Submitted:
21 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Occurrence of Pesticides in Pears
2.1. Pesticide Residues in Pears are Prevalent, with Multiple Pesticides Frequently Co-Occurring.
2.2. Occurrences of Pesticide Residue Levels Exceeding Regulatory Limits are Common in Pears, with a Low Overall Exceedance Rate
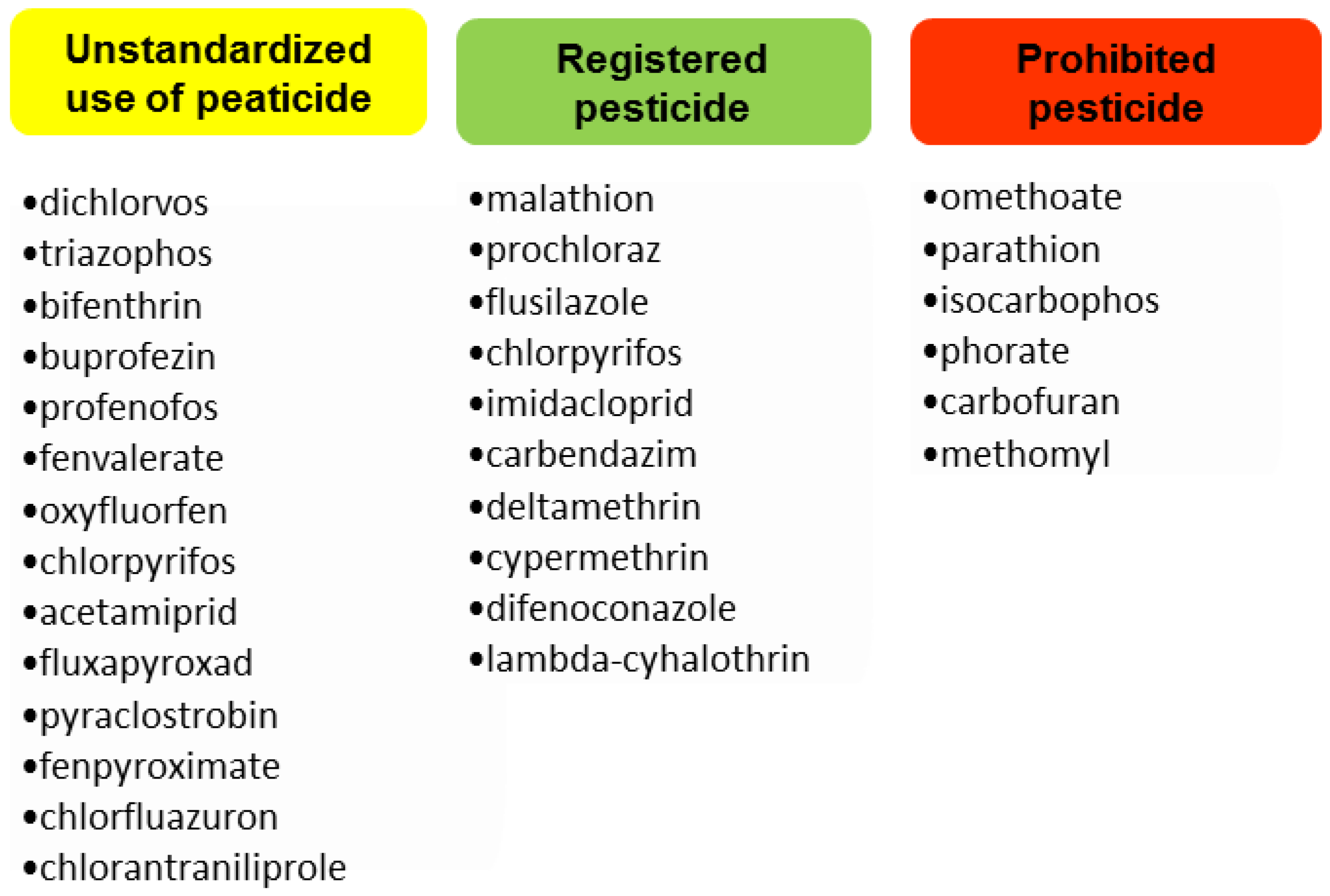
2.3. Multiple Residual Pesticides Occurr in Pears, with a Majority Being Unregistered Varieties
3. Dissipation of Pesticide in Pears
4. Pesticide Residue Detection in Pears
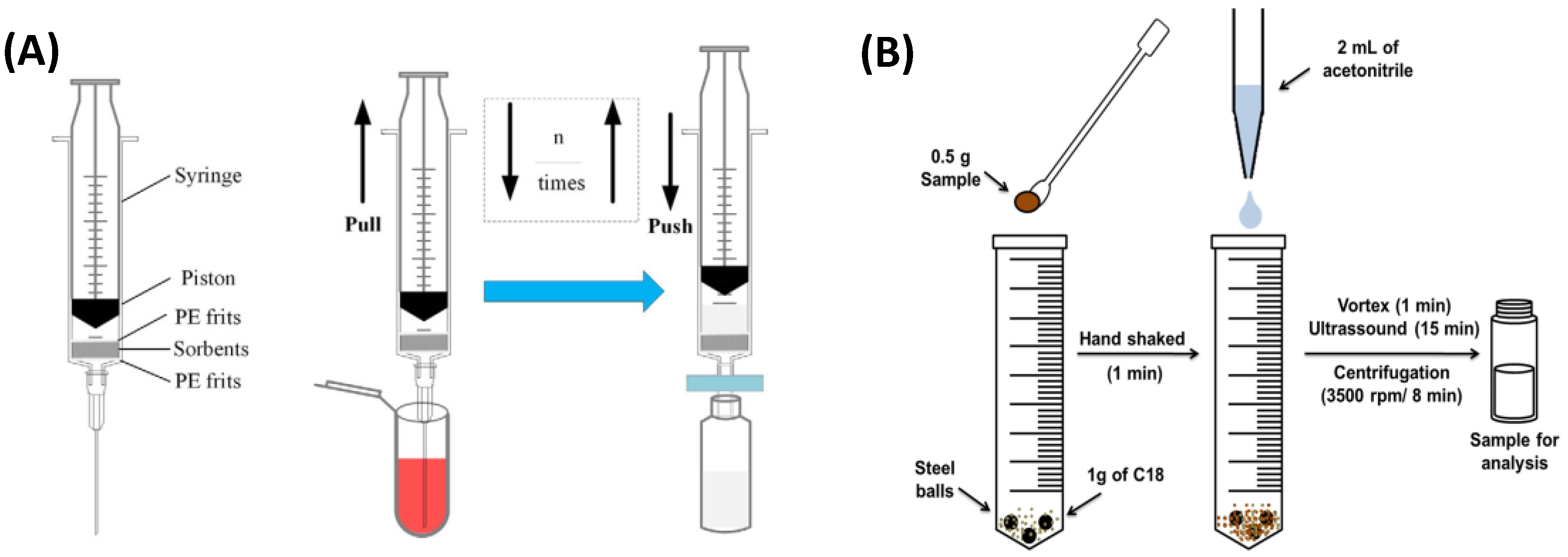
4.1. Sample Preparation
4.2. Detection Techniques
4.2.1. Chromatography and Mass Spectrometry
4.2.2. Other Techniques
| Sample | Analytes | Sample Pretreatment | Instrumental Techniques |
Instrumental Details | Analytical Performance | Ref. | |
|---|---|---|---|---|---|---|---|
| Pear | 34 pesticides | Vortex-assisted extraction with acetonitrile containing 1% acetic acid, purification by d-SPE using PSA as sorbent | GC-MS/MS | HP-5MS column (15 m × 0.25 mm i.d., 0.25 µm); programmed temperature; splitless injection; inlet, ion source, and transfer line temperature at 280 °C, 230 °C, and 280 °C, respectively | Recoveries: 83.3–109.4% RSDs: 1.3–10.8% LOQs: 5.0 μg/kg |
[12] | |
| Pear | 22 pesticides | Homogenization extraction with acetonitrile, without cleanup | UPLC-MS/MS | ReproSil 100 C18 column (25 cm × 2.1 mm i.d., 5 μm) at 35 °C with a gradient mobile phase of methanol and water containing 0.1% formic acid; positive electrospray ionization (ESI+); MRM. | Recoveries: 71.4–106.7% RSDs: 0.7–9.9% LODs: 0.9–4.6 μg/kg LOQs: 3.0–15.4 μg/kg |
[14] | |
| Pear | 21organophosphorus pesticides | Homogenization extraction with acetonitrile, purification by d-SPE using PSA as sorbent | GC-MS | DB-5MS column (30 m × 0.25 mm i.d., 0.25 µm); programmed temperature; splitless injection; inlet, ion source, and transfer line temperature at 280 °C, 230 °C, and 280 °C, respectively | Recoveries: 85.4–100.4% RSDs: 1.9–6.8% LODs: 0.2–2.6 μg/kg |
[16] | |
| pear | 31 pesticides | Vortex-assisted extraction with acetonitrile containing 1% acetic acid, purification by d-SPE using PSA and C18 as sorbents | HPLC-MS/MS | C18 column (10 cm × 2.1 mm i.d., 1.8 µm) at 30 °C with a gradient mobile phase of acetonitrile and water containing 0.1% formic acid; ESI+ at 350 °C; MRM. | Recoveries: 75.0–111.5% RSDs: 0.9–6.7% LODs: 0.25–25 μg/kg |
[33] | |
| Apple-pear | 19 organochlorine pesticides | Ultrasonic extraction with acetonitrile, purification by an SPE cartridge using NH2 as sorbent and eluting with methanol/dichloromethane (1:19, v/v) | GC-MS | TG-5MS capillary column (30 m × 0.25 mm i.d., 0.25 µm); programmed temperature; splitless injection; inlet and ion source temperature at 290 °C and 280 °C, respectively. | Recoveries: 86.1–108.9% RSDs: 4.0–9.5% LODs: 3.0–6.0 μg/kg LOQs: 10–20 μg/kg |
[58] | |
| Pear | Myclobutanil, diniconazole, epoxiconazole, methoxychlor | Ultrasonic extraction with acetonitrile, purification by d-SPE using PSA and GCB as sorbents | GC-MS/MS | DB-5MS column (30 m × 0.25 mm i.d., 0.25 µm); programmed temperature; splitless injection; inlet, transfer line, and ion source temperature at 250 °C, 250 °C, and 200 °C, respectively | Recoveries: 80–111% RSDs: 0.8–1.2% LOQs: 10.0 μg/kg |
[59] | |
| Pear and tomato | 9 pesticides | Ultrasonic extraction with acetonitrile, without cleanup | UPLC-MS/MS | BEH C18 column (5 cm × 2.1 mm i.d., 1.7 μm) at 35 °C with a gradient mobile phase of acetonitrile and water containing 0.1% formic acid; positive electrospray ionization (ESI+) at 110 °C; MRM. | Recoveries: 61.7–116.5% RSDs: 0.7–18.9% LODs: 0.1–4.0 μg/kg LOQs: 10 μg/kg |
[60] | |
| Pear, grape, and apple | 15 pesticides and adjuvants | Vortex-assisted extraction with acetonitrile, purification by an SPE cartridge using NH2 as sorbent and eluting with methanol/dichloromethane (5:95, v/v) | UPLC-MS/MS | Shim-pack XR-ODS column (7.5 cm × 2.0 mm i.d., 1.6 μm) at 40 °C with a gradient mobile phase of methanol and water containing 2 mmol/L ammonium acetate and 0.05% formic acid; ESI+/ESI; MRM. | Recoveries: 80–112% RSDs: 5.5–16% LOQs: 5-10 μg/kg |
[79] | |
| Grains and vegetables including pears | Metamifop | Vortex-assisted extraction with n-hexane and acetonitrile/water (5:5, v/v) containing 1% acetic acid, purification by d- SPE using PSA and polystyrene/divinylbenzene as sorbents | HPLC-MS/MS | JADE-PAK CB-C18 column (10 cm × 2.1 mm i.d., 3.0 μm) at 30 °C with a gradient mobile phase of acetonitrile and water containing 0.1% formic acid; ESI+; MRM. | Recoveries: 63.9–113.7% RSDs: 1.0–22.2% LODs: 0.2–0.3 μg/kg LOQs: 0.6–1.0 μg/kg |
[80] | |
| Pear | Polyoxin B and oxine-copper | Vortex-assisted extraction with methanol and water containing 1% acetic acid (5:95, v/v) containing 1% acetic acid, purification by d-SPE using PSA as sorbent | UPLC-MS/MS | SB-Aq column (10 cm × 3.0 mm i.d., 1.8 μm) at 35 °C with a gradient mobile phase of methanol and water containing 0.1% formic acid; ESI+ at 150 °C; MRM. | Recoveries: 78–99% RSDs ≤ 5.2% LOQs: 5–10 μg/kg |
[81] | |
| Pear, grape, jujube, and apricot | 99 pesticides | Ultrasonic extraction with acetonitrile, purification by d- SPE using PSA and C18 as sorbents | GC-MS/MS | TG-5MS column (30 m × 0.25 mm i.d., 0.25 µm); programmed temperature; splitless injection; inlet, ion source, and transfer line temperature at 260 °C, 280 °C and 280 °C, respectively. | Recoveries: 70–120% RSDs: 0.3–20% LOQs: 10–25 μg/kg |
[82] | |
6. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiland, H.; Slavin, J. , Systematic review of pears and health. Nutrition Today 2015, 50, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Hong, S. Y.; Lansky, E.; Kang, S. S.; Yang, M. H. , A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. [CrossRef]
- USDA Foreign Agricultural Service. Fresh apples, grapes, and pears: World markets and trade. Available online: https://www.fas.usda.gov/sites/default/files/2024-12/fruit.pdf (accessed on 1 February 2025).
- Wang, W. H.; Wang, G. P.; Tian, L. M.; Li, X. G.; Lv, X. L.; Zhang, Y. X.; Zhang, J. H.; Cao, Y. F. , Fruit scientific research in New China in the past 70 years: Pear. J. Fruit Sci. 2019, 36, 1273–1282. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Database. Crops and livestock products. Available online: https://www.fao.org/faostat/en/#data/TCL (accessed on 20 July 2024).
- Wang, X. C.; Yang, M. N.; Huang, D. S. , Research progress on pear diseases and insect pests. South China Agriculture 2022, 16, 110–114. [Google Scholar] [CrossRef]
- Eissa, F.; Zidan, N. E.-H.; Sebaei, A. S.; Mohamed, M. E. B. , Pesticide residues in fruits and vegetables: Analysis and risk assessment of EU RASFF notifications between 1999 and 2022. J. Food Compos. Anal. 2024, 134, 106556. [Google Scholar] [CrossRef]
- Jardim, A. N. O.; Caldas, E. D. , Pesticide residues in food of plant origin commercialized in Brazil from 2010 to 2020 – An update from the two national monitoring programs. Food Control 2024, 165, 110674. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Pesticide data program annual summary, Calendar Year 2022. Available online: https://www.ams.usda.gov/sites/default/files/media/2022PDPSummary.pdf (accessed on 5 August 2024).
- Li, H.; Chang, Q.; Bai, R.; Lv, X.; Cao, T.; Shen, S.; Liang, S.; Pang, G. , Simultaneous determination and risk assessment of highly toxic pesticides in the market-sold vegetables and fruits in China: A 4-year investigational study. Ecotox. Environ. Safe. 2021, 221, 112428. [Google Scholar] [CrossRef]
- Chen, M. H.; Zeng, Y.; Ouyang, W. M. , Current status and analysis of pesticide residues in fruits and vegetables grown in Huili region plant bases. Anal.Test.Technol. Instr. 2023, 29, 117–124. [Google Scholar] [CrossRef]
- Qian, X.; Yang, Q. R.; Zheng, Z. S.; Chen, Y. D. , Simultaneous determination of 34 kinds of pesticide residues in pears by gas chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2018, 9, 4555–4563. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Yin, D. Y.; Liang, X. C.; Qiao, H. O. , Current status and analysis of pesticide residues in fruits and vegetables grown in Huili region plant bases. Chin. J. Food Hygi. 2023, 35, 1749–1756. [Google Scholar] [CrossRef]
- Chi, M. Y.; Chen, Z. L.; Guo, C. Y.; Ding, R. Y.; Fang, L. P.; Zhang, W. J.; Mao, J. S.; Li, H. D. , Simultaneous determination of 22 kinds of pesticide residues in pear by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2019, 10, 1976–1981. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H. D.; Ding, R. Y.; Guo, C. Y.; Fang, L. P.; Zhang, W. J.; Mao, J. S.; Chen, Z. L. , Determination of seven pyrethroid pesticide residues in pear by GC-MS with QuEChERS. Agrochemicals 2019, 58, 356–358. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H. D.; Ding, R. Y.; Guo, C. Y.; Mao, J. S.; Chen, Z. L. , Simultaneous determination of 21 organophosphorus pesticide residues in pears by QuEChERS-GC-MS. The Food Ind. 2019, 40, 323–327. [Google Scholar]
- Hakme, E.; Herrmann, S. S.; Poulsen, M. E. , Processing factors of pesticide residues in biscuits and their relation to the physicochemical properties of pesticides. Food Addit. Contam. A 2020, 37, 1695–1706. [Google Scholar] [CrossRef]
- Albaseer, S. S. , Factors controlling the fate of pyrethroids residues during post-harvest processing of raw agricultural crops: An overview. Food Chem. 2019, 295, 58–63. [Google Scholar] [CrossRef]
- Farha, W.; Abd El-Aty, A. M.; Rahman, M. M.; Jeong, J. H.; Shin, H.-C.; Wang, J.; Shim, J.-H. , Analytical approach, dissipation pattern and risk assessment of pesticide residue in green leafy vegetables: A comprehensive review. Biomed. Chromatogr. 2018, 32, e4134. [Google Scholar] [CrossRef]
- Hakme, E.; Hajeb, P.; Herrmann, S. S.; Poulsen, M. E. , Processing factors of pesticide residues in cereal grain fractions. Food Control 2024, 161, 110369. [Google Scholar] [CrossRef]
- Ahmadi, S.; Khazaei, S.; mehri, F. , Determination of pesticide residues in fruits: A systematic review and meta-analyses. J. Food Compos. Anal. 2024, 128, 106012. [Google Scholar] [CrossRef]
- Luis, C. C.; Paula, M. P. , The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Luis, C. C.; Paula, M. P. , The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, e07215. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Pesticide data program annual summary, Calendar Year 2021. Available online: https:// www.ams.usda.gov/sites/default/files/media/2021PDPAnnualSummary.pdf (accessed on 5 August 2024).
- Gao, Y. Q.; Li, Q.; Fang, N. , Analysis of the monitoring results of 39 pesticide residues in the main fruits of Daxing district, Beijing from 2017 to 2019. J. Food Saf. Qual. 2021, 12, 827–831. [Google Scholar] [CrossRef]
- Liu, Y. H.; Bei, K.; Zheng, W. R.; Yu, G. G.; Sun, C. X. , Pesticide residues risk assessment and quality evaluation of four characteristic fruits in Zhejiang Province, China. Front. Environ. Sci. 2023, 11, 1124094. [Google Scholar] [CrossRef]
- Qin, D. P.; Yan, F.; Yang, Q. Z.; Huang, S. Y.; Chen, G.; Jing, L. Q. , Investigation of 208 pesticide residues in fruits in Chongqing in 2021. Environ. Chem. 2022, 41, 2146–2148. [Google Scholar] [CrossRef]
- Wang, S. W.; Wang, K. X.; Zhou, H. X.; Sun, Y. Z. , Investigation and analysis of pesticides use risk and safety in Shandong province. China Plant Protect. 2024, 44, 92–96. [Google Scholar] [CrossRef]
- Yang, Y. C.; Rebuji, S. B.; He, M.; Liu, X. , Current status and analysis of pesticide residues in fruits and vegetables grown in Huili region plant bases. Agric. Technol. 2023, 43, 48–52. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Q. Q.; Wang, X.; Yuan, P.; Su, Y. H.; Zhang, R. J. , Investigation on pesticides residues in common fruits and vegetables in Zhengzhou city. Henan J. Prev. Med. 2021, 32, 610–611. [Google Scholar] [CrossRef]
- Zeng, J.; Qiao, X. W. , A brief analysis of pesticide residues exceeding maximum residue limits in vegetables and fruits in China. Chin. J. Pestic. Sci. 2023, 25, 1206–1221. [Google Scholar] [CrossRef]
- Lan, F.; Wang, Z. X.; Lu, Z. Q.; Yao, J.; Jiang, W.; Liu, X.; Wang, X. C.; Zhou, X. X.; Liu, C. D. , Risk ranking of pesticide residues in apples and pears in Shandong Province. Plant Prot. 2017, 43, 181–186. [Google Scholar] [CrossRef]
- Duan, A. L.; Guo, F. C.; Zhang, N. J. , Determination of 31 pesticide residues in Huangguan pear by HPLC-MS/MS. China Food Saf. 2022, 17, 95–100. [Google Scholar] [CrossRef]
- Wang, Z. L.; Song, J.; Zhao, J. F.; Zhai, C. Y. , Current status and analysis of pesticide residues in fruits and vegetables grown in Huili region plant bases. Jiangsu Agric. Sci. 2023, 51, 28–35. [Google Scholar] [CrossRef]
- Farha, W.; Abd El-Aty, A. M.; Rahman, M. M.; Shin, H.-C.; Shim, J.-H. , An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188, 693. [Google Scholar] [CrossRef]
- Schusterova, D.; Horska, T.; Skalsky, M.; Stara, J.; Ourednickova, J.; Uttl, L.; Kocourek, V.; Hajslova, J. , Three-year monitoring study of pesticide dissipation in pears. J. Food Compos. Anal. 2024, 126, 105863. [Google Scholar] [CrossRef]
- Wang, J. T.; Fang, Y. J.; Yan, X. H.; Wang, H.; Yang, B. D.; Wang, X. W.; Zhang, Z. Y. , Residual dynamics of chlorpyrifos during the fruit inflating stage of Whangkeumbae pear. J. Food Saf. Qual. 2018, 9, 552–557. [Google Scholar] [CrossRef]
- Wu, X. W.; Xue, J. Y.; Pan, D. D.; Jin, L. J.; Shi, T. Z.; Cheng, X. X.; Li, Q. X.; Hua, R. M. , Dissipation and residue of acephate and its metabolite metamidophos in peach and pear under field conditions. Int. J. Environ. Res. 2017, 11, 133–139. [Google Scholar] [CrossRef]
- Lan, F.; Liu, X.; Li, X. L.; Zhou, X. X.; Liu, C. D.; Wang, Z. X.; Lu, Z. Q.; Jiang, W. , Residues and dissipation of clothianidin in pears. Chin. J. Pestic. Sci. 2018, 20, 814–818. [Google Scholar] [CrossRef]
- Kabir, M. H.; El-Aty, A. M. A.; Kim, S.-W.; Rahman, M. M.; Chung, H. S.; Lee, H. S.; Shin, H.-C.; Shim, J.-H. , Decline pattern and risk assessment of cyenopyrafen in different varieties of Asian pear using liquid chromatography and tandem mass spectrometry. Food Sci. Biotechnol. 2017, 26, 537–543. [Google Scholar] [CrossRef]
- Fang, Q. K.; Wu, R. F.; Hu, G. X.; Lai, A. P.; Wu, K. X.; Zhang, L. W.; Feng, J. J.; Cao, H. Q. , Dissipation behavior, residue distribution and risk assessment of three fungicides in pears. J. Sci. Food. Agric. 2020, 100, 1757–1763. [Google Scholar] [CrossRef]
- Tang, Y. F.; Hu, K. K.; Li, X. M.; Liu, C. G.; Xu, Y. H.; Zhang, Z. X.; Wu, X. W. , Dissipation dynamics and dietary risk assessment of four fungicides as preservatives in pear. Agriculture 2022, 12, 630. [Google Scholar] [CrossRef]
- Liang, Z.; Mahmoud Abdelshafy, A.; Luo, Z. S.; Belwal, T.; Lin, X. Y.; Xu, Y. Q.; Wang, L.; Yang, M. Y.; Qi, M.; Dong, Y. Y.; Li, L. , Occurrence, detection, and dissipation of pesticide residue in plant-derived foodstuff: A state-of-the-art review. Food Chem. 2022, 384, 132494. [Google Scholar] [CrossRef] [PubMed]
- Mao, J. S.; Chen, Z. L.; Li, H. D.; Guo, C. Y.; Ding, R. Y.; Zhang, W. J.; Yan, M. M. , Residues and dissipation dynamics of 4 pesticides in pear. Agrochemicals 2021, 60, 668–673. [Google Scholar] [CrossRef]
- Mao, J. S.; Chen, Z. L.; Li, H. D.; Zhang, W. J.; Ding, R. Y.; Fang, L. P.; Guo, C. Y. , Residues and dissipation dynamics of chlorpyrifos, imidacloprid, spirotetramat and its motablites, difenoconazole in pear. Chin. J. Pestic. Sci. 2019, 21, 395–400. [Google Scholar] [CrossRef]
- Lu, L. N.; Shen, Y.; Gao, M. J.; Zhong, J. F.; Lu, F.; Zheng, Z. T.; Zhang, Z. Y. , Residue and dissipation of matrine in pear and soil. Zhejiang Agric. Sci. 2024, 65, 2184–2189. [Google Scholar] [CrossRef]
- Li, Z. X.; Yan, Z.; Nie, J. Y.; Chen, Y.; Shen, Y. M. , Study on dissipation dynamics of fenbuconazole in pear and acute risk assessment of dietary intake. Qual. Saf. Agro-products. [CrossRef]
- Du, H. X.; Li, H. D.; Guan, S.; Chen, Z. L. , Study on residue and degradation of cyhalothrin in pear and soil. Shandong Agric. Sci. 2018, 50, 119–122. [Google Scholar] [CrossRef]
- Tang, S. H.; Huang, L.; Dai, X. F.; Deng, Y. S.; Pu, E. T.; Li, W. X.; Zhang, X. Y. , Residual behavior and dietary risk assessment of imibenconazole in pears. Agrochemicals 2021, 60, 196–200. [Google Scholar] [CrossRef]
- Gao, M. J.; Shen, Y.; Lu, L. N.; Zhong, J. F.; Zheng, Z. T.; Zhang, Z. Y. , Residues and dissipation characteristics of three fungicides in pear and soil. Chin. Agric. Sci. Bull. 2023, 39, 116–121. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Z. S.; Chen, Y. D.; Zhang, S. J.; Guan, J. F.; Fan, L. X.; Zhao, X. D.; Qian, M. Y. , Residues and dissipation dynamics of spirotetramat and its metabolites in pear and soil. Chin. J. Pestic. Sci. 2019, 21, 338–344. [Google Scholar] [CrossRef]
- Liu, Y. H.; Xu, M. F.; Teng, M. Y.; Pang, Y. J.; Sun, C. X. , Residue dissipation and safe medication recommendations of imidacloprid in honey pear. Zhejiang Agric. Sci. 2022, 63, 1422–1424. [Google Scholar] [CrossRef]
- Zhu, M. Q.; Mao, J. S.; Chen, Z. L.; Li, H. D.; Guo, C. Y.; Zhang, W. J. , Residue and dissipation dynamics of pyraclostrobin in pear. J. Anhui Agric. Sci. 2023, 51, 190–193. [Google Scholar] [CrossRef]
- Truong, L. T. B.; Kim, S. W.; El-Aty, A. M. A.; Kabir, H.; Rahman, M.; Choi, J. H.; Shin, H. C.; Kwon, C. H.; Lee, K. B.; Yoon, H. J.; Shim, J. H. , Current status and analysis of pesticide residues in fruits and vegetables grown in Huili region plant bases. Biomed. Chromatogr. 2016, 30, 1535–1540. [Google Scholar] [CrossRef]
- Šunjka, D.; Lazić, S.; Vuković, S.; Alavanja, A.; Nađ, Đ.; Mitrić, S. , Residue and dissipation dynamic of spinetoram insecticide in pear fruits. Plant Protect. Sci. 2021, 57, 326–332. [Google Scholar] [CrossRef]
- Tankiewicz, M. , Determination of selected priority pesticides in high water fruits and vegetables by modified QuEChERS and GC-ECD with GC-MS/MS confirmation. In Molecules, 2019; Vol. 24, p 417.
- Zhou, Q. Z.; Liu, Z. Q.; Liu, F. M.; Guo, Y. G.; Li, X. H. , Determination of desmedipham residue in 21 foods by HPLC-MS/MS combined with a modified QuEChERS and mixed-mode SPE clean-up method. Journal of Food Composition and Analysis 2021, 102, 104004. [Google Scholar] [CrossRef]
- Yao, Y. H.; Bai, L. L.; Wu, L. P.; Wu, X. Z. , Determination of 19 kinds of organchlorine pesticide residues in apple-pear by using gas chromatography-mass spectrometry coupled with solid-phase extraction. Food Mach. 2019, 35, 87–95. [Google Scholar] [CrossRef]
- Wang, F. H.; Hao, J.; M. , C.; Li, Z., Determination of 4 pesticide residues in pear by gas chromatography-triple quadrupole mass spectrometry method. China Food Saf. 2024, 90–93. [Google Scholar] [CrossRef]
- Zhang, H. Y. , Determination of nine pesticide residues in tomatoes and pears by UPLC-MS/MS. Agrochemicals 2018, 57, 192–195. [Google Scholar] [CrossRef]
- Cheng, Z. P.; Dong, F. S.; Xu, J.; Liu, X. G.; Wu, X. H.; Chen, Z. L.; Pan, X. L.; Gan, J.; Zheng, Y. Q. , Simultaneous determination of organophosphorus pesticides in fruits and vegetables using atmospheric pressure gas chromatography quadrupole-time-of-flight mass spectrometry. Food Chemistry 2017, 231, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. B. , Comparative study on the simultaneous determination of 21 pesticide residues in 8 fruits and vegetables by QuEChERS and SPE pre-treatment combined with GC-MS/MS. Cereal. Oil. 2024, 37, 128–132. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Yan, M. M.; Zhu, C.; Feng, J. J.; Tan, T. Y.; Liu, J. H.; Li, T. F. , Determination of 13 kinds of pesticide residues in agricultural products by array-thin film micro-extraction coupled with high performance liquid chromatography- tandem mass spectrometry. J. Food Saf. Qual. 2022, 13, 5401–5409. [Google Scholar] [CrossRef]
- Meng, Z. J.; Huang, Y. X.; Di, P. Y.; Zhao, L. M.; Niu, L. S.; Fan, S. F.; Li, Q.; Zhang, Y. , Rapid Screening of 234 pesticide residues in vegetables and fruits by multi-plug filtration cleanup method combined with gas chromatography-quadrupole time of flight mass spectrometry. Food Sci. 2020, 41, 272–285. [Google Scholar] [CrossRef]
- Shirani, M.; Ansari, F.; Shabanian, M.; Wagenknecht, U.; Salamat, Q.; Faraji, M.; Basij, M.; Adeli, M. , NiFe2O4 nanoparticles grafted sulfonated melamine for rapid magnetic dispersive µ-solid phase extraction of pesticides in apple and pear samples: Greenness evaluation. Microchem. J. 2024, 205, 111254. [Google Scholar] [CrossRef]
- Kemmerich, M.; Demarco, M.; Bernardi, G.; Prestes, O. D.; Adaime, M. B.; Zanella, R. , Balls-in-tube matrix solid phase dispersion (BiT-MSPD): An innovative and simplified technique for multiresidue determination of pesticides in fruit samples. J. Chromatogr. A 2020, 1612, 460640. [Google Scholar] [CrossRef]
- Du, L. J.; Tian, J. R.; Xue, Y.; Zhang, Y. F.; Chang, T. Y. , Rapid detection of 143 pesticide residues in agricultural products by QuEChERS and gas chromatography-mass spectrometry. China Port Sci. Technol. 2021, 43–53. [Google Scholar] [CrossRef]
- Kemmerich, M.; Bernardi, G.; Prestes, O. D.; Adaime, M. B.; Zanella, R. , Comprehensive method validation for the determination of 170 pesticide residues in pear employing modified QuEChERS without clean-up and ultra-high performance liquid chromatography coupled to tandem mass spectrometry. Food Anal. Methods 2018, 11, 556–577. [Google Scholar] [CrossRef]
- GB 23200. 123-2018; National Health Commission of the PRC, Ministry of Agriculture and Rural Affairs of the PRC, State Administration for Market Regulation. National Food Safety Standard—Determination of 208 pesticides and metabolitesresidues in foods of plant origin—Gas chromatography-tandem mass spectrometry method. China Agriculture Press: Beijing, China, 2018. [Google Scholar]
- GB 23200. 121-2021; National Health Commission of the PRC, Ministry of Agriculture and Rural Affairs of the PRC, State Administration for Market Regulation. National Food Safety Standard—Determination of 331 pesticides and metabolitesresidues in foods of plant origin—Liquid chromatography-tandem mass spectrometry method. China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Munaretto, J. S.; Viera, M. D. S.; Martins, M. L.; Adaime, M. B.; Zanella, R. , Quantitative multiclass pesticide residue analysis in apple, pear, and grape by modified QuEChERS and liquid chromatography coupled to high-resolution mass spectrometry. J. AOAC Int. 2016, 99, 1426–1435. [Google Scholar] [CrossRef]
- Cheng, Z. P.; Zhang, X. Z.; Geng, X.; Organtini, K. L.; Dong, F. S.; Xu, J.; Liu, X. G.; Wu, X. H.; Zheng, Y. Q. , A target screening method for detection of organic pollutants in fruits and vegetables by atmospheric pressure gas chromatography quadrupole-time-of-flight mass spectrometry combined with informatics platform. J. Chromatogr. A 2018, 1577, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gkountouras, D.; Boti, V.; Albanis, T. , High resolution mass spectrometry targeted analysis and suspect screening of pesticide residues in fruit samples and assessment of dietary exposure. Environ. Pollut. 2024, 352, 124143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Z. L.; Zhu, C.; Du, H. X.; Mao, J. S.; Qin, H. W.; She, Y. X.; Yan, M. M. , An interference-free SERS-based aptasensor for chlorpyrifos detection. Anal. Chim. Acta 2023, 1268, 341398. [Google Scholar] [CrossRef]
- Yu, X. D.; Li, Y. S.; Si, Z. Z.; Liu, Y.; Guan, N. Y.; Zhang, S. L.; Zha, Y. H.; Zhou, Y. , Development of an indirect competitive ELISA for the detection of imidacloprid. J. Yangzhou Univ. 2019, 40, 107–112. [Google Scholar] [CrossRef]
- Yue, Y. D.; Chen, J. Y.; Zhang, M. J.; Yin, Y. G.; Dong, Y. Y. , Determination of organophosphorus pesticides in vegetables and fruit by an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) and a lateral-flow immunochromatographic (LFIC) strip assay. Anal. Lett. 2022, 55, 1701–1718. [Google Scholar] [CrossRef]
- Zhang, Y. Y.; Yang, J. Y.; Zeng, D. P.; Xu, Z. L.; Wang, H.; Tian, Y. X.; Sun, Y. M.; Shen, Y. D. , Ultrasensitive immunoassay for paraquat residues in fruits and vegetables based on biotinylated nanobodies. J. Ins. Anal. 2024, 43, 1959–1964. [Google Scholar] [CrossRef]
- Jiang, M. D.; He, J. B.; Gong, J. J.; Gao, H. J.; Xu, Z. X. , Development of a quantum dot-labelled biomimetic fluorescence immunoassay for the simultaneous determination of three organophosphorus pesticide residues in agricultural products. Food Agr.Immunol. 2019, 30, 248–261. [Google Scholar] [CrossRef]
- Lan, F.; Li, X. L.; Duan, X. N.; Jiang, W.; Zang, H. W.; Liu, C. D.; Zhou, X. X.; Lu, Z. Q.; Wang, Z. X.; Hua, Z. K.; Wang, T. Y. , Determination of 15 high-risk pesticide and adjuvant residues in fruits from USA by solid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Pestic. Sci. 2019, 21, 475–482. [Google Scholar] [CrossRef]
- Lei, H. Q.; Su, Y. Z.; Asika, X. R. F. H.; Li, Y. M.; Li, F.; Zhou, J. , Determination of metamifop residues in fruits, vegetables and grains by dual-liquid extraction QuEChERS-high performance liquid chromatography-tandem mass spectrometry. Anal. Instrum. 2024, 31–36. [Google Scholar] [CrossRef]
- Hu, C. L.; Wang, Q. S.; Zhang, L.; Fu, Y.; Xu, F.; Chen, H. X.; Wu, Y. L. , Determination of polyoxin B and oxine-copper in pear by ultra performance liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2021, 12, 660–666. [Google Scholar] [CrossRef]
- Zhang, H. Y.; Li, Y. M.; hua, P. , Determination of 99 pesticide residues in four fruit products by gas chromatography-tandem mass spectrometry. Chin. J. Pestic. Sci. 2023, 25, 193–209. [Google Scholar] [CrossRef]
| Pesticide | Dosage, a.i. | Initial deposit (mg/kg) | Dissipation | Half-life (day) |
Comment (Trial time and location) |
Ref. | |
|---|---|---|---|---|---|---|---|
| Kinetic equation | Correlation coefficient | ||||||
| 45% chlorpyrifos EC | 600 mg/kg | 2.692 | Ct=1.5758e-0.166t | -0.98 | 4.2 | 2019, Anhui, Shandong, and Heibei, China | [44] |
| 25 g/L lambda-cyhalothrin EC | 50 mg/kg | 0.241 | Ct=0.2619e-0.096t | -0.99 | 7.1 | ||
| 10% imidacloprid SP | 100 mg/kg | 0.181 | Ct=0.1558e-0.056t | -0.97 | 12.2 | ||
| 50% carbendazim WP | 2000 mg/kg | 3.732 | Ct=3.9849e-0.057t | -0.91 | 11.9 | ||
| 480 g/L chlorpyrifos EC | 450 mg/kg | 4.68 | Ct=4.1289e-0.154t | -0.98 | 4.4 | 2018 | [45] |
| 10% imidacloprid SP | 30 mg/kg | 0.12 | Ct=0.1075e-0.056t | -0.96 | 12.2 | ||
| 22.4% spirotetramat SC | 90 mg/kg | 0.044 | Ct=0.0383e-0.052t | -0.98 | 13.1 | ||
| 10% difenoconazole WDG | 75 mg/kg | 0.082 | Ct=0.0586e-0.066t | -0.97 | 10.3 | ||
| 0.3% matrine EC | 0.27 g/m2 | 0.6633 | Ct=0.4352e-0.1418t | -0.9806 | 4.89 | Tianjing, China | [46] |
| 0.9140 | Ct=0.4394e-0.1761t | -0.9608 | 3.94 | Anhui, China | |||
| 24% fenbuconazole SC | 144 mg/kg | 0.6101 | Ct=0.4889e-0.073t | -0.9711 | 9.5 | 2017, Heibei, China | [47] |
| 0.6692 | Ct=0.5421e-0.057t | -0.9905 | 12.2 | 2017, Liaoning, China | |||
| 2.5% lambda-cyhalothrin EW | 18.75 g/hm2 | 0.159 | Ct=0.127e-0.03t | -0.9616 | 23.1 | 2016, Jinan, China | [48] |
| 1.050 | Ct=0.948e-0.09t | -0.9939 | 7.7 | 2016, Taiyuan, China | |||
| 0.424 | Ct=0.278e-0.07t | -0.9478 | 9.9 | 2016, Hangzhou, China | |||
| 15% imibenconazole WP | 75 mg/L | 0.23 | Ct=0.9461e-0.042t | -0.8859 | 16.5 | 2019, Yunnan, China | [49] |
| 0.15 | Ct=0.3097e-0.041t | -0.9385 | 16.9 | 2019, Tianjing, China | |||
| 10% flusilazole EW | 75 mg/kg | 0.223 | Ct=0.1547e-0.079t | -0.9763 | 8.83 | 2019, Shandong, China | [50] |
| 40% myclobutanil SC | 75 mg/kg | 1.310 | Ct=0.4875e-0.048t | -0.9669 | 14.44 | ||
| 250 g/L tebuconazole EW | 187.5 mg/kg | 0.581 | Ct=0.3720e-0.148t | -0.9517 | 4.70 | ||
| 22.4% spirotetramat SC | 112 mg/kg | 0.086 | Ct=0.0825e-0.056t | — | 12.4 | Hebei, China | [51] |
| 50% fenitrothion EC | 0.075 mL/m2 | 1.59 | Ct=1.1704e-0.226t | -0.9936 | 3.07 | 2020, Zhejiang, China | [52] |
| 250 g/Lpyraclostrobin SC | 50 g/kg | 0.466 | Ct=0.4053e-0.07t | -0.9855 | 9.9 | 2020, Anhui, Shandong, and Gnasu, China | [53] |
| 10% bistrifluron SC | 5 mL/20 L | 0.29 | Ct=0.3191e-0.068t | -0.9474 | 10.19 | Naju, Korea | [54] |
| 25% spinetoram WDG | 0.3 kg/hm2 | 0.51 | Ct=0.51e-0.321t | -0.9913 | 2.17 | Kula, Serbia | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





