recordings were collected in the morning, and the R–R intervals were exported to the Kubios HRV program (Kubios Oy, Kuopio, Finland, version 2.2) for analysis through time-domain and frequency-domain methods. The following parameters were calculated: mean R–R interval, standard deviation of normal-to-normal (NN) intervals (SDNN), rMSSD, and pNN50. In the frequency-domain analysis, spectral power was calculated in the physiologically significant range of 0.04 to 0.4 Hz. The low-frequency (LF) component (0.04–0.15 Hz) and the high-frequency (HF) component (0.15–0.4 Hz) were assessed in both absolute (ms²) and normalized units (nu). The LF and HF components were considered indicative of sympathetic and parasympathetic autonomic modulations, respectively. The LF/HF ratio was used as an index of cardiac sympathovagal balance [
27].
2.4. Anthropometric Measurements
Total body mass (TBM) in kilograms (kg) and height in centimeters (cm) were measured using a calibrated (NBR ISO/IEC 17025:2005) anthropometric scale (PL-200, Filizola S.A. Pesagem e Automação, São Paulo, SP, Brazil), with an accuracy of 0.05 kg and 0.1 cm. Body mass index (BMI, kg/m2) was calculated as TBM (kg) divided by the square of height in meters (m2).
2.5. Autonomic Nervous System Dysfunction
The assessment of ANS dysfunction was conducted through the evaluation of neurogenic orthostatic hypotension (OH), following the definition stipulated in the International Consensus Statement [
28]. The evaluation of OH involved the measurement of BP using the automated device HEM-7130 (OMRON Healthcare, Inc.) in both supine and standing positions. The supine BP measurement was obtained as the average of the third measurement taken after a 30-minute rest period in the supine position. Subsequently, participants were instructed to stand up unassisted, and BP measurements were recorded at the first, second, and third minute of active standing. During these assessments, participants were instructed to maintain a state of relaxation, avoiding any unnecessary movement or muscle contractions that could influence BP regulation. The OH was defined as a decrease in SBP of at least 20 mmHg and/or a decrease in DBP of at least 10 mmHg within three minutes following the assumption of an upright position.
2.6. Assessment of Activities of Daily Living and Instrumental Activities of Daily Living
The functional assessment employed the Katz Index of Independence in Activities of Daily Living [
29] and the Lawton Instrumental Activities of Daily Living Scale [
30], with the latter adapted for the Brazilian elderly population [
31].
The Katz Index is a tool used to evaluate an individual's level of independence in performing basic activities of daily living (ADLs). This scale assesses six self-care activities, which are organized according to a hierarchy of complexity: bathing, dressing, toileting, transferring, continence, and feeding. Each activity is scored dichotomously as independent (1 point) or dependent (0 points), and the total score ranges from zero to six points [
29]. In this analysis, a score of six points indicates full independence in all activities, while a score of zero points indicates total dependence on performing all assessed activities.
In contrast, Lawton Scale is a tool designed to evaluate an individual's capacity to perform instrumental ADLs (IADLs), which require a higher level of cognitive and physical capability [
30]. The Lawton scale adopted in this study was the adapted version for the Brazilian elderly population [
31]. It consists of nine items intended to assess an individual’s capability to perform IADLs. These include telephone use, shopping, food preparation, housekeeping, laundry, transportation, managing medications, financial handling, and performing small household repairs or manual tasks. The scoring system for the scale ranges from 9 to 27 points, with higher scores indicating better performance. In this study, participants with a score ≤ 14 were classified as dependent for IADLs.
2.7. Assessments of Information Processing Speed and Executive Function
In the evaluation of executive function and information processing speed, the Trail Making Test (TMT), in its Parts A and B, was employed [
32,
33]. TMT Part A was used to evaluate information processing speed, while TMT Part B was designed to assess executive function, particularly cognitive flexibility.
Each part of the test consists of twenty-five circles, which are distributed across a sheet of paper. In Part A, the circles are sequentially numbered from 1 to 25, and participants are instructed to draw lines connecting the numbers in ascending order. In Part B, the circles contain both numbers (1 to 13) and letters (A to L). As in Part A, participants must connect the circles in ascending order, alternating between numbers and letters (1 → A → 2 → B → 3 → C, and so on).
Participants were instructed to complete the task as expeditiously as possible without lifting the pen or pencil from the paper. In the event of an error, the evaluator immediately indicated it and allowed the participant to correct it. The time required for error correction was included in the total task completion time. The test was concluded when the participant successfully completed the sequence or voluntarily decided to stop [
33].
The classification of TMT performance was based on the normative values proposed by Ashendorf
et al.[
32]. The cut-off values for preserved ability on the TMT Part A were ≤ 42 seconds for individuals aged 60 to 74 years and ≤ 51 seconds for individuals aged 75 years or more. For TMT Part B, the cut-off values were set at 101 seconds for individuals aged 60 to 74 years and 128 seconds for those aged 75 years and older. Individuals who exceeded these thresholds were designated as having no preserved ability.
2.8. Statistical Analysis
The data were double-entered and analyzed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA, release 16.0.2, 2007). The normality of continuous variables was assessed using the Shapiro–Wilk test and Levene’s test was applied to evaluate the homogeneity of variances. Categorical variables were described as absolute (n) and relative (%) frequencies. Given that most continuous variables did not meet normality assumptions, they were reported as median (first quartile – third quartile). For comparisons between WCI and CIND groups, the Mann–Whitney U test was used for non-parametric data, and the independent t-test was used when parametric assumptions were satisfied. Additionally, to investigate differences in HRV parameters among older adults with and without preserved cognitive abilities (based on TMT performance thresholds), a Kruskal–Wallis test with Dunn’s post hoc test was conducted. To quantify the magnitude of these group differences, effect sizes (ES) were computed following the guidelines of Erceg-Hurn and Mirosevich [
34] and Grissom [
35]. Furthermore, to control potential confounding effects of age, years of education, and SAH on cardiac autonomic modulation, an Analysis of Covariance (ANCOVA) was performed separately for each HRV parameter. Adjusted means were estimated for each group, and the statistical significance of these differences was assessed using F-tests, with partial eta squared (
η2p) reported to interpret effect sizes. All p-values and 95% confidence intervals (95%CI) were calculated and reported exactly. A two-tailed significance level of 5% was adopted for all analyses.
3. Results
3.1. Sample Characteristics
A total of 101 older adults participated in the study, with a mean age of 69.1 ± 6.6 years, and 67.3% of the subjects were female.
Table 1 summarizes the demographic, clinical, and cognitive characteristics of the sample according to cognitive status (WCI vs. CIND). The CIND group exhibited significantly higher age (p < 0.001), lower educational level (p = 0.001), and lower MMSE scores (p < 0.001) compared to the WCI group. Furthermore, individuals in the CIND group demonstrated lower performance in ADLs (p < 0.001) and IADLs (p < 0.001), as well as longer times on information processing speed (p < 0.001) and executive function (p < 0.001). No significant differences were observed in BMI, obesity prevalence, alcohol or tobacco use, or history of systemic arterial hypertension.
3.2. Hemodynamic Parameters
The OH was observed in 39.6% of the total sample (
Table 2), with a similar distribution (p = 0.814) between groups (40.9% in WCI vs. 38.6%). No statistically significant differences were found for resting heart rate (p = 0.378,
d = 0.18), SBP (p = 0.828,
d = 0.04), or DBP (p = 0.667,
d = 0.08).
3.3. Heart Rate Variability
Significant differences were observed in time-domain HRV indices, with the CIND group exhibiting lower values than the WCI (
Table 3). Specifically, SDNN was significantly lower in CIND (p < 0.05, d = 0.44), indicating a moderate effect size. Similar reductions were found in rMSSD (p < 0.05, d = 0.39) and pNN50 (p < 0.05, d = 0.40), both also suggesting moderate effects. In contrast, no significant differences emerged in frequency-domain parameters (LF, HF, or LF/HF ratio).
To account for potential confounders, an ANCOVA was performed, adjusting for age, years of education, and SAH. Separate models were tested for time-domain and frequency-domain HRV indices. Even after adjustment for covariates, ANCOVA confirmed significant group differences in SDNN, rMSSD, and pNN50, consistent with the initial Mann–Whitney U test results. SDNN remained significantly lower in CIND (p = 0.035, η2p = 0.044), indicating a moderate effect size. rMSSD was also significantly reduced in CIND (p = 0.049, η2p = 0.039), suggesting reduced parasympathetic activity. pNN50 similarly showed a significant reduction in CIND (p = 0.047, η2p = 0.04), strengthening the association between vagal modulation and cognitive function. In contrast, no significant differences were detected in frequency-domain HRV measures after ANCOVA. Neither LF (p = 0.561, η2p = 0.007) nor HF (p = 0.751, η2p = 0.001) varied significantly between groups, and the LF/HF ratio (p = 0.509, η2p = 0.003) also remained unchanged. These findings suggest that reduced time-domain HRV parameters are associated with cognitive impairment, independent of age, education, and SAH, whereas spectral measures of autonomic balance do not appear to differ between WCI and CIND groups.
3.4. Heart Rate Variability and Cognitive Performance
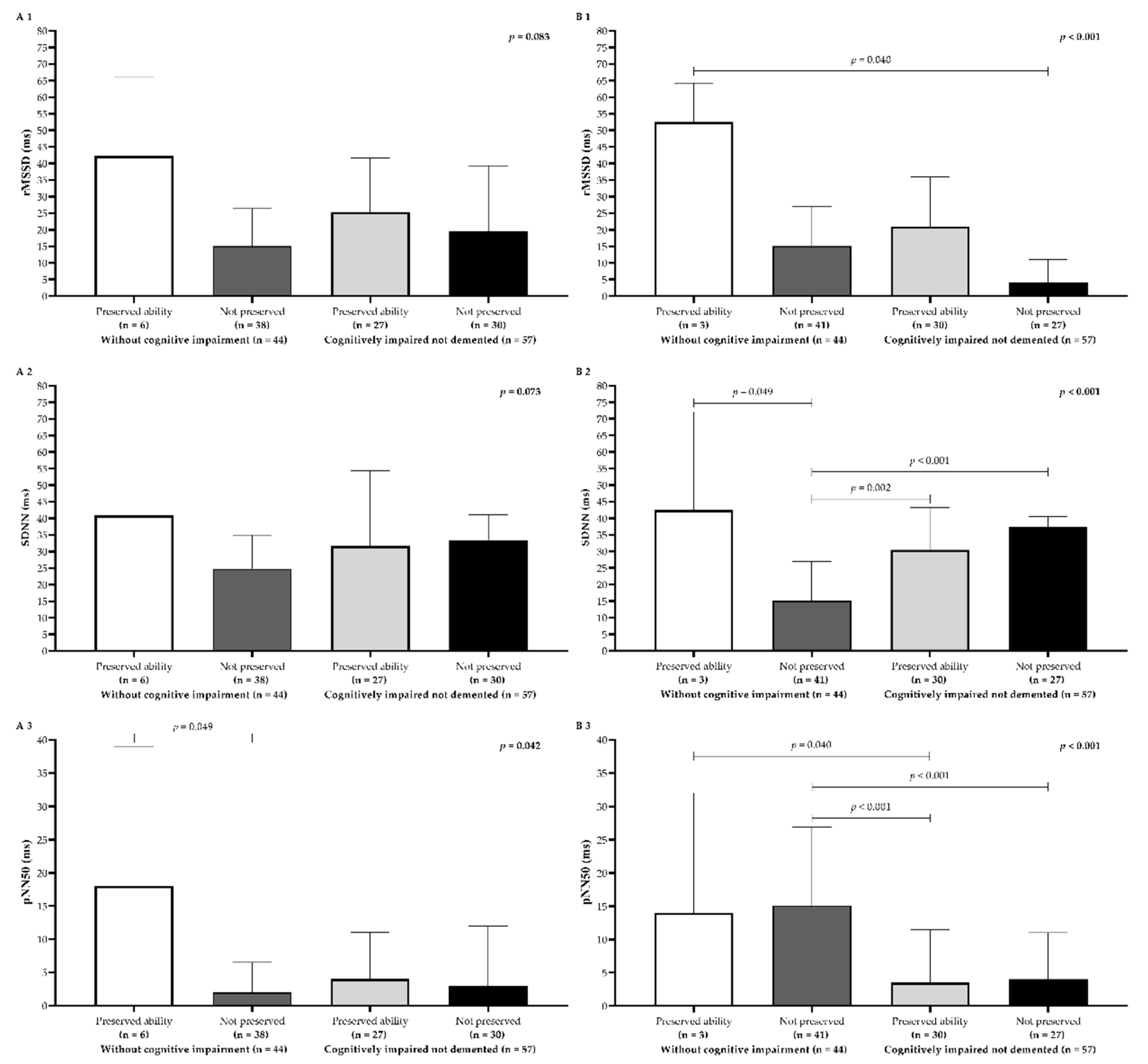
Older adults with WCI and preserved processing speed showed the highest median HRV values for all time-domain metrics (
Figure 2A1–A3). In addition, significant differences emerged among the groups, particularly for pNN50 (p = 0.04). Dunn’s post hoc test indicated that older adults with WCI and preserved processing speed had significantly higher pNN50 scores than those with WCI but without preserved processing speed (
Figure 2A3). A similar pattern was observed for executive function, where older adults with WCI and preserved executive function exhibited higher HRV medians for all metrics analyzed. Notably, the rMSSD parameter was significantly higher (p < 0.05) in the WCI group with preserved executive function compared to the CIND group without preserved executive function (
Figure 2B1). Furthermore, older adults with WCI but without preserved executive function had a statistically lower median SDNN (p < 0.001) than all other groups (
Figure 2B2). Additionally, older adults with WCI demonstrated higher median pNN50 (p < 0.05) than those with CIND but preserved executive function (
Figure 2B3). Finally, Dunn’s post hoc test also confirmed that the WCI group without preserved executive function had significantly higher pNN50 scores than the CIND group without preserved executive function (
Figure 2B3).
4. Discussion
The present study highlights a robust association between cognitive performance – specifically, information processing speed and executive function – and cardiac autonomic regulation, as reflected in time-domain HRV parameters. Older adults with preserved processing speed and executive function demonstrated higher HRV values, particularly for rMSSD, SDNN, and pNN50, suggesting a more adaptive parasympathetic modulation. In contrast, those with impaired cognitive performance exhibited markedly lower HRV indices, reinforcing the potential role of autonomic function in cognitive health. These findings align with previous research linking diminished HRV to cognitive deficits [
15,
36,
37] and suggest that parasympathetic activity plays a critical role in supporting higher-order cognitive processes, including attention, memory, and inhibitory control. Consequently, HRV may serve as a physiological marker of cognitive resilience in aging populations [
38,
39].
The subgroup analyses further elucidate this relationship, demonstrating that older adults with preserved processing speed exhibited the highest HRV values, whereas those with impaired performance displayed the lowest. Among the time-domain indices, pNN50 was particularly sensitive to differences in processing speed, reinforcing the role of parasympathetic modulation in rapid cognitive operations. Similarly, executive function impairments were associated with reductions in SDNN and rMSSD, suggesting that autonomic flexibility may be crucial for tasks requiring cognitive control, decision-making, and attentional regulation. These findings align with broader evidence that intact autonomic modulation supports cognitive efficiency and adaptability [
10].
Multiple studies have documented that cognitive impairment is associated with reduced HRV, particularly in short-term measures of vagal modulation [
9,
40]. In the present investigation, the clear reductions in SDNN, rMSSD, and pNN50 among cognitively impaired participants mirror prior observations demonstrating the sensitivity of these time-domain parameters to cognitive decline [
41]. Since SDNN captures overall autonomic influence, its decline in older adults with CIND may reflect a loss of dynamic control resulting from neurodegenerative processes affecting central autonomic pathways. The neurovisceral integration model [
42] helps illuminate why lower HRV and poorer cognitive status co-occur, emphasizing how the prefrontal cortex, anterior cingulate cortex, and insular cortex play integral roles in both higher-order cognition and autonomic regulation. Deterioration in these brain regions can manifest concurrent deficits in cognitive performance and HRV, a relationship supported by evidence that cholinergic dysfunction, implicated in neurodegenerative conditions, contributes to both reduced vagal tone and cognitive impairment [
4,
14].
In contrast to these findings in time-domain measures, the study did not reveal significant group differences in frequency-domain parameters (LF, HF, or LF/HF ratio) between older adults with and without cognitive impairment. Some prior investigations have found that lower HF power or changes in LF/HF ratio correlate with cognitive deficits [
9,
39]. However, the absence of significant differences here suggests that frequency-domain measures may be less sensitive – or become sensitive only later in disease progression – than short-term time-domain indices when detecting early or mild cognitive impairment. It is plausible that time-domain metrics capture more immediate fluctuations in autonomic tone closely tied to executive control and processing speed, whereas frequency-domain parameters may reflect more stable or long-term autonomic states [
28,
43]. Furthermore, methodological variations, including recording conditions, sample characteristics, and cognitive tasks employed, have the potential to contribute to the observed discrepancies across different studies [
44].
Several potential mechanisms may explain why reduced HRV often accompanies cognitive impairment. First, cholinergic dysfunction, commonly observed in Alzheimer’s disease and related dementias, compromises both parasympathetic regulation and cognitive performance [
14,
38]. Second, ANS dysfunction has been linked to vascular issues (e.g., arterial stiffness, endothelial dysfunction) that can limit cerebral blood flow, thereby affecting neural structures responsible for cognition [
44]. Third, chronic low-grade inflammation and oxidative stress can impair both the autonomic network and cognitive function, establishing a bidirectional relationship that exacerbates neurodegeneration [
45]. Finally, reduced HRV characterizes maladaptive stress responses, including hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, which in turn correlates with impaired memory and executive functioning [
10].
Given its non-invasive nature and relatively simple measurement protocols, HRV shows promise as a biomarker for identifying older adults at risk of cognitive decline. The current findings reveal moderate effect sizes (e.g., Cohen’s d around 0.39–0.44, partial eta squared ~0.04) in comparing cognitively intact and impaired groups, underscoring the practical significance of these differences. Future applications could involve screening tools that incorporate time-domain HRV indices, especially pNN50, rMSSD, and SDNN, as early indicators of subtle neurodegenerative processes. Moreover, incorporating HRV into routine evaluations could catalyze earlier interventions – such as autonomic biofeedback, vagal nerve stimulation, or mindfulness-based therapies – that bolster parasympathetic function and potentially mitigate further cognitive decline.
Nonetheless, certain limitations temper the generalizability of this study. Its cross-sectional design precludes conclusions about causality, leaving open questions about whether reduced HRV predicts future cognitive impairment or is simply a concurrent manifestation of degenerative changes. Additionally, the sample comprised community-dwelling older adults and may not reflect the broader population of individuals in long-term care facilities or those with more advanced dementia. While adjustments were made for age, education, and SAH, other factors like physical activity, sleep quality, medication, or comorbidities might confound HRV measures. Therefore, more controlled, longitudinal studies are warranted to elucidate the precise temporal relationship between changes in HRV and subsequent cognitive decline. Finally, employing more expansive neuropsychological batteries could help discriminate specific cognitive domains linked most strongly to autonomic dysregulation.
In summary, the present study strengthens the notion that time-domain HRV metrics are lower in older adults with cognitive impairment, signifying compromised parasympathetic modulation and reduced autonomic adaptability. The lack of significant differences in frequency-domain measures suggests that the relationship between autonomic regulation and cognition may manifest more consistently in short-term HRV fluctuations rather than in spectral components. Taken together, these findings underscore the potential for HRV to serve as an accessible, informative biomarker for cognitive health, warranting further research that integrates autonomic measures with neuroimaging, biochemical markers, and longitudinal designs to uncover the neurophysiological underpinnings of cognitive aging more comprehensively.
5. Conclusions
This study demonstrates a significant association between cardiac autonomic modulation and cognitive performance in older adults. Older adults with preserved processing speed and executive function showed higher time-domain HRV indices (SDNN, rMSSD, and pNN50), indicative of enhanced parasympathetic regulation. In contrast, those with cognitive impairment exhibited lower HRV, suggesting diminished autonomic flexibility.
The results underscore pNN50 as a particularly sensitive indicator of executive function, while SDNN appears closely linked to information processing efficiency. Notably, the absence of significant frequency-domain differences highlights the relevance of short-term HRV measures in capturing early cognitive changes. Given the growing interest in autonomic-cognitive interactions, incorporating HRV assessments into clinical evaluations could improve the identification of at-risk individuals. Future work should employ longitudinal designs and multimodal approaches to clarify causal pathways and explore interventions targeting autonomic function as potential strategies to bolster cognitive resilience.
Author Contributions
Conceptualization: P.A.M., L.A.R.M, P.E.L., M.J.S.R., J.M.D.M, and P.A.S.; Data curation: P.A.M. and J.M.D.M.; Formal analysis: P.E.L., A.C.C.S., R.d.C.M.d.S., M.J.S.R., J.M.D.M. and B.B.G.; Funding acquisition: P.A.S.; Investigation: P.A.M., L.A.R.M., P.E.L., A.C.C.S., A.S.L.R., J.M.D.M. and B.B.G.; Methodology: P.A.M., L.A.R.M., B.B.G. and P.A.S.; Project administration: P.A.S.; Resources: P.A.M., L.A.R.M., A.C.C.S. and A.S.L.R.; Software: R.d.C.M.d.S. and A.S.L.R.; Supervision: L.A.R.M. and M.J.S.R.; Validation: P.A.M. and R.d.C.M.d.S.; Visualization: A.C.C.S.; Writing – original draft: P.A.M., L.A.R.M., A.C.C.S., B.B.G. and P.A.S.; Writing – review & editing: P.E.L., R.d.C.M.d.S., A.S.L.R., M.J.S.R., J.M.D.M. and P.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco – FACEPE [grant numbers APQ-0246-4.06/14, APQ-1413-4.08/21]. This study also received partial financial support from the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) [Finance Code 001]. In addition, CAPES awarded a doctoral scholarship to Paula Andreatta Maduro, and Alaine Souza Lima Rocha was granted a doctoral scholarship by the FACEPE [grant number IBPG-0393-4.01/17]. Finally, Paulo Adriano Schwingel was awarded a Research Productivity Grant (BPP) from the FACEPE [grant number BPP-0003-4.01/24], and Ana Clara Castro Silva was supported by a scholarship from the EBSERH Technological Initiation Program.
Institutional Review Board Statement
The research adhered to the ethical principles of the Declaration of Helsinki (1964, revised in 2013) and complied with all regulatory requirements outlined in Resolutions 466/2012 and 510/2016 of the Brazilian National Health Council. The study was approved by the Research Ethics Committee of the School of Medical Sciences of Pernambuco (CEP-FCM/PE) under Evaluation Report Number 4.389.686 and Certificate of Presentation for Ethical Appraisal (CAAE) Number 38942320.4.0000.5192.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all the study participants for their invaluable contributions. We would also like to thank Dr. Godson Sebastião Chaves Teixeira Junior, MD (HU-UNIVASF/EBSERH), whose data collection and ongoing support were instrumental to the research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 1Q |
first quartile |
| 3Q |
third quartile |
| ADLs |
activities of daily living |
| ANCOVA |
Analysis of Covariance |
| ANS |
autonomic nervous system |
| BMI |
body mass index |
| BP |
blood pressure |
| CIND |
cognitively impaired not demented |
| EBSERH |
Brazilian Hospital Services Company |
| HF |
high frequency |
| HRV |
heart rate variability |
| HU |
Dr. Washington Antônio de Barros Teaching Hospital |
| IADLs |
instrumental activities of daily living |
| ICF |
Informed Consent Form |
| LF |
low frequency |
| MCI |
mild cognitive impairment |
| MMSE |
Mini-Mental State Examination |
| OH |
orthostatic hypotension |
| pNN50 |
percentage of successive R–R intervals that differ by more than 50 milliseconds |
| rMSSD |
root mean square of successive R–R interval differences |
| SDNN |
standard deviation of normal-to-normal intervals |
| TBM |
total body mass |
| TMT-A |
Trail Making Test Part A |
| TMT-B |
Trail Making Test Part B |
| UNIVASF |
Federal University of Vale do São Francisco |
| WCI |
without cognitive impairment |
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Nelson, M.E.; Jester, D.J.; Petkus, A.J.; Andel, R. Cognitive Reserve, Alzheimer’s Neuropathology, and Risk of Dementia: A Systematic Review and Meta-Analysis. Neuropsychol Rev 2021, 31, 233–250. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimers Dement 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Zulli, R.; Nicosia, F.; Borroni, B.; Agosti, C.; Prometti, P.; Donati, P.; De Vecchi, M.; Romanelli, G.; Grassi, V.; Padovani, A. QT Dispersion and Heart Rate Variability Abnormalities in Alzheimer’s Disease and in Mild Cognitive Impairment. J Am Geriatr Soc 2005, 53, 2135–2139. [Google Scholar] [CrossRef]
- Frewen, J.; Finucane, C.; Savva, G.M.; Boyle, G.; Coen, R.F.; Kenny, R.A. Cognitive Function Is Associated with Impaired Heart Rate Variability in Ageing Adults: The Irish Longitudinal Study on Ageing Wave One Results. Clin Auton Res 2013, 23, 313–323. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front Psychol 2014, 5. [Google Scholar] [CrossRef]
- Collins, O.; Dillon, S.; Finucane, C.; Lawlor, B.; Kenny, R.A. Parasympathetic Autonomic Dysfunction Is Common in Mild Cognitive Impairment. Neurobiol Aging 2012, 33, 2324–2333. [Google Scholar] [CrossRef]
- Galluzzi, S.; Nicosia, F.; Geroldi, C.; Alicandri, A.; Bonetti, M.; Romanelli, G.; Zulli, R.; Frisoni, G.B. Cardiac Autonomic Dysfunction Is Associated with White Matter Lesions in Patients with Mild Cognitive Impairment. J Gerontol A Biol Sci Med Sci 2009, 64, 1312–1315. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Are Changes in Heart Rate Variability in Middle-Aged and Older People Normative or Caused by Pathological Conditions? Findings From a Large Population-Based Longitudinal Cohort Study. J Am Heart Assoc 2016, 5. [Google Scholar] [CrossRef]
- McIntosh, R.C.; Khambaty, T.; Llabre, M.M.; Perreira, K.M.; Gonzalez, H.M.; Kansal, M.M.; Tarraf, W.; Schneiderman, N. Paradoxical Effect of Cumulative Stress Exposure on Information Processing Speed in Hispanics/Latinos with Elevated Heart Rate Variability. Int J Psychophysiol 2021, 164, 1–8. [Google Scholar] [CrossRef]
- Salthouse, T. Consequences of Age-Related Cognitive Declines. Annu Rev Psychol 2012, 63, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu Rev Psychol 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Pettigrew, C.; Soldan, A. Defining Cognitive Reserve and Implications for Cognitive Aging. Curr Neurol Neurosci Rep 2019, 19. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; Asmundis, C.D.E.; Magrini, F.; Brugada, P. The Prognostic Value of Heart Rate Variability in the Elderly, Changing the Perspective: From Sympathovagal Balance to Chaos Theory. Pacing Clin Electrophysiol 2012, 35, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Schaich, C.L.; Malaver, D.; Chen, H.; Shaltout, H.A.; Hazzouri, A.Z. Al; Herrington, D.M.; Hughes, T.M. Association of Heart Rate Variability With Cognitive Performance: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2020, 9. [Google Scholar] [CrossRef]
- Malta, M.; Cardoso, L.O.; Bastos, F.I.; Magnanini, M.M.F.; da Silva, C.M.F.P. STROBE Initiative: Guidelines on Reporting Observational Studies. Rev Saude Publica 2010, 44, 559–565. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int J Surg 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Ann Intern Med 2007, 147. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Exact Computation of Minimum Sample Size for Estimating Proportion of Finite Population. ArXiv 2007. [CrossRef]
- Brucki, S.M.D.; Nitrin, R.; Caramelli, P.; Bertolucci, P.H.F.; Okamoto, I.H. [Suggestions for Utilization of the Mini-Mental State Examination in Brazil]. Arq Neuropsiquiatr 2003, 61, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment; 3rd ed.; International Society for the Advancement of Kinanthropometry: Lower Hutt, 2011; ISBN 9780620362078.
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; de Magalhães Feitosa, A.D.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines of Hypertension - 2020. Arq Bras Cardiol 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Feitosa, A.D. de M.; Barroso, W.K.S.; Mion Junior, D.; Nobre, F.; Mota-Gomes, M.A.; Jardim, P.C.B.V.; Amodeo, C.; Oliveira, A.C.; Alessi, A.; Sousa, A.L.L.; et al. Brazilian Guidelines for In-Office and Out-of-Office Blood Pressure Measurement - 2023. Arq Bras Cardiol 2024, 121. [Google Scholar] [CrossRef]
- Gambassi, B.B.; Neves, V.R.; Brito, E.Z.A.; da Silva Fernandes, D.S.; Sá, C.A.; da Rocha Nogueira, R.M.; de Jesus Furtado Almeida, F.; de Araújo Cavalcanti, P.A.; Gomes Gonçalves E Silva, D.C.; Neto, D.S.; et al. A Validation Study of a Smartphone Application for Heart Rate Variability Assessment in Asymptomatic Adults. Am J Cardiovasc Dis 2020, 10, 219–229. [Google Scholar]
- Neves, V.R.; Takahashi, A.C.M.; Do Santos-Hiss, M.D.B.; Kiviniemi, A.M.; Tulppo, M.P.; De Moura, S.C.G.; Karsten, M.; Borghi-Silva, A.; Porta, A.; Montano, N.; et al. Linear and Nonlinear Analysis of Heart Rate Variability in Coronary Disease. Clin Auton Res 2012, 22, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996, 17, 354–381. [Google Scholar]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus Statement on the Definition of Orthostatic Hypotension, Neurally Mediated Syncope and the Postural Tachycardia Syndrome. Clin Auton Res 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. STUDIES OF ILLNESS IN THE AGED. THE INDEX OF ADL: A STANDARDIZED MEASURE OF BIOLOGICAL AND PSYCHOSOCIAL FUNCTION. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar]
- Brasil; Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica Ageing and Health of the Elderly Person; Ministério da Saúde: Brasília, 2006; ISBN 85-334-1273-8.
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test Errors in Normal Aging, Mild Cognitive Impairment, and Dementia. Arch Clin Neuropsychol 2008, 23, 129–137. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch Clin Neuropsychol 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Erceg-Hurn, D.M.; Mirosevich, V.M. Modern Robust Statistical Methods: An Easy Way to Maximize the Accuracy and Power of Your Research. Am Psychol 2008, 63, 591–601. [Google Scholar] [CrossRef]
- Grissom, R.J. Probability of the Superior Outcome of One Treatment over Another. Journal of Applied Psychology 1994, 79, 314–316. [Google Scholar] [CrossRef]
- Grässler, B.; Dordevic, M.; Darius, S.; Herold, F.; Forte, G.; Langhans, C.; Halfpaap, N.; Müller, P.; Glanz, W.; Dantas, E.H.M.; et al. Is There a Link between Heart Rate Variability and Cognitive Decline? A Cross-Sectional Study on Patients with Mild Cognitive Impairment and Cognitively Healthy Controls. Arq Neuropsiquiatr 2023, 81, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, C.U.D.; Hanson, L.M.; Theorell, T.; Osika, W.S. Executive Cognitive Functioning and Cardiovascular Autonomic Regulation in a Population-Based Sample of Working Adults. Front Psychol 2016, 7. [Google Scholar] [CrossRef]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural Correlates of Heart Rate Variability during Emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Rocha, A.S.L.; Siqueira, V. de B.; Maduro, P.A.; Batista, L. da S.P.; Schwingel, P.A. Reference Values for Heart Rate Variability in Older Adults: A Systematic Review. Psychophysiology 2024, 61. [Google Scholar] [CrossRef]
- Alharbi, E.A.; Jones, J.M.; Alomainy, A. Non-Invasive Solutions to Identify Distinctions Between Healthy and Mild Cognitive Impairments Participants. IEEE J Transl Eng Health Med 2022, 10. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; Malfatto, G.; Abbate, C.; Mari, D.; Rossi, P.D.; Pettenuzzo, E.; Magrini, F.; Consonni, D.; Lombardi, F. Autonomic Dysfunction in Mild Cognitive Impairment: Evidence from Power Spectral Analysis of Heart Rate Variability in a Cross-Sectional Case-Control Study. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the Heart-Brain Connection: Further Elaboration of a Model of Neurovisceral Integration. Neurosci Biobehav Rev 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Czopek-Rowinska, J.; de Bruin, E.D.; Manser, P. Diagnostic Accuracy of Heart Rate Variability as a Screening Tool for Mild Neurocognitive Disorder. Front Aging Neurosci 2024, 16. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Jurivich, D.A.; Gao, W.; Singer, D.H. Relation of High Heart Rate Variability to Healthy Longevity. Am J Cardiol 2010, 105, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of Neurodegenerative Diseases on Human Adult Hippocampal Neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).