Submitted:
21 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Development and Maintenance of CLEs in Root Apical Meristem
3. Development and Maintenance of CLEs in Shoot Apical Meristem
3.1. CLV3-CLV1
3.2. CLV3-CLV2 CRN/SOL2
3.3. CLV3-RPK2
3.4. CLV1-BAMs
4. Development and Maintenance of CLEs in Stem and Root Cambium
5. Development and Maintenance of CLEs in Leaf
6. Development and Mai[71,122–125ntenance of CLEs in Floral Meristem
7. Compensation Mechanism of CLEs in Plant
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khavinson, V.; Linkova, N.; Diatlova, A.; Dudkov; Aleksandr. Peptide regulation of plant cells differentiation and growth. BIO Web of Conferences 2024. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. The rudimentary embryo: An early angiosperm invention that contributed to their dominance over gymnosperms. Seed Science Research 2023. [Google Scholar] [CrossRef]
- Kathryn Barton, M. Cell type specification and self renewal in the vegetative shoot apical meristem. Current Opinion in Plant Biology 1998, 1, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, N.; Xie, L. Molecular and hormonal regulation of leaf morphogenesis in Arabidopsis. International Journal of Molecular Sciences 2020. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Benfey, P.N. Plant stem cell niches: standing the test of time. Cell 2008. [Google Scholar] [CrossRef]
- Scofield, S.; Murray, J.A.H. KNOX gene function in plant stem cell niches. Plant Molecular Biology 2006. [Google Scholar] [CrossRef]
- Scofield, S.; Dewitte, W.; Murray, J.A. STM sustains stem cell function in the Arabidopsis shoot apical meristem and controls KNOX gene expression independently of the transcriptional repressor AS1. Plant Signaling & Behavior, 2014. [Google Scholar]
- Kwon, C.S.; Chen, C.; Wagner, D. WUSCHEL is a primary target for transcriptional regulation by SPLAYED in dynamic control of stem cell fate in Arabidopsis. Genes & Development, 2005. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Yadav, S.; Bajaj, I.; Kumar, S.; Jain, A.; Sarkar, A.K. Role of chromatin modification and remodeling in stem cell regulation and meristem maintenance in Arabidopsis. Journal of Experimental Botany 2020. [Google Scholar] [CrossRef]
- Kaya, H.; Shibahara, K.I.; Taoka, K.I.; Iwabuchi, M.; Stillman, B.; Araki, T. FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 2001. [Google Scholar] [CrossRef]
- Cock, J.M.; McCormick, S. A large family of genes that share homology with CLAVATA3. Plant Physiology 2001. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Yu, X.; Wu, Q.; Man, X.; Diao, Z.; You, H.; Shen, J.; Cai, Y. Identification and application of CLE peptides for drought resistance in Solanaceae Crops. Journal of Agricultural and Food Chemistry 2024. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, X.; Wen, L.; Deng, Z.; Liu, T.; Guo, Y. Characterization of the CLE Family in three nicotiana species and potential roles of CLE peptides in osmotic and salt stress responses. Agronomy 2023. [Google Scholar] [CrossRef]
- Gao Xiaoming; Yongfeng, G. CLE Peptides in Plants: Proteolytic processing structure-activity relationship, and ligand-receptor interaction. Journal of Integrative Plant Biology, 2012, 54 (10): 738–745. [CrossRef]
- Murphy, E.; Smith, S.; De Smet, I. Small signaling peptides in Arabidopsis development: how cells communicate over a short distance. The Plant Cell 2012. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A. Ligand mimicry? Plant-parasitic nematode polypeptide with similarity to CLAVATA3. Trends in Plant Science 2003. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, Y. CLE peptides in plants: proteolytic processing, structure-activity relationship, and ligand-receptor interaction. Journal of Integrative Plant Biology 2012. [Google Scholar] [CrossRef]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 1993. [Google Scholar] [CrossRef]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 1995, 121, 2057–2067. [Google Scholar] [CrossRef]
- Schoof; Lenhard; Haecker; Mayer; K, F.; Jurgens; Laux. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell, 2000.
- Rojo, E.; Sharma, V.K.; Kovaleva, V.; Raikhel, N.V.; Fletcher, J.C. CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. The Plant Cell 2002. [Google Scholar] [CrossRef]
- Ito, Y.; Nakanomyo, I.; Motose, H.; Iwamoto, K.; Sawa, S.; Dohmae, N.; Fukuda, H. Dodeca-CLE Peptides as suppressors of plant stem cell differentiation. Science 2006. [Google Scholar] [CrossRef]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef]
- Lopes, F.L.; Galvan-Ampudia, C.; Landrein, B. WUSCHEL in the shoot apical meristem: old player, new tricks. Journal of Experimental Botany 2021, 72, 1527–1535. [Google Scholar] [CrossRef]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A Signaling module controlling the stem cell niche in Arabidopsis root meristems. Current Biology 2009. [Google Scholar] [CrossRef] [PubMed]
- Whitford, R.; Fernandez, A.; De Groodt, R.; Ortega, E.; Hilson, P. Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proceedings of the National Academy of Sciences of the United States of America 2008. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Hirakawa, Y.; Sawa, S. Peptide signaling in vascular development. Current Opinion in Plant Biology 2007. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, M.S.; Losev, M.R.; Dodueva, I.E.; Lutova, L.A. Phloem-expressed CLAVATA3/ESR-like genes in Potato. Horticulturae 2023. [Google Scholar] [CrossRef]
- Skripnikov, A. Bioassays for Identifying and Characterizing Plant Regulatory Peptides. Biomolecules 2023. [Google Scholar] [CrossRef]
- Han, H.; Zhang, G.; Wu, M.; Wang, G. Identification and characterization of the Populus trichocarpa CLE family. BMC Genomics 2016. [CrossRef]
- Brumfield, R.T.J.A.J.o.B. cell-lineage studies in root meristems by means of chromosome rearrangements induced by x-rays. Am. J. Bot. 1943, 30, 101–110. [Google Scholar]
- Zhang, H.; Mu, Y.; Zhang, H.; Yu, C. Maintenance of stem cell activity in plant development and stress responses. Frontiers in Plant Science 2023. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, W.S.; Kim, S.-H. Hormonal regulation of stem cell maintenance in roots. Journal of Experimental Botany 2013, 64, 1153–1165. [Google Scholar] [CrossRef]
- Kumpf, R.P.; Nowack, M.K. The root cap: a short story of life and death. Journal of Experimental Botany 2015, 66, 5651–5662. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.P.; Sozzani, R. Uncovering the networks involved in stem cell maintenance and asymmetric cell division in the Arabidopsis root. Current Opinion in Plant Biology 2015. [Google Scholar] [CrossRef] [PubMed]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Current Biology 2009, 19, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Olt, P.; Ding, W.; Schulze, W.X.; Ludewig, U. The LaCLE35 peptide modifies rootlet density and length in cluster roots of white lupin. Plant, Cell & Environment. [CrossRef]
- Hobe, M.; Müller, R.; Grünewald, M.; Brand, U.; Simon, R. Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Development Genes and Evolution 2003. [Google Scholar] [CrossRef]
- Gancheva, M.S.; Lutova, L.A. Nitrogen-activated CLV3/ESR-Related 4 (CLE4) regulates shoot, root, and stolon growth in Potato. Plants 2023, 12. [Google Scholar] [CrossRef]
- Nakagami, S.; Aoyama, T.; Sato, Y.; Kajiwara, T.; Ishida, T.; Sawa, S. CLE3 and its homologs share overlapping functions in the modulation of lateral root formation through CLV1 and BAM1 in Arabidopsis thaliana. The Plant Journal 2023. [Google Scholar] [CrossRef]
- Miwa, H.; Betsuyaku, S.; Iwamoto, K.; Kinoshita, A.; Fukuda, H.; Sawa, S. The receptor-like kinase SOL2 mediates CLE Signaling in Arabidopsis. Plant and Cell Physiology 2008, 49, 1752–1757. [Google Scholar] [CrossRef]
- Fiers, M.; Golemiec, E.; Xu, J.; van der Geest, L.; Heidstra, R.; Stiekema, W.; Liu, C.-M. The 14–amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through aCLAVATA2-Dependent Pathway. The Plant Cell 2005, 17, 2542–2553. [Google Scholar] [CrossRef]
- Casamitjana-Martínez, E.; Hofhuis, H.F.; Xu, J.; Liu, C.-M.; Heidstra, R.; Scheres, B. Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Current Biology 2003. [Google Scholar] [CrossRef]
- Fiers, M.; Hause, G.; Boutilier, K.; Casamitjana-Martinez, E.; Weijers, D.; Offringa, R.; van der Geest, L.; van Lookeren Campagne, M.; Liu, C.-M. Mis-expression of the CLV3/ESR-like gene CLE19 in Arabidopsis leads to a consumption of root meristem. Gene 2004. [Google Scholar] [CrossRef]
- Barton, M.K. Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Developmental Biology 2010, 341, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y. Evolution of meristem zonation by CLE gene duplication in land plants. Nature Plants 2022. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, D.M. A reconstruction of cell development in the shoot apex of maize. American Journal of Botany 1968. [Google Scholar] [CrossRef]
- Itoh, J.I.; Kitano, H.; Matsuoka, M.; Nagato, Y. Shoot organization genes regulate shoot apical meristem organization and the pattern of leaf primordium initiation in rice. Plant Cell 2000, 12, 2161–2174. [Google Scholar] [CrossRef]
- Meyerowitz, E.M. Genetic control of cell division patterns in developing plants. Cell 1997. [Google Scholar] [CrossRef]
- Gallois, J.-L.; Woodward, C.; Reddy, G.V.; Sablowski, R. Combined shoot meristemless and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 2002. [Google Scholar] [CrossRef]
- Vernoux, T.; Autran, D.; Traas, J. Developmental control of cell division patterns in the shoot apex. Plant Molecular Biology 2000. [Google Scholar] [CrossRef]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999. [Google Scholar] [CrossRef]
- Meyerowitz, E.M. Genetic control of cell division patterns in developing plants. Cell 1997. [CrossRef]
- Klaus F., X. Mayer, H.S., Achim Haecker,; Michael Lenhard, G.J.r., and Thomas Laux*. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell, 1998, Vol. 95, 805–815.
- Clark, S.E.; Jacobsen, S.E.; Levin, J.Z.; Meyerowitz, E.M. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development 1996. [Google Scholar] [CrossRef]
- Yadav, R.K.; Perales, M.; Gruel, J.; Girke, T.; Jönsson, H.; Reddy, G.V. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes & Development 2011, 25, 2025–2030. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Laux, T.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proceedings of the National Academy of Sciences of the United States of America 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, Z.; Li, Y.; Shang, X.; Cao, Y.; Duan, L.; Ma, L. Ski-interacting protein interacts with shoot meristemless to regulate shoot apical meristem formation. Plant Physiology 2022. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Fiume, E.; Roeder, A.H.K.; Meng, L.; Sharma, V.K.; Osmont, K.S.; Baker, C.; Ha, C.M.; Meyerowitz, E.M.; Feldman, L.J.; et al. Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis. Plant Physiology 2010. [Google Scholar] [CrossRef]
- Xu, T.-T.; Song, X.-F.; Ren, S.-C.; Liu, C.-M. The sequence flanking the N-terminus of the CLV3 peptide is critical for its cleavage and activity in stem cell regulation in Arabidopsis. BMC Plant Biology 2013. [Google Scholar] [CrossRef]
- Hirakawa, Y. CLAVATA3, a plant peptide controlling stem cell fate in the meristem. Peptides 2021. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhou, C.; Li, Y.J.; Yu, Y.; Tang, L.P.; Zhang, W.J.; Yao, W.J.; Huang, R.; Laux, T.; Zhang, X.S. Integration of pluripotency pathways regulates stem cell maintenance in the Arabidopsis shoot meristem. Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 22561–22571. [Google Scholar] [CrossRef]
- Stuurman, J.; Jäggi, F.; Kuhlemeier, C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes & Development 2002, 16, 2213–2218. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, A.; Han, H.; Li, T.; Geng, Y.; Liu, X.; Meyerowitz, E.M. HAIRY MERISTEM with WUSCHEL confines CLAVATA3 expression to the outer apical meristem layers. Science 2018. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Engstrom, E.M.; Nimchuk, Z.L.; Pruneda-Paz, J.L.; Tarr, P.T.; Yan, A.; Kay, S.A.; Meyerowitz, E.M. Control of plant stem cell function by conserved interacting transcriptional regulators. Nature 2014. [Google Scholar] [CrossRef]
- Han, H.; Liu, X.; Zhou, Y. Transcriptional circuits in control of shoot stem cell homeostasis. Current Opinion in Plant Biology 2019. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Rodriguez, K.; Snipes, S.; Yadav, R.K.; Diaz-Mendoza, M.; Reddy, G.V. Threshold-dependent transcriptional discrimination underlies stem cell homeostasis Proceedings of the National Academy of Sciences of the United States of America 2016. [CrossRef]
- Engstrom, E.M.; Andersen, C.M.; Gumulak-Smith, J.; Hu, J.; Orlova, E.; Sozzani, R.; Bowman, J.L. Arabidopsis homologs of the petunia hairy meristem gene are required for maintenance of shoot and root indeterminacy. Plant Physiology 2010. [Google Scholar] [CrossRef]
- Brand, U.; Grünewald, M.; Hobe, M.; Simon, R. Regulation of CLV3 expression by two homeobox genes in Arabidopsis. Plant Physiology 2002. [Google Scholar] [CrossRef] [PubMed]
- Brand, *!!! REPLACE !!!*; Fletcher, *!!! REPLACE !!!*; J, C.; Hobe, *!!! REPLACE !!!*; Meyerowitz, *!!! REPLACE !!!*; E, M. Brand; Fletcher; J, C.; Hobe; Meyerowitz; E, M.; Simon. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science, 2000. [CrossRef]
- Hu, C.; Zhu, Y.; Cui, Y.; Cheng, K.; Liang, W.; Wei, Z.; Zhu, M.; Yin, H.; Zeng, L.; Xiao, Y.; et al. A group of receptor kinases are essential for CLAVATA signalling to maintain stem cell homeostasis. Nature Plants 2018, 4, 205–211. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, Y. Cell signaling in the shoot apical meristem. Plant Physiology 2023. [Google Scholar] [CrossRef]
- Clark; S, E. ; Williams; R, W.; Meyerowitz; E, M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 1997. [Google Scholar] [CrossRef]
- Nimchuk, Z.L.; Tarr, P.T.; Ohno, C.; Qu, X.; Meyerowitz, E.M. Plant stem cell signaling involves ligand-dependent trafficking of the CLAVATA1 receptor kinase. Current Biology 2011. [Google Scholar] [CrossRef]
- Ogawa, M.; Shinohara, H.; Sakagami, Y.; Matsubayashi, Y. Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 2008. [Google Scholar] [CrossRef]
- Ni, J.U.N.; Clark, S.E. CHAPTER 3 - CLAVATA3: A putative peptide ligand controlling Arabidopsis stem cell specification. In Handbook of Biologically Active Peptides, Kastin, A.J., Ed.; Academic Press: Burlington, 2006; pp. 9–15. [Google Scholar]
- Julie, M. Stone2, Amy E. Trotochaud2, John C. Walker, and Steven E. Clark. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiology, 1998, 1217±1225.
- Nimchuk, Z.L.; Tarr, P.T.; Meyerowitz, E.M. An evolutionarily conserved pseudokinase mediates stem cell production in plants. The Plant Cell 2011. [Google Scholar] [CrossRef]
- Bleckmann, A.; Weidtkamp-Peters, S.; Seidel, C.A.M.; Simon, R. Stem Cell Signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiology 2010, 152, 166–176. [Google Scholar] [CrossRef]
- Sangho Jeong, A.E.T. , and Steven E. Clark1. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Cell, 1999, 1925–1933.
- Müller, R.; Bleckmann, A.; Simon, R.d. The receptor kinase CORYNE of Arabidopsis transmits the stem cell–limiting signal CLAVATA3 independently of CLAVATA1. The Plant Cell 2008, 20, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Diévart, A.; Dalal, M.; Tax, F.E.; Lacey, A.D.; Huttly, A.; Li, J.; Clark, S.E. CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. The Plant Cell 2003, 15, 1198–1211. [Google Scholar] [CrossRef]
- Mizuno, S.; Osakabe, Y.; Maruyama, K.; Ito, T.; Osakabe, K.; Sato, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Receptor-like protein kinase 2 (RPK 2) is a novel factor controlling anther development in Arabidopsis thaliana. The Plant Journal 2007, 50, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Betsuyaku, S.; Takahashi, F.; Kinoshita, A.; Miwa, H.; Shinozaki, K.; Fukuda, H.; Sawa, S. Mitogen-activated protein kinase regulated by the CLAVATA receptors contributes to shoot apical meristem homeostasis. Plant and Cell Physiology 2011, 52, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Matsubayashi, Y. Reevaluation of the CLV3-receptor interaction in the shoot apical meristem: dissection of the CLV3 signaling pathway from a direct ligand-binding point of view. The Plant Journal 2015, 82, 328–336. [Google Scholar] [CrossRef]
- DeYoung, B.J.; Bickle, K.L.; Schrage, K.J.; Muskett, P.; Patel, K.; Clark, S.E. The CLAVATA1-related BAM1, BAM2 and BAM3 receptor kinase-like proteins are required for meristem function in Arabidopsis. The Plant Journal 2006. [Google Scholar] [CrossRef]
- Guo, Y.; Han, L.; Hymes, M.; Denver, R.; Clark, S.E. CLAVATA2 forms a distinct CLE-binding receptor complex regulating Arabidopsis stem cell specification. The Plant Journal 2010. [Google Scholar] [CrossRef]
- Lee, H.; Jun, Y.S.; Cha, O.-K.; Sheen, J. Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem. Plant Cell Reports 2018, 38, 311–319. [Google Scholar] [CrossRef]
- Yu LP, M.A. , Clark SE. Poltergeist encodes a PROTEIN PHOSPHATASE 2C that regulates CLAVATA pathways controlling stem cell identity at Arabidopsis shoot and flower meristems. Current Biology, 2003, 13(13):179-188.
- Chaffey, N. Esau's Plant anatomy, meristems, cells, and tissues of the plant body: their structure, function, and development. 3rd edn. Annals of Botany 2006. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Chen, W.; Xu, M.; Zhou, R.; Shou, H.; Chen, J. High-resolution anatomical and spatial transcriptome analyses reveal two types of meristematic cell pools within the secondary vascular tissue of poplar stem. Molecular Plant 2023. [Google Scholar] [CrossRef]
- Larson, P.R. Procambium vs. Cambium and Protoxylem vs. Metaxylem in Populus deltoides seedlings. American Journal of Botany 1976, 63, 1332–1348. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.-R.; Helariutta, Y.; He, X.-Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: evolution, development and functions. Journal of Integrative Plant Biology 2013. [Google Scholar] [CrossRef] [PubMed]
- Etchells, J.P.; Turner, S.R. The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 2010. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Shinohara, H.; Kondo, Y.; Inoue, A.; Nakanomyo, I.; Ogawa, M.; Sawa, S.; Ohashi-Ito, K.; Matsubayashi, Y.; Fukuda, H. Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proceedings of the National Academy of Sciences of the United States of America 2008. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Nakagami, H.; Hirakawa, Y.; Saito, M.; Tamaki, T.; Shirasu, K.; Fukuda, H. Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signalling. Nature Communications 2014. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Xu, L.; Chu, S.; Yan, X.; Lin, L.; Wen, J.; Zheng, B.; Chen, S.; Li, Q. Transcription factor PagMYB31 positively regulates cambium activity and negatively regulates xylem development in poplar. The Plant Cell 2024. [Google Scholar] [CrossRef]
- Han, S.; Cho, H.; Noh, J.; Qi, J.; Jung, H.-J.; Nam, H.; Lee, S.; Hwang, D.; Greb, T.; Hwang, I. BIL1-mediated MP phosphorylation integrates PXY and cytokinin signalling in secondary growth. Nature Plants 2018. [Google Scholar] [CrossRef]
- Etchells, J.P.; Turner, S.R. The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 2010, 137, 767–774. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, D.; Zhang, R.; Luo, L.; Cao, S.; Huang, C.; Sun, J.; Gui, J.; Li, L. A xylem-produced peptide PtrCLE20 inhibits vascular cambium activity in Populus. Plant Biotechnology Journal 2019. [Google Scholar] [CrossRef]
- Kucukoglu, M.; Chaabouni, S.; Zheng, B.; Mähönen, A.P.; Helariutta, Y.; Nilsson, O. Peptide encoding Populus CLV3/ESR-RELATED 47 (PtCLE47) promotes cambial development and secondary xylem formation in hybrid aspen. New Phytologist 2019. [Google Scholar] [CrossRef]
- Song, X.-F.; Hou, X.-L.; Liu, C.-M. CLE peptides: critical regulators for stem cell maintenance in plants. Planta 2021. [Google Scholar] [CrossRef] [PubMed]
- Bauby, H.; Divol, F.; Truernit, E.; Grandjean, O.; Palauqui, J.-C. Protophloem differentiation in early Arabidopsis thaliana development. Plant & Cell Physiology, 2007. [CrossRef]
- Qian, P.; Song, W.; Yokoo, T.; Minobe, A.; Wang, G.; Ishida, T.; Sawa, S.; Chai, J.; Kakimoto, T. The CLE9/10 secretory peptide regulates stomatal and vascular development through distinct receptors. Nature Plants 2018, 4, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, S.; Cornelis, S.; Hazak, O. The CLE33 peptide represses phloem differentiation via autocrine and paracrine signaling in Arabidopsis. Communications Biology 2023. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-C.; Song, X.-F.; Chen, W.-Q.; Lu, R.; Lucas, W.J.; Liu, C.-M. CLE25 peptide regulates phloem initiation in Arabidopsis through a CLERK-CLV2 receptor complex. Journal of Integrative Plant Biology 2019. [Google Scholar] [CrossRef]
- Anne, P.; Amiguet-Vercher, A.; Brandt, B.; Kalmbach, L.; Geldner, N.; Hothorn, M.; Hardtke, C.S. CLERK is a novel receptor kinase required for sensing of root-active CLE peptides in Arabidopsis. Development 2018. [Google Scholar] [CrossRef]
- Depuydt, S.; Rodriguez-Villalon, A.; Santuari, L.; Wyser-Rmili, C.; Ragni, L.; Hardtke, C.S. Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor-like kinase BAM3. Proceedings of the National Academy of Sciences of the United States of America 2013, 110, 7074–7079. [Google Scholar] [CrossRef]
- Kang, Y.H.; Hardtke, C.S. Arabidopsis MAKR5 is a positive effector of BAM3-dependent CLE45 signaling. EMBO reports. 2016, 17, 1145-1154-1154. [CrossRef]
- Hang, Z.; Qian, W.; Noel, B.-T.; Christian, S.H. Antagonistic CLE peptide pathways shape root meristem tissue patterning. Nature Plants 2024. [Google Scholar] [CrossRef]
- Qian, P.; Song, W.; Zaizen-Iida, M.; Kume, S.; Wang, G.; Zhang, Y.; Kinoshita-Tsujimura, K.; Chai, J.; Kakimoto, T. A Dof-CLE circuit controls phloem organization. Nature Plants 2022. [Google Scholar] [CrossRef]
- Araya, T.; Miyamoto, M.; Wibowo, J.; Suzuki, A.; Kojima, S.; Tsuchiya, Y.N.; Sawa, S.; Fukuda, H.; von Wirén, N.; Takahashi, H. CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proceedings of the National Academy of Sciences 2014, 111, 2029–2034. [Google Scholar] [CrossRef]
- Pazourek, J. T.A. Steeves & I.M. Sussex patterns in plant development. Folia Geobotanica et Phytotaxonomica 1992, 27, 136–136. [Google Scholar] [CrossRef]
- Berná, G.; Robles, P.; Micol, J.L. A mutational analysis of leaf morphogenesis in Arabidopsis thaliana. GENETICS 1999. [Google Scholar] [CrossRef] [PubMed]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of stomatal development. Annual Review of Plant Biology 2012. [Google Scholar] [CrossRef]
- Geisler, M.; Nadeau, J.; Sack, F.D. Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. The Plant Cell 2000. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Jun, S.E.; Jeong, S.J.; Lee, Y.K.; Kim, G.T. Developmental processes of leaf morphogenesis in Arabidopsis. Journal of Plant Biology 2007, 50, 282–290. [Google Scholar] [CrossRef]
- Kalve, S.; De Vos, D.; Beemster, G.T.S. Leaf development: a cellular perspective. Frontiers in Plant Science 2014. [Google Scholar] [CrossRef]
- Bennett, T.; Hines, G.; van Rongen, M.; Waldie, T.; Sawchuk, M.G.; Scarpella, E.; Ljung, K.; Leyser, O. Connective auxin transport in the shoot facilitates communication between shoot apices. PLOS Biology 2016. [Google Scholar] [CrossRef]
- de Reuille, P.B.; Bohn-Courseau, I.; Ljung, K.; Morin, H.; Carraro, N.; Godin, C.; Traas, J. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 2006. [Google Scholar] [CrossRef]
- Vidaurre, D.P.; Ploense, S.; Krogan, N.T.; Berleth, T. AMP1 and MP antagonistically regulate embryo and meristem development in Arabidopsis. Development 2007. [Google Scholar] [CrossRef]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal control of the shoot stem-cell niche. Nature 2010. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Möller, B.; Lokerse, A.S.; Llavata-Peris, C.I.; van den Berg, W.; Weijers, D. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. The Plant Journal 2011. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of molecular mechanisms of plant leaf development: a systematic review. Frontiers in Plant Science 2023. [Google Scholar] [CrossRef] [PubMed]
- DiGennaro, P.; Grienenberger, E.; Dao, T.Q.; Jun, J.H.; Fletcher, J.C. Peptide signaling molecules CLE5 and CLE6 affect Arabidopsis leaf shape downstream of leaf patterning transcription factors and auxin. Plant Direct 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, S.; Gao, Y.; Kan, C.; Wang, H.-L.; Yang, Q.; Xia, X.; Ishida, T.; Sawa, S.; Guo, H.; et al. CLE42 delays leaf senescence by antagonizing ethylene pathway in Arabidopsis. New Phytologist 2022. [Google Scholar] [CrossRef]
- Han, H.; Zhuang, K.; Qiu, Z. CLE peptides join the plant longevity club. Trends in Plant Science 2022. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Li, K.; Li, X.; Xu, M.; Guo, Y. CLE14 functions as a “brake signal” to suppress age-dependent and stress-induced leaf senescence by promoting JUB1-mediated ROS scavenging in Arabidopsis. Molecular Plant 2021. [Google Scholar] [CrossRef]
- Dennis, L.; Peacock, J. Genes directing flower development in Arabidopsis. The Plant Cell 2019. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, K.; Guo, L.; Liu, X.; Zhang, Z. AUXIN RESPONSE FACTOR3 plays distinct role during early flower development. Plant Signaling & Behavior, 2018. [CrossRef]
- Nakajima, K.; Benfey, P.N. Signaling in and out: control of cell division and differentiation in the shoot and root. The Plant Cell 2002. [Google Scholar] [CrossRef]
- Takeda, S.; Iwasaki, A.; Matsumoto, N.; Uemura, T.; Tatematsu, K.; Okada, K. Physical interaction of floral organs controls petal morphogenesis in Arabidopsis. Plant Physiology 2013, 161, 1242–1250. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Tu, Z.; Zhu, S.; Zhang, C.; Li, H. Genome-wide identification of MIKC-type genes related to stamen and gynoecium development in Liriodendron. Scientific Reports 2021. [Google Scholar] [CrossRef]
- Jones, D.S.; John, A.; VanDerMolen, K.R.; Nimchuk, Z.L. CLAVATA signaling ensures reproductive development in plants across thermal environments. Current Biology 2021, 31, 220–227.e225. [Google Scholar] [CrossRef] [PubMed]
- Kayes JM, C.S. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development, 1998, 125(119):3843-3851. [CrossRef]
- Steven, E. Clark, M.P. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development, 1995, 121 (127): 2057–2067. [CrossRef]
- Nidhi, S.; Preciado, J.; Tie, L. Knox homologs shoot meristemless (STM) and KNAT6 are epistatic to CLAVATA3 (CLV3) during shoot meristem development in Arabidopsis thaliana. Molecular Biology Reports 2021, 48, 6291–6302. [Google Scholar] [CrossRef] [PubMed]
- Box, M.S.; Huang, B.E.; Domijan, M.; Jaeger, K.E.; Khattak, A.K.; Yoo, S.J.; Sedivy, E.L.; Jones, D.M.; Hearn, T.J.; Webb, A.A.R.; et al. ELF3 Controls thermoresponsive growth in Arabidopsis. Current Biology 2014. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020. [Google Scholar] [CrossRef]
- Lindsay, R.J.; Stelzl, L.S.; Pietrek, L.; Hummer, G.; Wigge, P.A.; Hanson, S.M. Helical region near poly-Q tract in prion-like domain of Arabidopsis ELF3 plays role in temperature-sensing mechanism. Biophysical Journal 2022. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes & development 2006, 20, 1790–1799. [Google Scholar] [CrossRef]
- Rodriguez-Leal, D.; Xu, C.; Kwon, C.-T.; Soyars, C.; Demesa-Arevalo, E.; Man, J.; Liu, L.; Lemmon, Z.H.; Jones, D.S.; Van Eck, J.; et al. Evolution of buffering in a genetic circuit controlling plant stem cell proliferation. Nature Genetics 2019, 51, 786–792. [Google Scholar] [CrossRef]
- John, A.; Smith, E.S.; Jones, D.S.; Soyars, C.L.; Nimchuk, Z.L. A network of CLAVATA receptors buffers auxin-dependent meristem maintenance. Nature Plants 2023, 9, 1306–1317. [Google Scholar] [CrossRef]
- Bashyal, S.; Gautam, C.K.; Müller, L.M. CLAVATA signaling in plant–environment interactions. Plant Physiology 2023. [Google Scholar] [CrossRef]
- Diss, G.; Ascencio, D.; DeLuna, A.; Landry, C.R. Molecular mechanisms of paralogous compensation and the robustness of cellular networks. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 2013, 322, 488–499. [Google Scholar] [CrossRef]
- Hanada, K.; Sawada, Y.; Kuromori, T.; Klausnitzer, R.; Saito, K.; Toyoda, T.; Shinozaki, K.; Li, W.H.; Hirai, M.Y. Functional compensation of primary and secondary metabolites by duplicate genes in Arabidopsis thaliana. Molecular Biology and Evolution 2010, 28, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Moens, C.; El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLOS Genetics 2017, 13. [Google Scholar] [CrossRef]
- Goad, D.M.; Zhu, C.; Kellogg, E.A. Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytologist 2016. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.Q.; Weksler, N.; Liu, H.M.H.; Leiboff, S.; Fletcher, J.C. Interactive CLV3, CLE16, and CLE17 signaling mediates stem cell homeostasis in the Arabidopsis shoot apical meristem. Development 2022. [Google Scholar] [CrossRef]
- Nimchuk, Z.L.; Zhou, Y.; Tarr, P.T.; Peterson, B.A.; Meyerowitz, E.M. Plant stem cell maintenance by transcriptional cross-regulation of related receptor kinases. Development 2015, 142, 1043–1049. [Google Scholar] [CrossRef]
- Shimizu, N.; Ishida, T.; Yamada, M.; Shigenobu, S.; Tabata, R.; Kinoshita, A.; Yamaguchi, K.; Hasebe, M.; Mitsumasu, K.; Sawa, S. BAM 1 and RECEPTOR-LIKE PROTEIN KINASE 2 constitute a signaling pathway and modulate CLE peptide-triggered growth inhibition in Arabidopsis root. New Phytologist 2015. [Google Scholar] [CrossRef]
- Ni, J.; Clark, S.E. Evidence for functional conservation, sufficiency, and proteolytic processing of the CLAVATA3 CLE domain. Plant Physiology 2006. [Google Scholar] [CrossRef]
- Wulf, K.; Sun, J.; Wang, C.; Ho-Plagaro, T.; Kwon, C.-T.; Velandia, K.; Correa-Lozano, A.; Tamayo-Navarrete, M.I.; Reid, J.B.; García Garrido, J.M.; et al. The role of CLE peptides in suppression of mycorrhizal colonisation of tomato. Plant & Cell Physiology, 2023. [CrossRef]
- Liu, L.; Gallagher, J.; Arevalo, E.D.; Chen, R.; Skopelitis, T.; Wu, Q.; Bartlett, M.; Jackson, D. Enhancing grain-yield-related traits by CRISPR–Cas9 promoter editing of maize CLE genes. Nature Plants 2021, 7, 287–294. [Google Scholar] [CrossRef]
- Selby, R.; Jones, D.S. Complex peptide hormone signaling in plant stem cells. Current Opinion in Plant Biology 2023. [Google Scholar] [CrossRef]
- Strabala, T.J.; Phillips, L.; West, M.; Stanbra, L. Bioinformatic and phylogenetic analysis of the CLAVATA3/EMBRYO-SURROUNDING REGION (CLE) and the CLE-LIKE signal peptide genes in the Pinophyta. BMC Plant Biology 2014. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Kucukoglu, M.; Tian, D.; Larkin, R.M.; Shi, X.; Zheng, B. Predicting and clustering plant CLE genes with a new method developed specifically for short amino acid sequences. BMC Genomics 2020, 21(1), 709. [Google Scholar] [CrossRef]

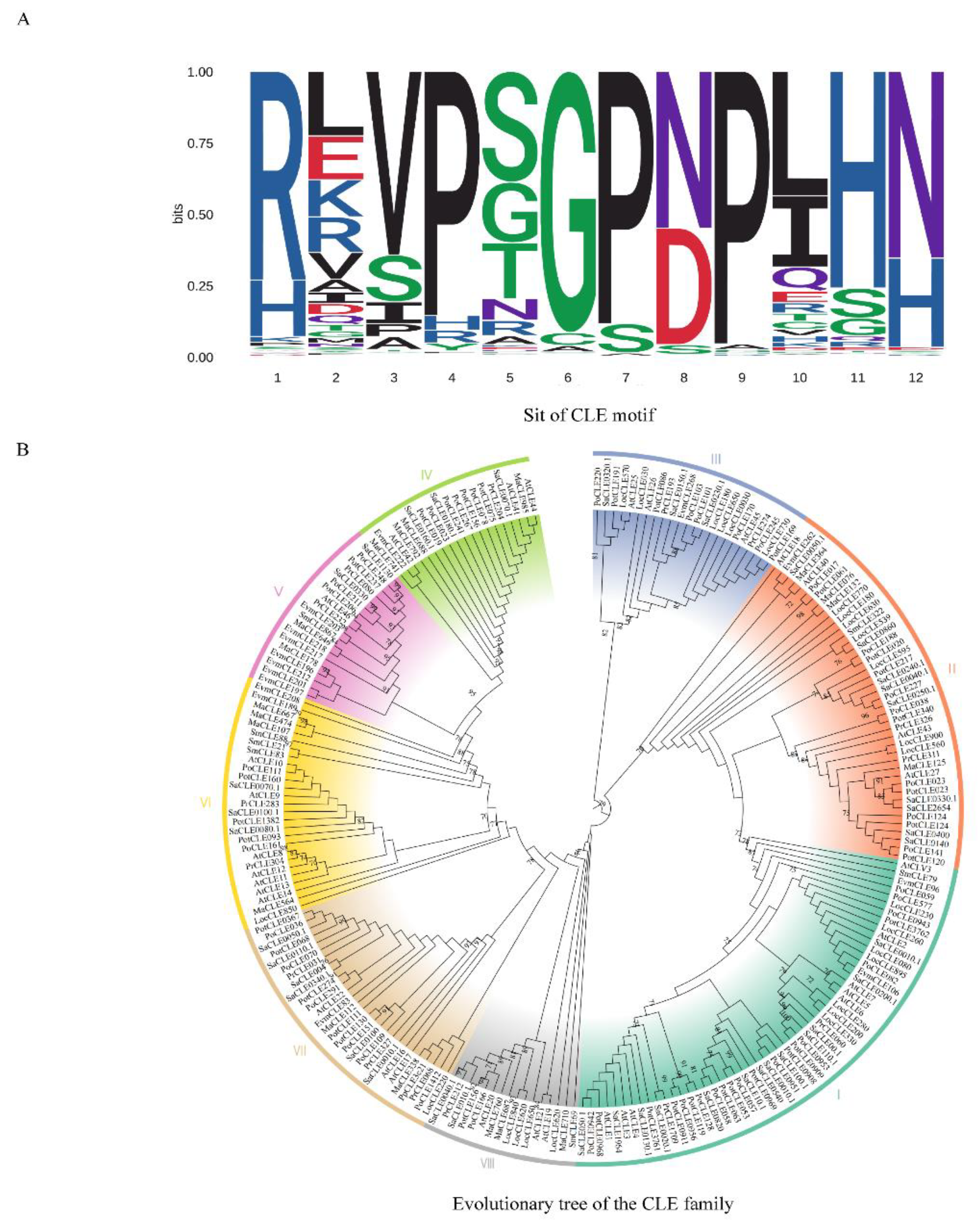
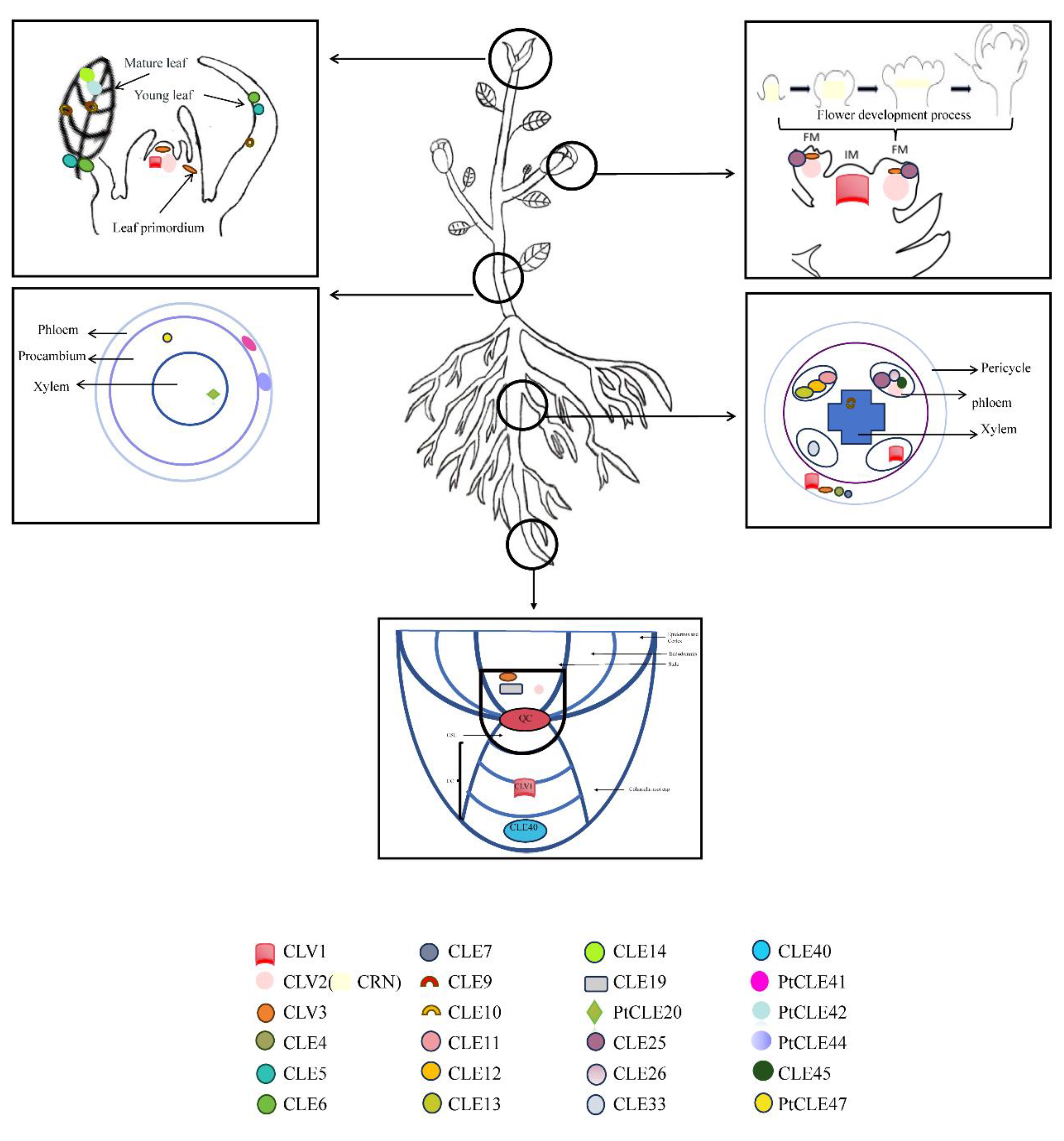
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



