1. Introduction
Pseudorabies (PR), a highly infectious disease affecting swine's nervous and respiratory systems, is caused by the Pseudorabies virus (PRV) - a double-stranded DNA virus from the herpes subfamily [
1]. PRV, primarily residing in swine, leads to neurological disorders in newborn piglets and reproductive failures in sows [
2]. Notably, since 2017, PRV-specific sequences have been detected in human tissues in China, with the first human-originated strain, hSD-1/2019, isolated from a patient's cerebrospinal fluid [
3,
4] Despite control efforts through the Bartha-K61 strain vaccine, the evolving PRV strains have reduced vaccine efficacy, underscoring the need for alternative control measures. Since the approval of the first antiviral drug in 1963, many drugs have been developed for clinical use. However, the emergence of drug-resistant PRV strains and unclear antiviral mechanisms necessitate new antiviral agents and targets for effective PRV management.
Recent trends in medical research favor extracting active ingredients from natural plants for disease prevention [
5]. It has been documented to regulate immune responses and inhibit cellular damage post-viral infection [
6]. Natural plants medicines like
Chrysanthemum indicum L. (
C.indicum L.),
Nepeta coerulescens,
Tribulus terrestris L,
Aconitum tanguticum, and
Belamcanda chinensis have long been used for various therapeutic purposes including anti-inflammatory, ativiral, and pain-relieving effects.
Belamcanda chinensis has been used in the form of antipyretic agents, antidote, expectorant, antiphlogistic, analgesic and antiviral agents [
7,
8,
9].Tectoridin, the primary compound in
Belamcanda chinensis, has been demonstrated to possess a spectrum of pharmacological effects, including recent findings of its ability to mitigate radiotherapy side effects [
6,
10]. As the mother root of the
ranunculaceae plant
Aconitum (wild species),
Aconitum tanguticum is traditionally used to alleviate colds, abdominal pain, fever, influenza, and food poisoning [
11,
12]. Most of the pharmacologically relevant compounds in
Aconitum tanguticum, including heteratisine, benzoyl heteratisine, atisine, hordenine, and tanulusine et al, belong to diterpenoid alkaloids [
13].
Aconitum tanguticum, with key compounds like heteratisine, has been noted for its antiviral efficacy against H1N1 and its therapeutic impact on acute lung injury through anti-inflammatory action [
14]. Total alkaloids of
Aconitum tanguticum improved the pathological changes in the lungs, and reduced inflammatory cell infiltration and pro-inflammatory cytokine release via inhibiting the NF-κB activation in LPS-induced acute lung injury models of rats [
15]. The 14-O-acetylneoline, isolated by Aconitum laciniatum, demonstrated significant inflammatory inhibition in mice treated with colitis [
16]. Additionally, certain studies have indicated effective anti-herps or other viruses from
Aconitum tanguticum [
13,
17,
18] and
Belamcanda chinensis [
9]. However, the mechanism of anti-herpes virus activity is still unclear.
This study investigates the anti-PRV potential of these natural plants medicines . We demonstrate that Aconitum tanguticum and Belamcanda chinensis exhibit optimal antiviral activity in vitro, limiting PRV transmission and pathology in infected mice. These findings highlight the anti-PRV bioactivity and therapeutic potential of natural plants medicines, providing a foundation for developing novel anti-PRV drugs and leveraging natural plants medicine resources.
2. Materials and Methods
2.1. Animals
Specific pathogen-free BALB/c mice (6-8 weeks old, 18-22g) were obtained from vitalriver (Beijing) Biotechnology Co. Ltd. All animals were housed under pathogen-free conditions, adhering to guidelines approved by the Management Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences.
2.2. Cells, Viruses, and Drugs
Vero cells, derived from African green monkey kidney cells, support the replication of various viruses like PEDV, PRV, HSV, VSV, etc. Vero cells obtained from ATCC were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, Cat 11965092, USA) supplemented with 10% foetal bovine serum (FBS) (BI, Cat 04-007-1A, Israel), penicillin (100 μg/mL) and streptomycin (100 μg/mL) (Gibco, Cat 15140-122, USA).
Pseudorabies virus (PRV) strain Fa was conserved in our laboratory. PRV Fa was propagated in Vero cells cultivated in DMEM supplemented with 2% FBS, penicillin (100 μg/mL) and streptomycin (100 μg/mL).
Plant extracts from Chrysanthemum indicum, Nepeta coerulescens, Tribulus terrestris, Aconitum tanguticum, and Belamcanda chinensis were prepared by water extraction. Soak 20 g Chinese herbs in 200 mL distilled water for 2 h, heat and boil and then simmer for 30 min. Filter the Chinese medicine liquid with 12 layers of gauze, then add 150 mL distilled water and repeat the above steps. The filtrate obtained from above two steps was combined and centrifuged at 4500 r/min for 20 min. The drug was heated and concentrated to 1 g/mL, and stored at 4℃ for use.
2.3. Antibodies and Reagents
Western blot was performed using the following antibodies: mouse anti-PRV VP5 monoclonal antibody (prepared by our laboratory, 1:1000), mouse anti-GAPDH monoclonal antibody (Beyotime Institute of Biotechnology AG019, China, 1:1000).
Immunohistochemistry was performed using mouse anti-PRV VP5 monoclonal antibody (prepared by our laboratory, 1:500).
2.4. Determination of Half Maximal Tissue Culture Infective Dose (TCID50)
Vero cells were seeded in 96-well plates with a density of 4×104 cells per well one day before compounds were added, and then added 100 μL virus in different dilution to each well with 8 repeat wells per dilution. In the control group, 100 μL PBS was used to replace the disease venom, and then 100 μL fresh DMEM culture solution was added to each well. Cell culture plates were placed in an incubator of 37℃ and 5% CO2. The cytopathic and noncytopathic plate wells has been observed for 5-7 days. The TCID50 was calculated by Reed-Muench formula method.
2.5. Cytotoxicity Assay
The cytotoxic activity was firstly evaluated by checking the morphological change of cells incubated with drugs at different concentrations. The cell toxicity was measured using CCK-8 kit (Beyotime), which is a water-soluble tetrazolium salt-8 (WST-8) reagent. Briefly, Vreo cells were seeded in 96-well plates with a density of 4×104 cells per well one day before compounds were added. Then 100 μL of two-fold dilution of liquid medicine was added to each well and incubated for another 48 h. After incubation, 10 μL of CCk-8 reagent was added into each well and incubated for 60 min at 37℃ with 5% CO2. The absorbance at 450 nm was measured using the Enspire 2300 Multiable reader (PerkinElmer). The 50% cytotoxic concentration (CC50) was calculated by comparing the viability of test compounds-treated cells with that of DMSO-treated cells.
2.6. Test of Inhibition of PRV Infection In Vitro
To investigate the drugs antiviral effect during Vero cells infection of PRV, three modes of actions were designed, including prevent virus invasion (cell pre-treat), inhibition of PRV replication (cell post-treat) and direct targeting PRV (virus pre-treat).
2.6.1. Prevention of PRV Infection
After cell culture plates were filled with Vero cells, different concentrations of drug solution were added, then the cell culture plates were cultured in a 37 ℃ incubator with 5% CO2 for 1 h. The drug solution was discarded, and then 100 μL of 100 TCID50/0.1 mL PRV was added to each well. After incubation for 2 h, the virus was discarded. Wash with PBS for 3 times, then add freshly prepared 2% maintenance solution and culture in a 37 ℃ incubator with 5% CO2.
2.6.2. Inhibition Effect of PRV Replication
After culture plates were filled with Vero cells, the 100 TCID50/0.1 mL of PRV was inoculated for 2 h. The venom was sucked and washed with PBS for 3 times, then different concentrations of the drug liquid were added, and then cultured in a 37 ℃ incubator with 5% CO2 until the samples collection.
2.6.3. Direct Direct Targeting PRV
After cell culture plates were filled with Vero cells, a mixture of 100 μL/well of the same amount of drug and virus were at 37℃ for 1 h, then washed the cells for 3 times with PBS and added above mixture. The cell culture plates were incubated for 2 h, and then the virus was discarded. Wash with PBS for 3 times, then add freshly prepared 2% maintenance solution and culture in a 37 ℃ incubator with 5% CO2.
2.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from the cells using the TRIzol Reagent (Invitrogen 15596-018, USA). Reverse transcription was performed using the HiScript III RT SuperMix for qPCR (+gDNA wiper) Kit (Vazyme R323-01, China), and qPCR amplification was carried out using an ABI QuantStudio 7 Real-Time PCR System (Applied Biosystems, USA) with power SYBR Green PCR Master Mix (Takara, RR820B, Japan) to determine the mRNA levels of the targeting genes (the Wip1 gene of Vero, the gC gene of PRV). The thermal cycling conditions were 95 ℃ for 30 s, followed by 39 cycles of 95 ℃for 5 s, 50-60℃ for 30 s, and 72℃ for 30 s. The relative amounts of RNAs in each sample were normalized to the expression of β-actin as an internal control by 2
-ΔΔCt method. Primers were designed using Oligo 7 software and are shown in
Table 1.
2.8. Western Blotting
Cell samples were lysed in RIPA cell lysis buffer (Beyotime P0013B, China) containing protease inhibitor (Beyotime P1005, China) on ice for 30 min. Cell lysates were then centrifuged at 10,000 rpm and 4 °C for 10 min to remove the insoluble fraction. The supernatant was then mixed with 5×SDS-PAGE loading buffer and boiled at 100℃ for 10 min. Extracted proteins were subjected to 12% SDS-PAGE and transferred onto polyvinyl difluoride (PVDF) membranes (Merck-Millipore ISEQ00010, Germany). The blots were blocked using PBS + 0.05% Tween 20 (PBST) with 3% bovine serum albumin (BSA) (Yuanye S12012, China) for 1 h at room temperature. Then membranes were incubated with the indicated primary antibodies overnight at 4˚C. After washing in PBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. The membranes were washed again with PBST, and the indicated proteins were detected with Pierce™ ECL Western Blotting Substrate (ThermoFisher Scientific 32106, USA) using the Tanon 5200 automatic chemiluminescence analysis system. The level of target protein expression was analyzed using ImageCal software (Tanon, China).
2.9. PRV Infection of Mice
BALB/c mice were randomly assigned into six groups, including mock, normal saline (NS), (150 and 300 mg/kg) of Aconitum tanguticum and (800 and 1600 mg/kg) of Belamcanda chinensis. Two days before PRV challenge, mice in groups were given drugs via intragastric administration every 24 hours. On the third day, PRV was intraperitoneally injected with the doses of 100 μl virus/mouse (N=8/group), and the drugs was administered 1 h after the challenge. Then the drugs were intragastric administration every 24 h in every groups. Clinical symptoms were observed every 12 h after infection, and changes in body weight were recorded every 24 h.
2.10. Immunohistochemical Analysis and H&E Staining
Brains, lungs, livers and kidneys were removed from euthanized mice and fixed in 4% formalin at room temperature for 48 h. Serial tissue sections 3 μm thick were obtained after embedding in paraffin. Each slide was stained with hematoxylin and eosin (H&E) and subjected to immunohistochemical analysis using antibodies to PRV-gC, and then examined under light microscopy (Olympus BX43, Olympus Optical Co., Tokyo, Japan). Criteria for histopathological changes were as follows: 0 = no microscopic lesions; 1 = extremely mild, characterized by mild cellular degeneration and desquamation of epithelial cells; 2 = mild, characterized by desquamation of rare epithelial cells, hyperemia; 3 = moderate, characterized by desquamation of epithelial cells, hyperemia, loosen and obvious edema of blood vessel walls and slight inflammatory cell infiltration; 4 = severe, which is characterized by hyperemia, hemorrhage, loosen and edema of blood vessel wall, more inflammatory cell infiltration and sloughed epithelial cells. PRV-gC antigen was measured using a ranked score of 0–4 to evaluate of positive cells per section from each block. Scores were as follows: 0 = no positive cells, 1 = 1–20 positive cells, 2 = 21–50 positive cells, 3 = 51–100 positive cells and 4 = or > 100 positive cells.
2.11. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). All data are presented as mean ± standard deviation (SD). Differences in indicators between the treatment groups and controls were assessed using a t-test. A two-tailed probability (p) value of < 0.05 was considered statistically significant.
3. Results
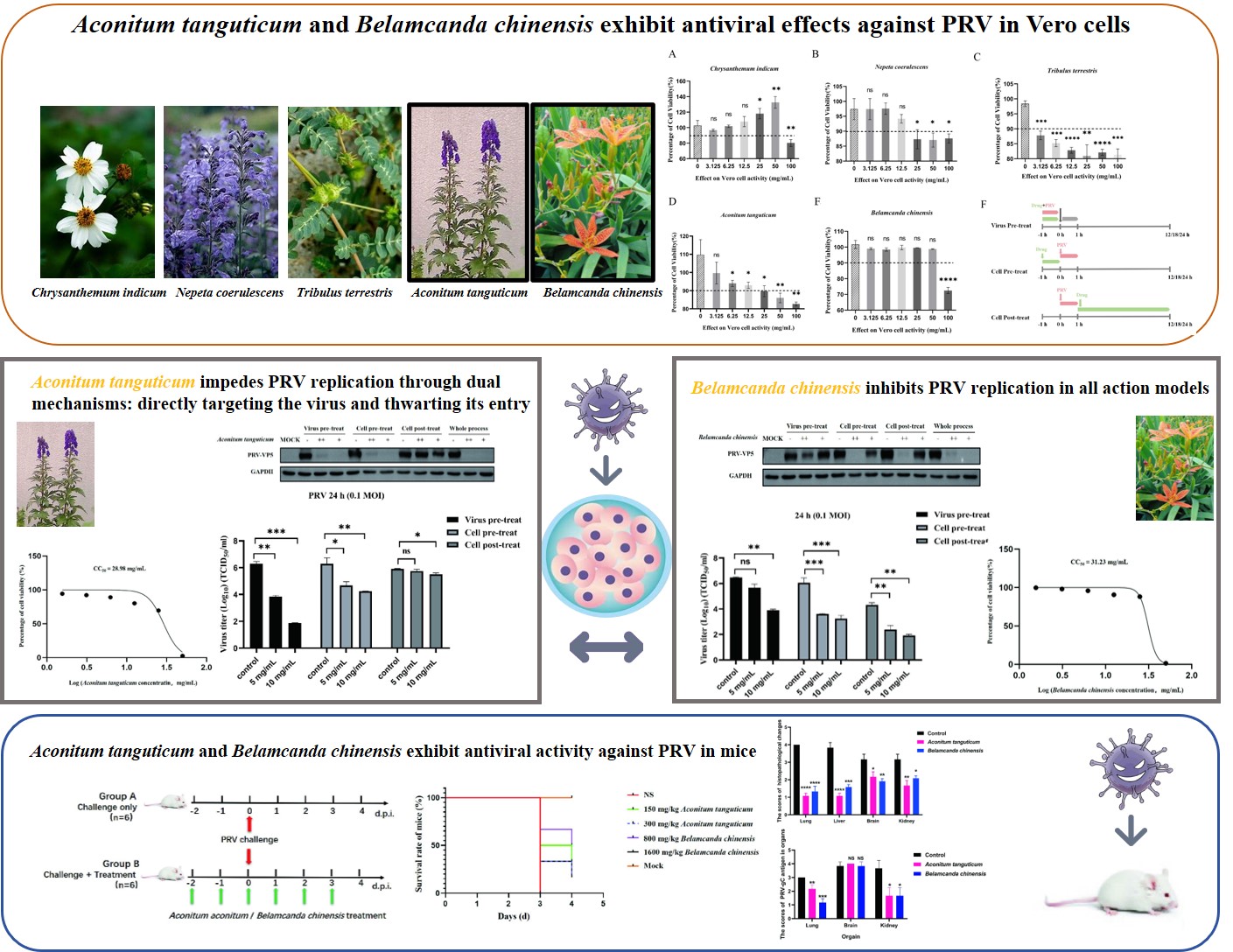
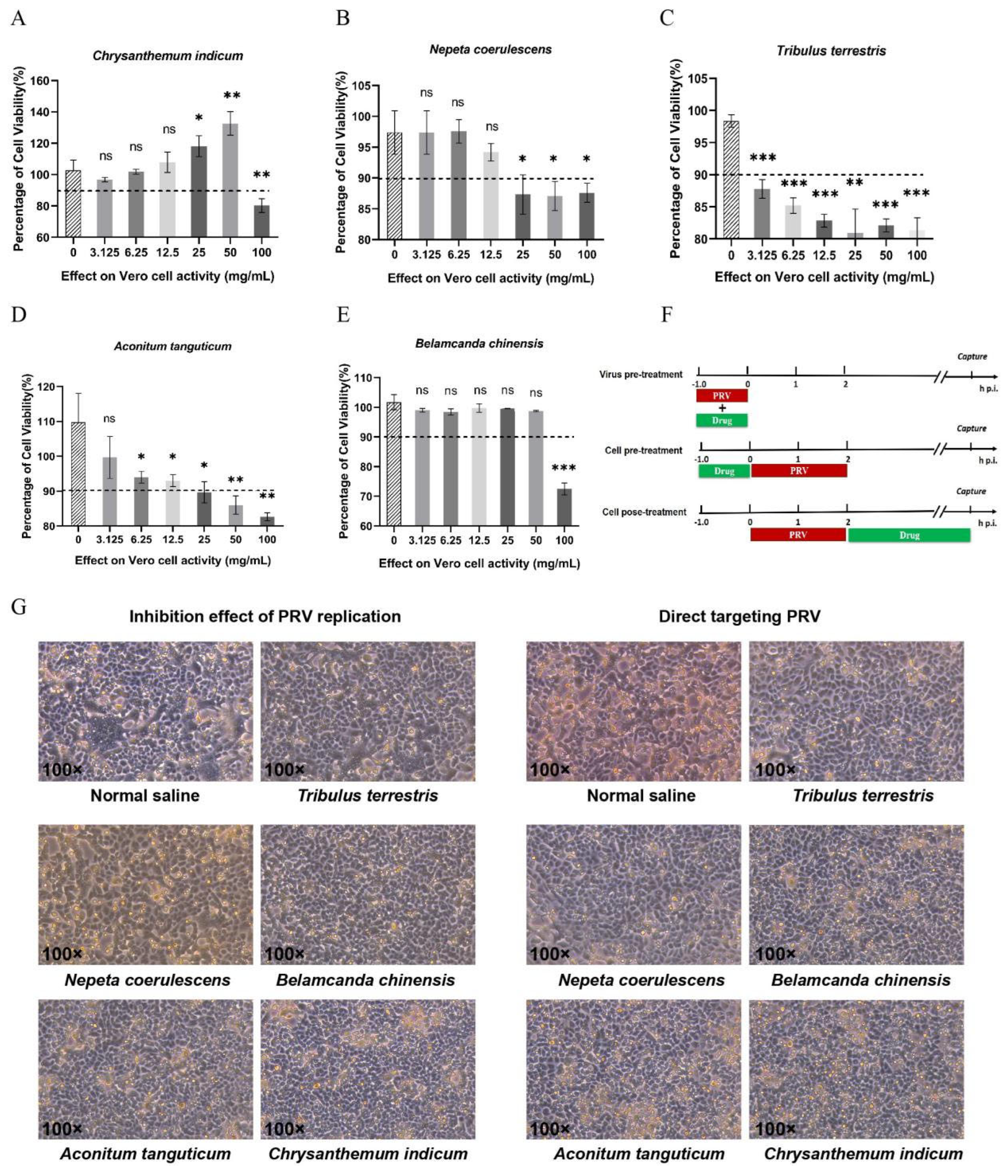
3.1. Aconitum tanguticum and Belamcanda chinensis Exhibit Antiviral Effects Against PRV in Vero Cells
Using the CCK-8 assay, non-toxic concentrations of various herbal extracts were determined, ensuring cell viability above 90%. The effective ranges included
Chrysanthemum indicum (0-50 mg/mL)(
Figure 1A),
Nepeta coerulescens (0-12.5 mg/mL) (
Figure 1B),
Tribulus terrestris (0-3.125 mg/mL) (
Figure 1C),
Aconitum tanguticum (0-25 mg/mL) (
Figure 1D) and
Belamcanda chinensis (0-50 mg/mL) (
Figure 1E). Three drugs action models were designed for the mechanism exploration (
Figure 1F). In the models of inhibition of replication (cell post-treat) and direct killing effect on PRV (virus pre-treat), five drugs displayed antiviral effects, alleviating cytopathy of syncytial lesions induced by PRV infection.
Aconitum tanguticum and
Belamcanda chinensis have obvious inhibitory effect on the cytopathic effect caused by PRV (
Figure 1G), warranting further research into their antiviral mechanisms and therapeutic potential.
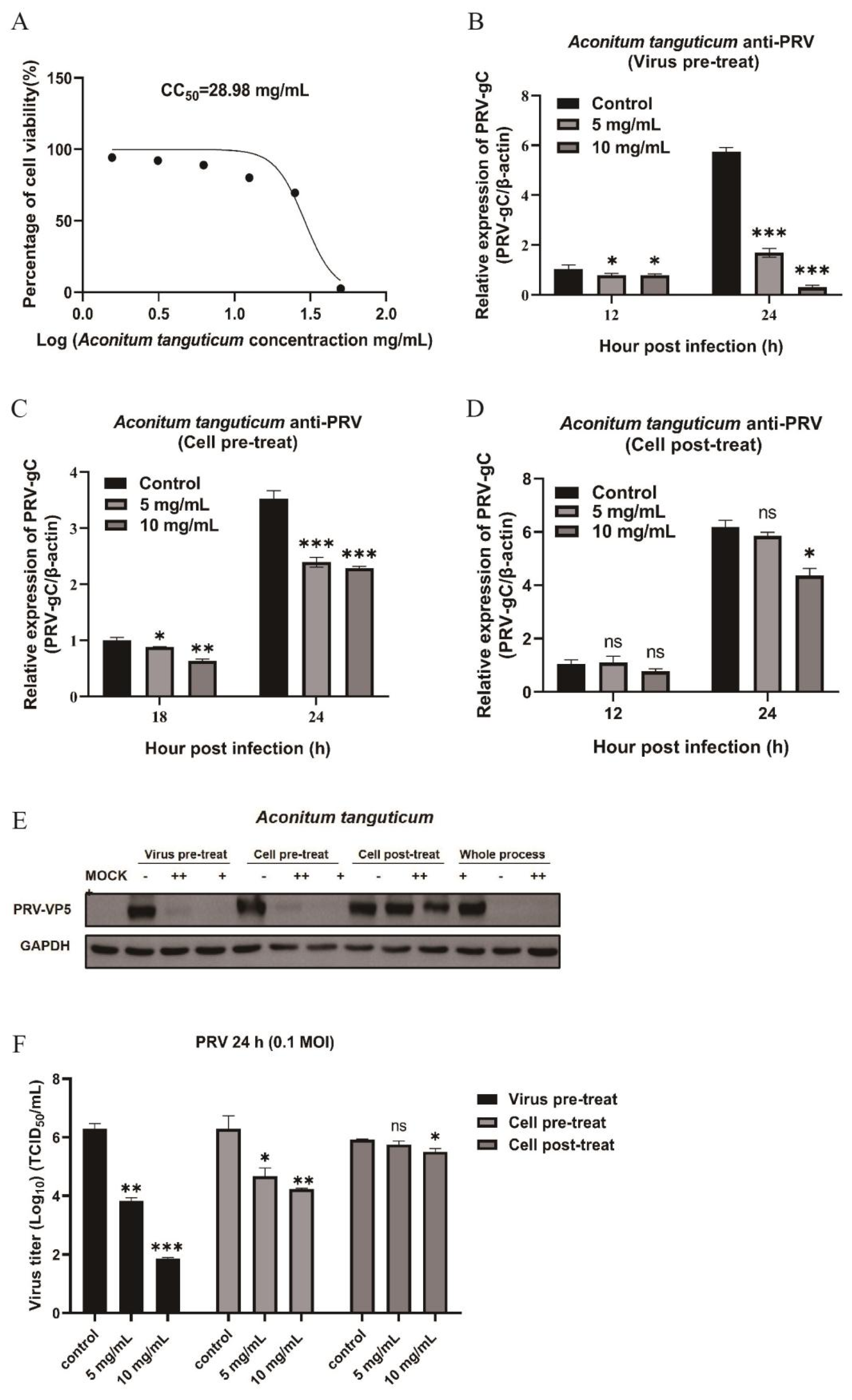
3.2. Aconitum tanguticum Impedes PRV Replication Through Dual Mechanisms: Directly Targeting the Virus and Thwarting Its Entry
For the purpose of evaluating antiviral action of
Aconitum tanguticum, we detected the cytotoxicity using the CCK-8 assay. Experiments confirm its CC
50 of 28.98 mg/mL (
Figure 2A) and dose-dependent antiviral action, with a significant reduction in viral titers observed at 5 mg/mL and 10 mg/mL concentrations especially in the action model of directly targeting the virus (
Figure 2F). The herb's effect is more pronounced in preventing virus entry rather than combating PRV post-infection, suggesting its primary mode of action is pre-entry interference (
Figure 2B, 2C, 2D). Concurrent drug treatment of the virus and cells markedly hampers infection, as corroborated by western blot analysis (
Figure 2E). Therefore,
Aconitum tanguticum's primary antiviral strategy appears to be direct viral neutralization and blockade of viral entry.
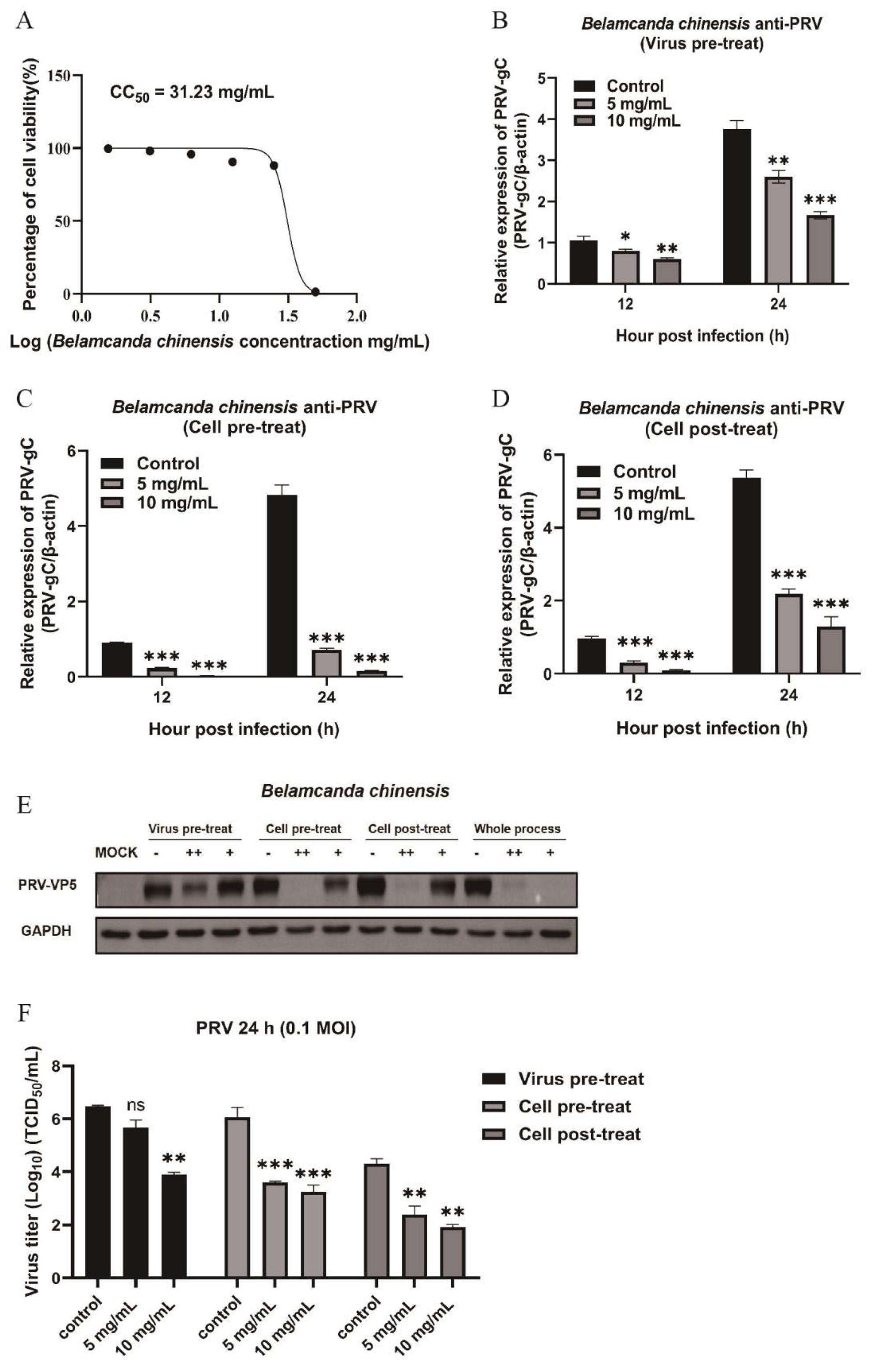
3.3. Belamcanda chinensis Inhibits PRV Replication in All Action Models
Belamcanda chinensis demonstrates comprehensive antiviral effects against PRV, impacting all stages of viral infection in Vero cells. The CCK-8 assay confirmed its CC
50 of 31.23 mg/mL (
Figure 3A). It significantly lowers PRV-gC mRNA (
Figure 3B, 3C, 3D) and protein (
Figure 3E) levels whether administered cell pre- or post-infection, significantly reducing viral titers at a concentration of 10 mg/mL (
Figure 3F).
Belamcanda chinensis also displayed directly targeting PRV virion to inhibit PRV replication at the condition of high drug concertation in the virus pretreatment mode. (
Figure 3B, E, F).
Belamcanda chinensis acts by directly targeting virus, preventing virus invasion and inhibiting replication process after virus entry into cells, suggesting a robust, multiphase antiviral effect.
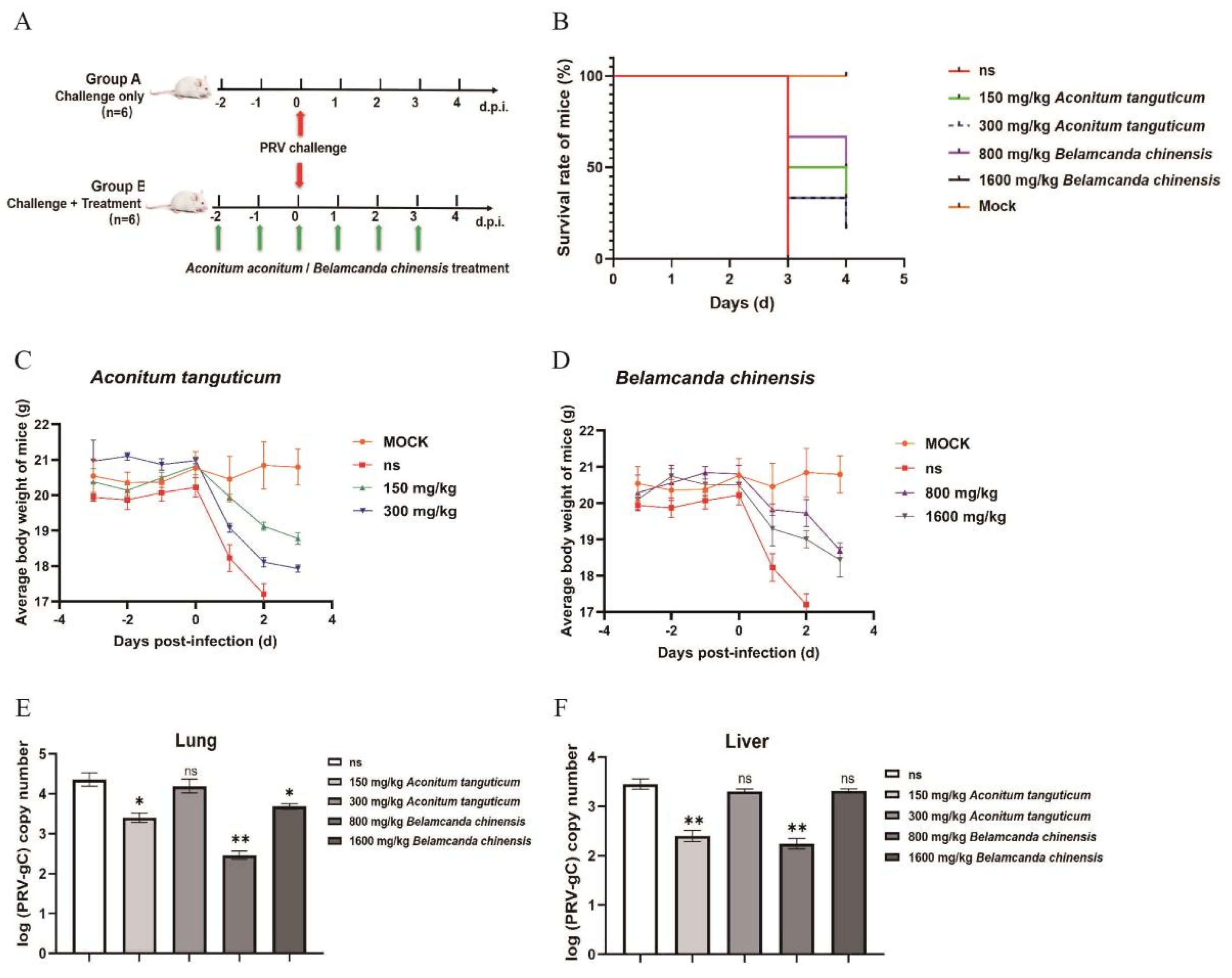
3.4. Aconitum tanguticum and Belamcanda chinensis Exhibit Antiviral Activity Against PRV in Mice
In our investigation into the antiviral properties of
Aconitum tanguticum and
Belamcanda chinensis against Pseudorabies virus (PRV) in mice, we observed that these natural medicines have a notable impact on the course of viral infection. Our study, conducted on 6-8 week old Balb/c mice, revealed a stark contrast between untreated and treated groups (
Figure 4A). Mice exhibiting severe clinical symptoms such as depression, loss of appetite, and neurological impairments after PRV exposure showed remarkable improvement following treatment with either herb at a dosage of 25 mg/kg. Not only did these treatments enhance the body weight (
Figure 4C, 4D) and prolong the survival of the infected mice (
Figure 4B), but they also substantially decreased the viral burden in the lungs (
Figure 4E) and livers (
Figure 4F), as illustrated in our findings. Both of
Aconitum tanguticum and
Belamcanda chinensis antiviral ability are related to the drug dose in vitro validation. However, no significant antiviral enhancement effect has been discovered in vivo with the increased doses. This may be attributed to the comprehensive mechanism of drugs action and the body complexity. In addition, we chose the oral dosage according to acute toxicity data. The lack of data on chronic toxicity of drugs may be one of the reasons for this phenomenon. Inappropriate doses may cause potential unknown damage to the body, thus diminishing the efficacy of antiviral therapy.
After two days of drug treatment, mice infected by PRV were euthanized by cervical dislocation and dissected to observe the lesions of organs. PRV infection typically leads to acute lesions, including lung hemorrhage, hepatomegaly, spleen and kidney atrophy, and cerebral edema (
Figure 5A). The lesions of organs were significantly mitigated upon treatment with
Aconitum tanguticum and
Belamcanda chinensis. This mitigation is evidenced by reduced inflammation and erythrocyte infiltration in the affected organs as determined by hematoxylin and eosin (H&E) staining (
Figure 5B), and reduced PRV virions as determined by immunohistochemical analyses (
Figure 5C). Severe pathological changes of multi-organs were observed in PRV infected mice, which characterized by massive hemorrhaging in the alveolar cavities and alveolar septa widened, hepatocyte steatosis and necrosis, cerebral cortical inflammatory cell aggregation, glial degeneration, necrosis and disintegration, renal hemorrhage, and renal tubular epithelial cell necrosis and shedding. However, monotherapy using
Aconitum tanguticum or
Belamcanda chinensis treatment alleviated above organs damage. There was no bleeding in the alveolar cavity, and only slightly thickening of the alveolar septum was observed. Both of above drugs alleviated the degeneration and necrosis of hepatocytes and renal tubular epithelial cells, as well as reduced inflammation in the cerebral cortex (
Figure 5B). The histopathological changes and number of positive signals in the organs differed significantly between challenge group and treatment groups (
Figure 5D, 5E). Notably, PRV gC protein-positive signals were observed in Brain pyramidal cells, which transmit excitatory signals. While both herbs proved effect in alleviating the damage to the lungs, livers, and kidneys, a notable observation was none PRV positive signals reduction in the brain during drugs treatment (
Figure 5C). This could be attributed to the difficulty these compounds face in traversing the blood-brain barrier. Despite this limitation, the treated mice displayed fewer PRV gC protein-positive signals in more accessible organs, suggesting the drug efficacy in areas reachable via systemic circulation.
Our results highlight the potential of Aconitum tanguticum and Belamcanda chinensis as antiviral agents in the treatment of PRV. Their ability to ameliorate symptoms and reduce viral replication in peripheral organs is promising, albeit their limited action in neural tissues due to the blood-brain barrier. The scope of their impact calls for further exploration, particularly in overcoming the challenges of delivering these compounds to the central nervous system.
4. Discussion
This study explores the antiviral potential of natural plant medicine, specifically
Aconitum tanguticum and
Belamcanda chinensis, against the Pseudorabies virus (PRV). The natural drugs have been discovered the advantages in stimulating and mobilizing the immune defense system, playing an indirect antiviral role [
14,
19]. Pharmacological studies have revealed multifaceted effects of
Aconitum tanguticum, including anti-inflammatory, analgesic, anti-arrhythmic, anti-tumor, and insecticidal properties.
Belamcanda chinensis has been used to treat conditions such as tonsillitis and lower back pain. Its antiviral properties extend to targeting viruses like influenza, HSV-1, and bovine viral diarrhea virus [
8,
9,
20]. The antiviral capacity of these herbs against PRV is a novel finding of our study. Our research not only elucidates their inhibitory effects and mechanisms on PRV but also underscores their potential as broad-spectrum antiviral candidates.
The selected natural plants, including
Chrysanthemum indicum,
Nepeta coerulescens, and
Tribulus terrestris, along with
Aconitum tanguticum and
Belamcanda chinensis, have exhibited varied antiviral effect
in vitro (
Figure 1). Notably,
Aconitum tanguticum showed a pronounced inhibition rate of over 90% at a concentration of 10 mg/mL in virus pretreatment models and produced an inhibition of virus titer of 10
4 orders of magnitude for PRV (
Figure 2).
Belamcanda chinensis, on the other hand, displayed a distinct mechanism, significantly reducing expression levels of PRV-gC protein and the virus titer through its action on host cells, particularly by inhibiting the replication process after virus entry into cells (
Figure 3).
In vivo testing on Balb/c mice demonstrated that oral administration of these drugs before and after PRV challenge extended survival times and mitigated clinical symptoms (
Figure 4). Notably, PRV infection, which predominantly targets pyramidal cells in the brain, induced severe symptoms like hemorrhage in the alveolar cavity, hepatocyte steatosis, and liver and kidney congestion. Treatment with
Aconitum tanguticum and
Belamcanda chinensis alleviated these symptoms, although they were less effective in preventing PRV infection of brain pyramidal cells (
Figure 5). Interestingly, a higher drug dosage correlated with a reduced protective effect, suggesting a need for further evaluation of the drugs' toxicity.
While the primary antiviral components of Aconitum tanguticum and Belamcanda chinensis warrant further investigation, the diverse antiviral action models of these drugs have been substantiated in this study. Their ability to alleviate clinical symptoms and the potential synergistic effects with other antiviral drugs suggest promising avenues for enhanced clinical treatment strategies against PRV. Our findings align with the millennia-old use of natural plant medicines in epidemic disease prevention and treatment, highlighting their significant role as antiviral agents.
5. Conclusions
We demonstrate that Aconitum tanguticum and Belamcanda chinensis exhibit antiviral activity in vitro with different action mechanism, limiting PRV transmission and pathology in infected mice. Aconitum tanguticum showed a pronounced inhibition effect in virus pretreatment models. Belamcanda chinensis, on the other hand, displayed a distinct mechanism particularly by inhibiting the replication process after virus entry into cells.
Author Contributions
Zhaohua Wang: Conceptualization, writing-original draft, writing-review & editing; Wei Dai: data curation, formal analysis; Xiaohuan Yan: investigation, validation; Tianyi Leng: writing-original draft; Zhenya Su: investigation, methodology; Zhenni Yan: investigation, methodology; Songda Li: investigation; Ming Li: data curation, formal analysis, writing-review & editing; Songli Li: Conceptualization, project administration, resource, supervision, funding acquisition.
Funding
This study received no specific grants from any funding agency.
Institutional Review Board Statement
The experimental design and protocols used in this study were approved by the Ethics Committee and Animal Research Committee of the IAS, CAAS (IAS2023-64).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The funders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Conflicts of Interest
The authors have no financial conflicts of interest.
References
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005, 69, 462-500. [CrossRef]
- Liu, Q.; Kuang, Y.; Li, Y.; Guo, H.; Zhou, C.; Guo, S.; Tan, C.; Wu, B.; Chen, H.; Wang, X. The Epidemiology and Variation in Pseudorabies Virus: A Continuing Challenge to Pigs and Humans. Viruses. 2022, 14. [CrossRef]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused By Pseudorabies Virus Infection, China, 2017. Emerg Infect Dis. 2018, 24, 1087-1090. [CrossRef]
- Zhao, W.L.; Wu, Y.H.; Li, H.F.; Li, S.Y.; Fan, S.Y.; Wu, H.L.; Li, Y.J.; Lü, Y.L.; Han, J.; Zhang, W.C.; Zhao, Y.; Li, G.L.; Qiao, X.D.; Ren, H.T.; Zhu, Y.C.; Peng, B.; Cui, L.Y.; Guan, H.Z. [Clinical experience and next-generation sequencing analysis of encephalitis caused by pseudorabies virus]. Zhonghua Yi Xue Za Zhi. 2018, 98, 1152-1157. [CrossRef]
- Liu, D.; Zhong, W.; Zhu, Y.; Wu, H.; Du, S. Research progress on pharmacological effects of high-frequency drugs of Tibetan medicine in treating novel coronavirus pneumonia. Chinese Traditional Patent Medicine. 2022, 44, 2249-2256.
- Zhang, K.; Wang, L.; Peng, J.; Sangji, K.; Luo, Y.; Zeng, Y.; Zeweng, Y.; Fan, G. Traditional Tibetan medicine to fight against COVID-19: Basic theory and therapeutic drugs. Front Pharmacol. 2023, 14, 1098253. [CrossRef]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2016, 186, 1-13. [CrossRef]
- Shi, L.; Ji, Z.; Kang, W. Clinical application of Shegan antiviral Belamcanda chinensis injection. China Pharmacy. 2012, 23, 3359-3360.
- Guan, Z.; Li, G.; Meng, L.; Chen, H.; Zhao, J. antiviral research in vitro of Belamcanda chinensis active components Chinese Archives of Traditional Chinese Medicine. 2015, 33, 1814-1816.
- Wen, W.; Ma, Y.; Zhu, J.; Zou, G.; Qin, W.; Li, G. Traditional efficacy research and experimental pharmacology verification of Belamcanda chinensis. World Science and Technology-Modernization of Traditional Chinese Medicine 2017, 19, 846-850.
- Chunjiang, Z.; Xiaobin, H.E.; Fengkong, D.U.; Aiping, L.I.U.; Hongyu, L.I. Antibacterial Activity of Eight Tibetan Medicinal Plants in Vitro. Chinese Journal of Modern Applied Pharmacy. 2010, 27, 99-104.
- Yang, L.; Lin, L.; Wang, Z.; Li, C.; Li, Z. Research Progress on Aconitum tanguticum. Chinese Journal of Experimental Traditional Medical Formulae. 2016, 22, 43-49.
- Zhang, C.-J.; Li, W.; Li, H.-Y.; Wang, Y.-L.; Yun, T.; Song, Z.-P.; Song, Y.; Zhao, X.-W. In vivo and in vitro antiviral activity of five Tibetan medicinal plant extracts against herpes simplex virus type 2 infection. Pharmaceutical Biology. 2009, 47, 598-607. [CrossRef]
- Fan, X.; Yang, L.; Liu, Z.; Lin, L.; Li, C.; Guo, S.; Wang, Z.; Wang, Z.; Sui, F. Diterpenoid alkaloids from the whole plant of Aconitum tanguticum (Maxim.) Stapf. Phytochemistry. 2019, 160, 71-77. [CrossRef]
- Wu, G.; Du, L.; Zhao, L.; Shang, R.; Liu, D.; Jing, Q.; Liang, J.; Ren, Y. The total alkaloids of Aconitum tanguticum protect against lipopolysaccharide-induced acute lung injury in rats. J Ethnopharmacol. 2014, 155, 1483-1491. [CrossRef]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci Rep. 2015, 5, 12845. [CrossRef]
- Wang, D.; Zhang, K.; Wang, X.; Wang, H.; Wang, L.; Zhang, K.; Zhang, J.; Li, J.; Wang, X. Inhibitory effects of extracts from Terminalia chebula, Corydalis hendersonii, Aconitum tanguticum on bovine viral diarrhea virus in vitro. Acta Veterinaria et Zootechnica Sinica. 2018, 49, 2036-2043.
- Zhang, C.J.; Li, W.; Li, H.Y. In vivo and in vitro antiviral activity of five Tibetan medicinal plant extracts against herpes simplex virus type 2 infection. Pharmaceutical Biology. 2009, 47, 598-607. [CrossRef]
- Zhou, Y.; He, Y.J.; Wang, Z.J.; Hu, B.Y.; Xie, T.Z.; Xiao, X.; Zhou, Z.S.; Sang, X.Y.; Luo, X.D. A review of plant characteristics, phytochemistry and bioactivities of the genus Glechoma. J Ethnopharmacol. 2021, 271, 113830. [CrossRef]
- Li, B.; Zhang, X.; Guan, Z.; Han, T. Clinical observation of Belamcanda chinensis antiviral injection combined with ribavirin in the treatment of herpes zoster. Qingdao Medical Journal 2007, 39, 15-16.
Figure 1.
Anti-PRV effects of five drugs in Vero cells. (A-E) Toxic effects of Chrysanthemum indicum, Nepeta coerulescens, Tribulus terrestris, Aconitum tanguticum, and Belamcanda chinensis were detected on Vero cells. Five medicines were diluted to different concentrations (two-fold dilution of liquid medicine from 0 mg/mL to 100 mg/mL) and added to Vero cells laid on 96-well plate. Forty-eight hours later, the cells viability was detected by CCK-8 regent. (F) Three antiviral action models of drugs in host cells. (G) Chrysanthemum indicum (50 mg/mL), Nepeta coerulescens (12.5 mg/mL), Tribulus terrestris (3 mg/mL), Aconitum tanguticum (25 mg/mL) and Belamcanda chinensis (50 mg/mL) were added to Vero cells laid on 12-well plates, with the action models of inhibition of replication (cell post-treat) and direct targeting PRV (virus pre-treat). Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 1.
Anti-PRV effects of five drugs in Vero cells. (A-E) Toxic effects of Chrysanthemum indicum, Nepeta coerulescens, Tribulus terrestris, Aconitum tanguticum, and Belamcanda chinensis were detected on Vero cells. Five medicines were diluted to different concentrations (two-fold dilution of liquid medicine from 0 mg/mL to 100 mg/mL) and added to Vero cells laid on 96-well plate. Forty-eight hours later, the cells viability was detected by CCK-8 regent. (F) Three antiviral action models of drugs in host cells. (G) Chrysanthemum indicum (50 mg/mL), Nepeta coerulescens (12.5 mg/mL), Tribulus terrestris (3 mg/mL), Aconitum tanguticum (25 mg/mL) and Belamcanda chinensis (50 mg/mL) were added to Vero cells laid on 12-well plates, with the action models of inhibition of replication (cell post-treat) and direct targeting PRV (virus pre-treat). Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 2.
Inhibition effects of Aconitum tanguticum at different stages of PRV infection in Vero cells. (A) The toxicity of Aconitum tanguticum extract at different concentrations on Vero cells detected by CCK-8 method. (B-D) The mRNA expression levels of PRV-gC were detected by RT-qPCR under three antiviral action models of Aconitum tanguticum extract (concentration at 5 mg/mL and 10 mg/mL). (E) Expression of viral protein VP5 was detected by Western blot at PRV (0.1 MOI) infection for 24 h. (F) Cell supernatants were collected for the detection of virus titer using TCID50 method at PRV (0.1 MOI) infection for 24 h. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 2.
Inhibition effects of Aconitum tanguticum at different stages of PRV infection in Vero cells. (A) The toxicity of Aconitum tanguticum extract at different concentrations on Vero cells detected by CCK-8 method. (B-D) The mRNA expression levels of PRV-gC were detected by RT-qPCR under three antiviral action models of Aconitum tanguticum extract (concentration at 5 mg/mL and 10 mg/mL). (E) Expression of viral protein VP5 was detected by Western blot at PRV (0.1 MOI) infection for 24 h. (F) Cell supernatants were collected for the detection of virus titer using TCID50 method at PRV (0.1 MOI) infection for 24 h. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 3.
Inhibition effects of Belamcanda chinensis at different stages of PRV infection in Vero cells. (A) The toxicity of Belamcanda chinensis extract at different concentrations on Vero cells detected by CCK-8 method. (B-D) The mRNA expression levels of PRV-gC were detected by RT-qPCR under three antiviral action models of Belamcanda chinensis extract (concentration at 5 mg/mL and 10 mg/mL). (E) Expression of viral protein VP5 was detected by Western blot at PRV (0.1 MOI) infection for 24 h. (F) Cell supernatants were collected for the detection of virus titer using TCID50 method at PRV (0.1 MOI) infection for 24 h. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 3.
Inhibition effects of Belamcanda chinensis at different stages of PRV infection in Vero cells. (A) The toxicity of Belamcanda chinensis extract at different concentrations on Vero cells detected by CCK-8 method. (B-D) The mRNA expression levels of PRV-gC were detected by RT-qPCR under three antiviral action models of Belamcanda chinensis extract (concentration at 5 mg/mL and 10 mg/mL). (E) Expression of viral protein VP5 was detected by Western blot at PRV (0.1 MOI) infection for 24 h. (F) Cell supernatants were collected for the detection of virus titer using TCID50 method at PRV (0.1 MOI) infection for 24 h. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 4.
Aconitum tanguticum and Belamcanda chinensis had therapeutic effects in PRV infected mice. (A) PRV challenge and drugs treatment animal models. (B) The survival rates of PRV infected mice with Aconitum tanguticum or Belamcanda chinensis treatment (n = 6). (C-D) The changes of body weight of PRV virus-infected mice with different doses of Aconitum tanguticum or Belamcanda chinensis treatment. (E-F) The relative expression of viral gC gene in lung and liver in each group after 72 h of challenge. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01.
Figure 4.
Aconitum tanguticum and Belamcanda chinensis had therapeutic effects in PRV infected mice. (A) PRV challenge and drugs treatment animal models. (B) The survival rates of PRV infected mice with Aconitum tanguticum or Belamcanda chinensis treatment (n = 6). (C-D) The changes of body weight of PRV virus-infected mice with different doses of Aconitum tanguticum or Belamcanda chinensis treatment. (E-F) The relative expression of viral gC gene in lung and liver in each group after 72 h of challenge. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01.
Figure 5.
Histological and pathological changes of PRV infected mice with the treatment of Aconitum tanguticum or Belamcanda chinensis. (A) Changes of the organs in challenge and treatment groups. (B) Antiviral effects of Aconitum tanguticum and Belamcanda chinensis on histopathology. Representative organ sections from each group were subjected to H&E staining (B) and immunohistochemical analysis with antibody against PRV-VP5 (C). White arrow indicates hemorrhaging in the alveolar cavities, hepatocyte steatosis and necrosis, cerebral cortical inflammatory cell aggregation, glial degeneration, necrosis and disintegration, renal hemorrhage, and renal tubular epithelial cell necrosis and shedding. (D) The scores of pathological changes of lungs, livers, brains and kidneys in PRV virus-infected mice on days 2 with or without drugs treatment. (E) The scores of PRV antigen in lungs, brains and kidneys from PRV virally infected mice on days 2 with or without drugs treatment. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
Figure 5.
Histological and pathological changes of PRV infected mice with the treatment of Aconitum tanguticum or Belamcanda chinensis. (A) Changes of the organs in challenge and treatment groups. (B) Antiviral effects of Aconitum tanguticum and Belamcanda chinensis on histopathology. Representative organ sections from each group were subjected to H&E staining (B) and immunohistochemical analysis with antibody against PRV-VP5 (C). White arrow indicates hemorrhaging in the alveolar cavities, hepatocyte steatosis and necrosis, cerebral cortical inflammatory cell aggregation, glial degeneration, necrosis and disintegration, renal hemorrhage, and renal tubular epithelial cell necrosis and shedding. (D) The scores of pathological changes of lungs, livers, brains and kidneys in PRV virus-infected mice on days 2 with or without drugs treatment. (E) The scores of PRV antigen in lungs, brains and kidneys from PRV virally infected mice on days 2 with or without drugs treatment. Data are presented as mean and SD (N = 3). * represents P <0.05, ** represents P <0.01, *** represents P <0.001.
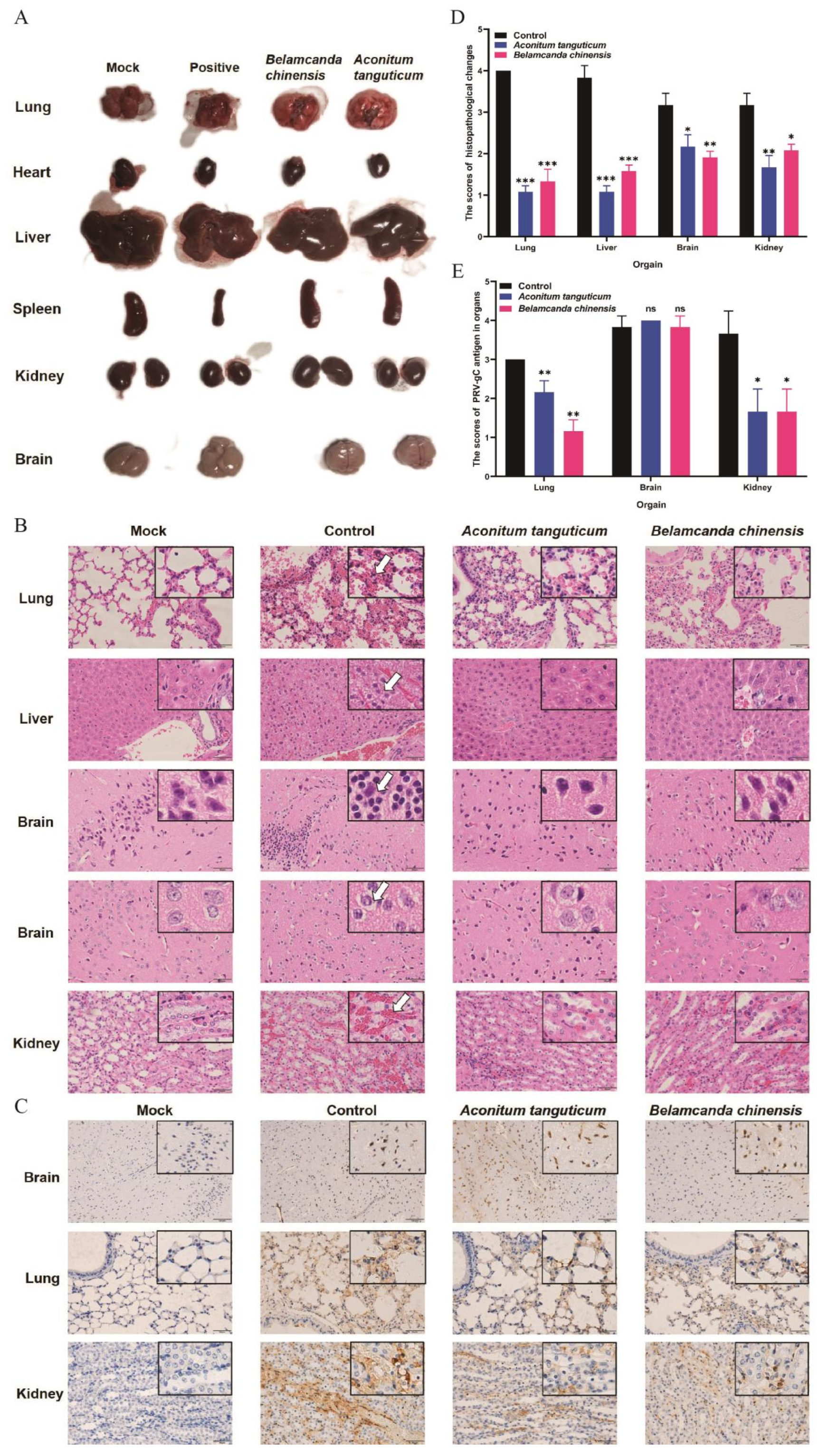
Table 1.
Primers used in this study.
Table 1.
Primers used in this study.
| Primer name |
Primer sequence |
Application |
| RT-PRV-gC |
F: AGCTGCCCATCTTCGAGGAC
R: GCCATGATGACCAGCACGAT |
For qPCR |
| RT-β-Actin |
F: CTCTCTTCCAACCTTCCTTC
R: ATCTTGATTTTCATCGTGCT |
For qPCR |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).