Submitted:
21 March 2025
Posted:
24 March 2025
You are already at the latest version
Abstract
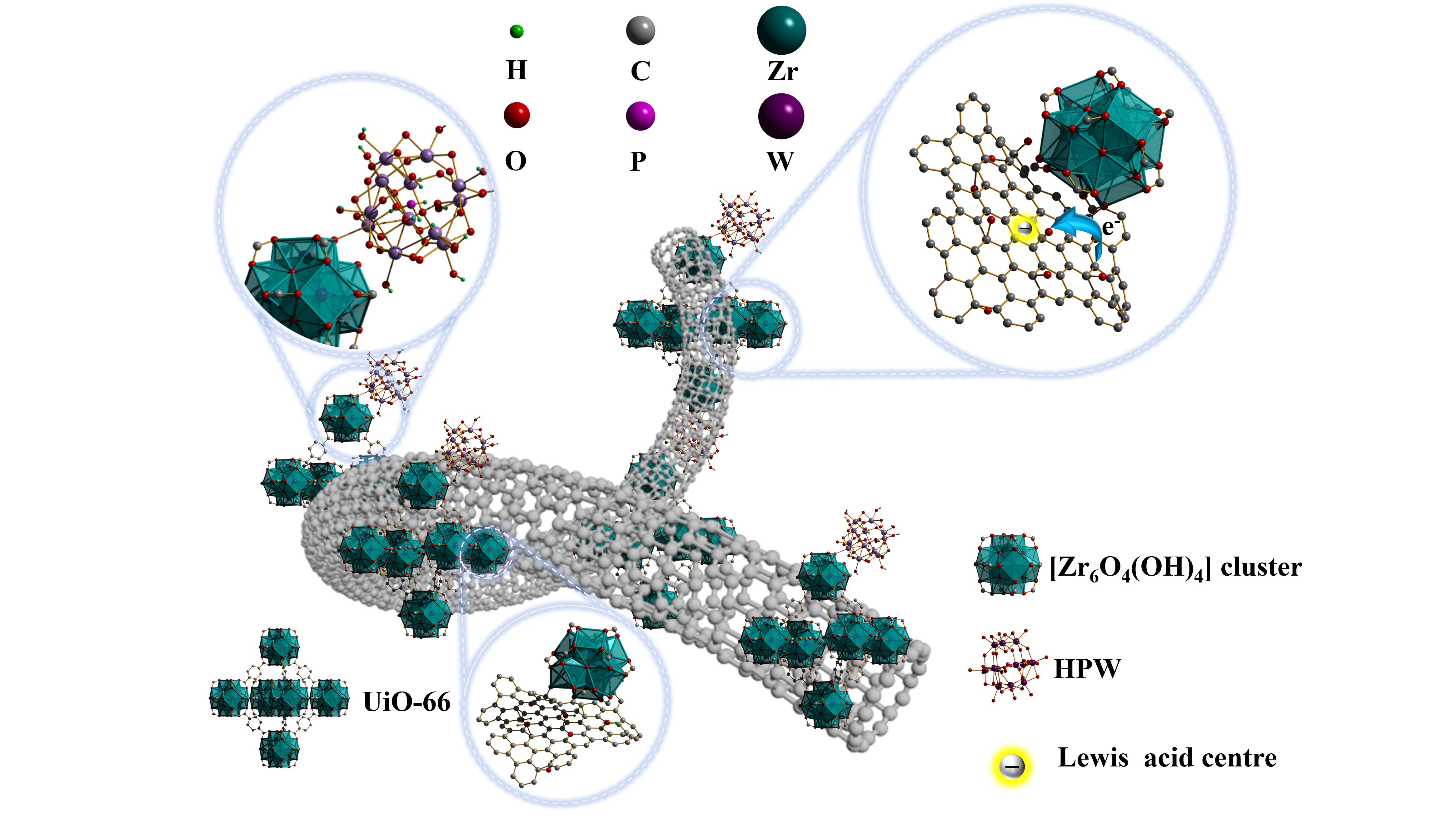
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. PW/UiO/CNTs-OH Catalyst Preparation
2.4. Characterization of the Catalysts
2.5. Esterification Process Catalyzed by PW/UiO/CNTs-OH
2.6. Gas Chromatographic Conditions
2.7. Experimental Design
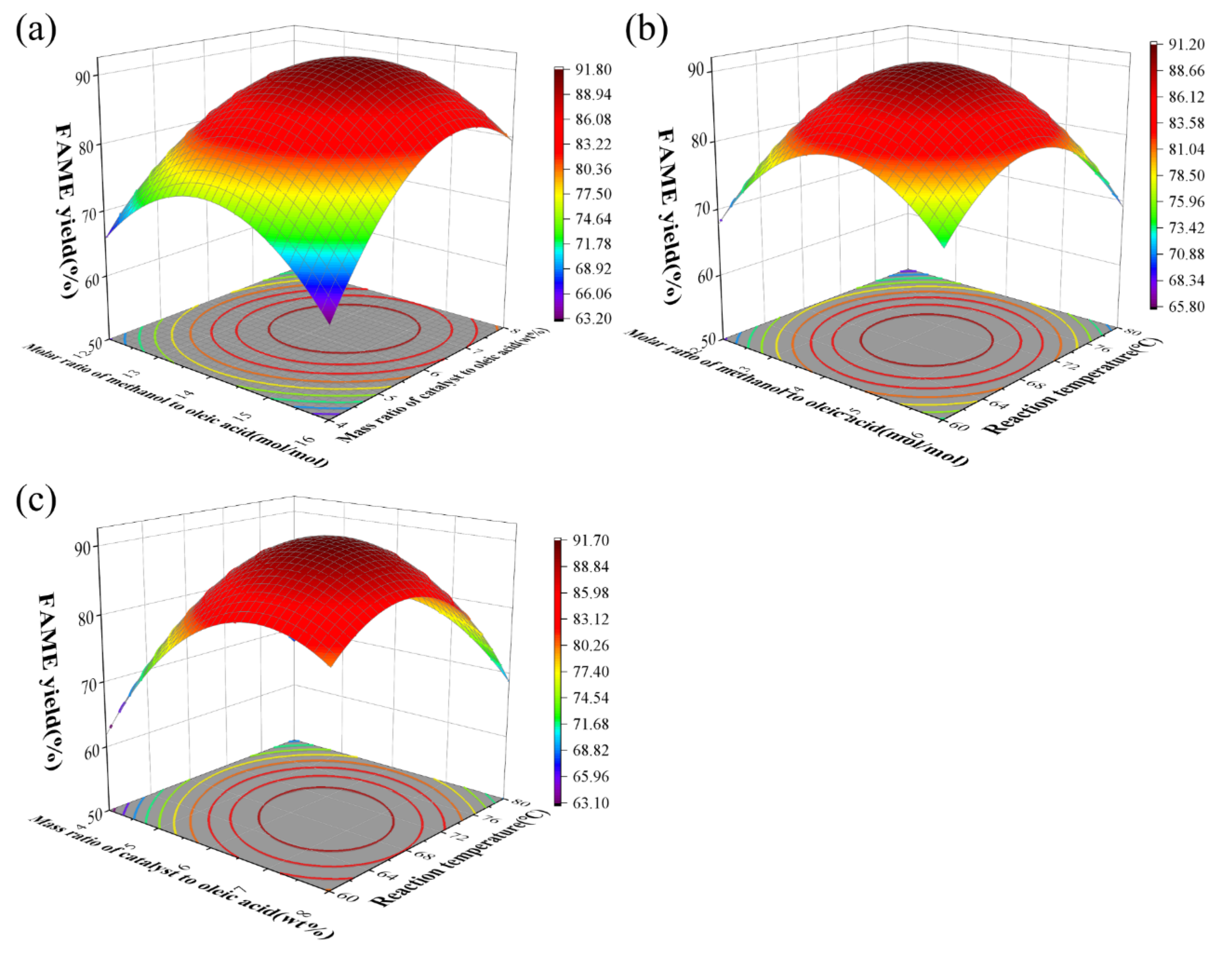
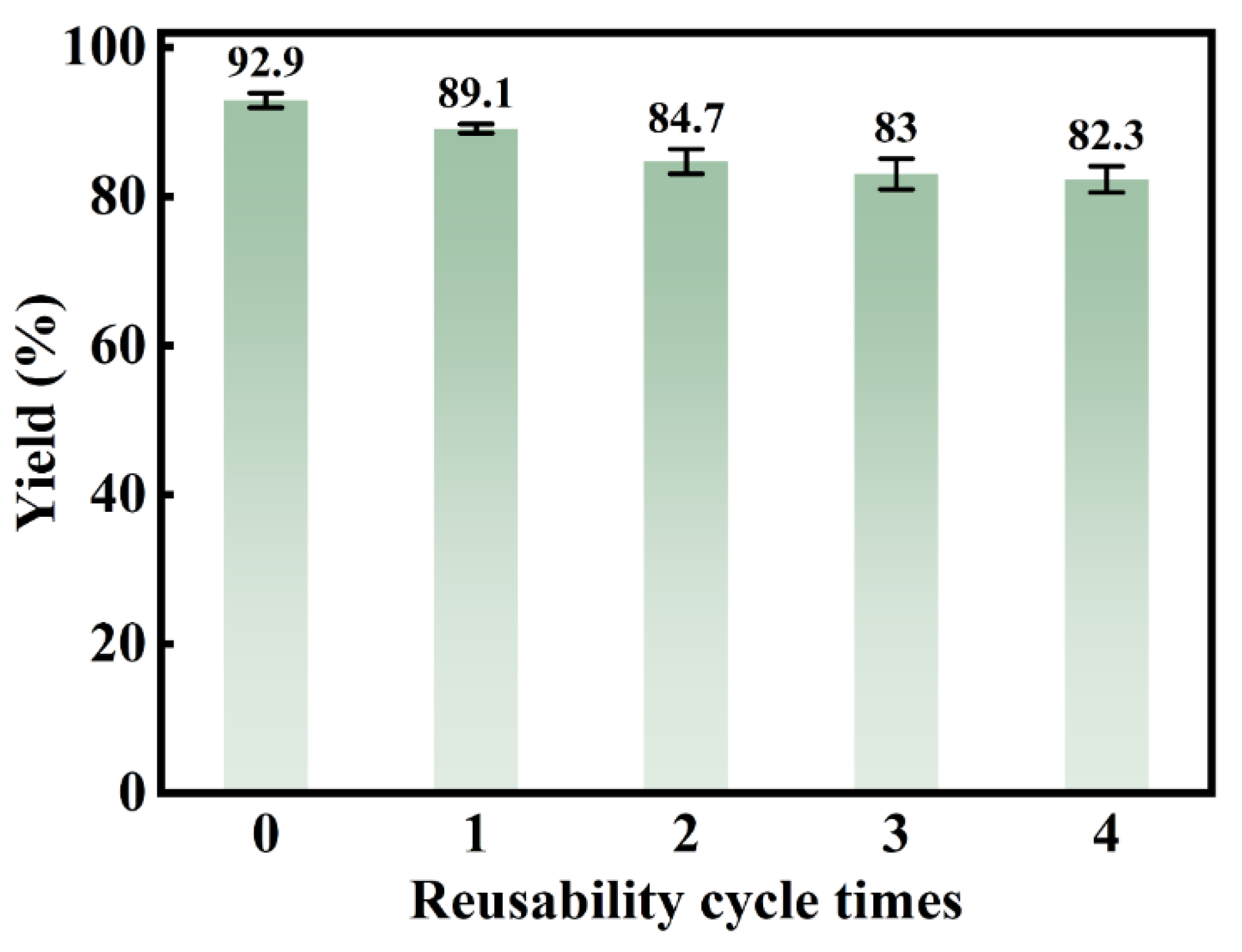
3. Results
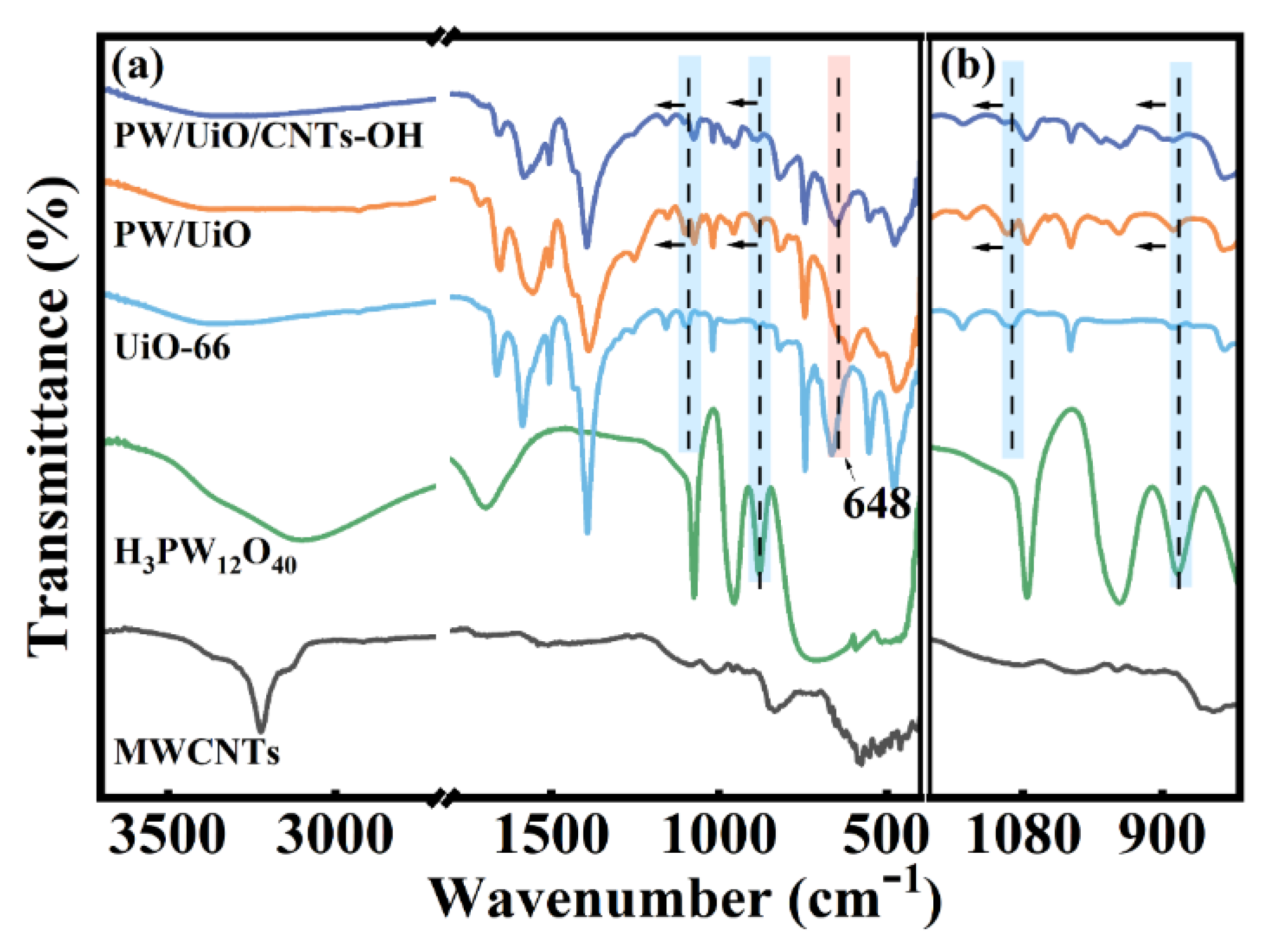
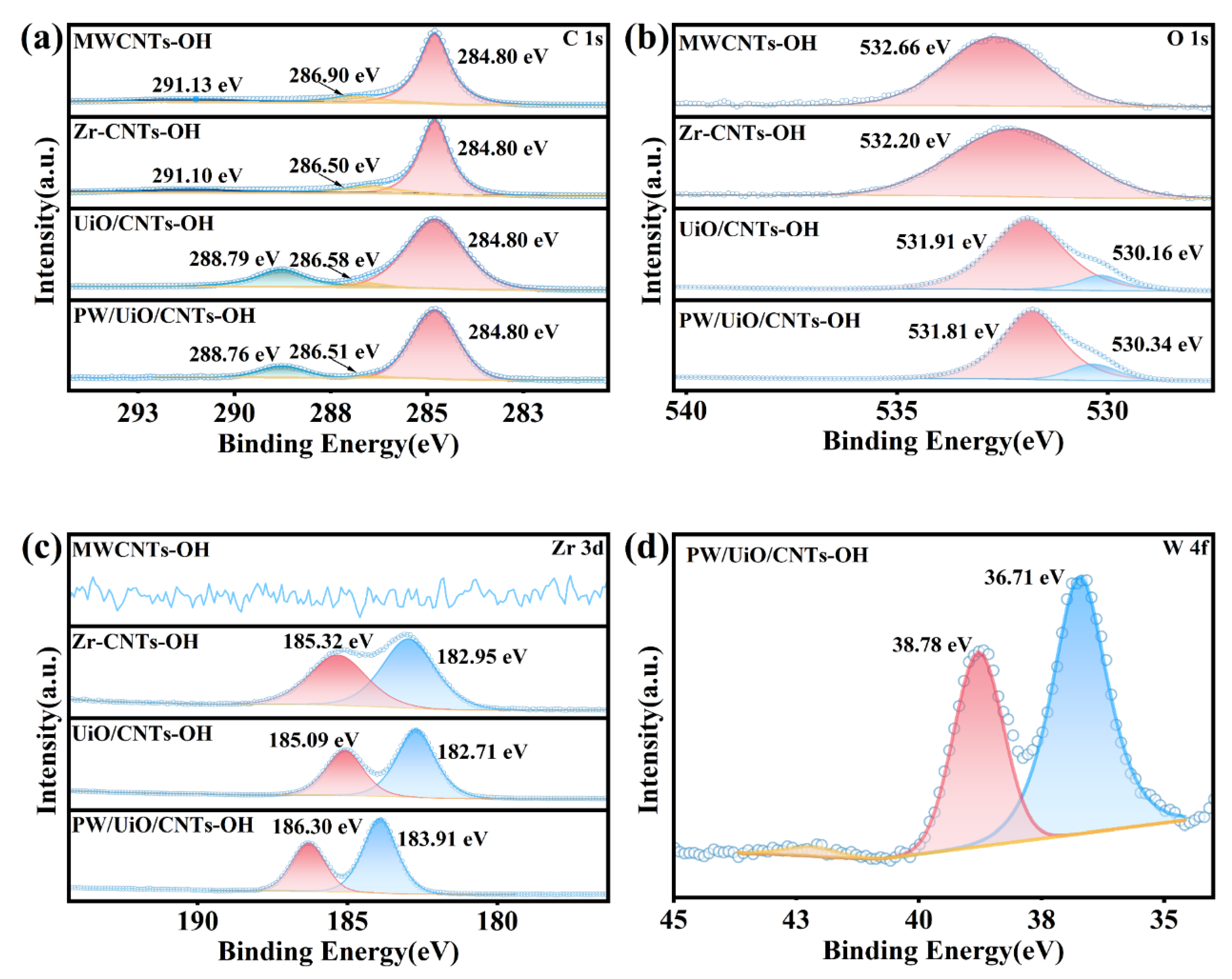
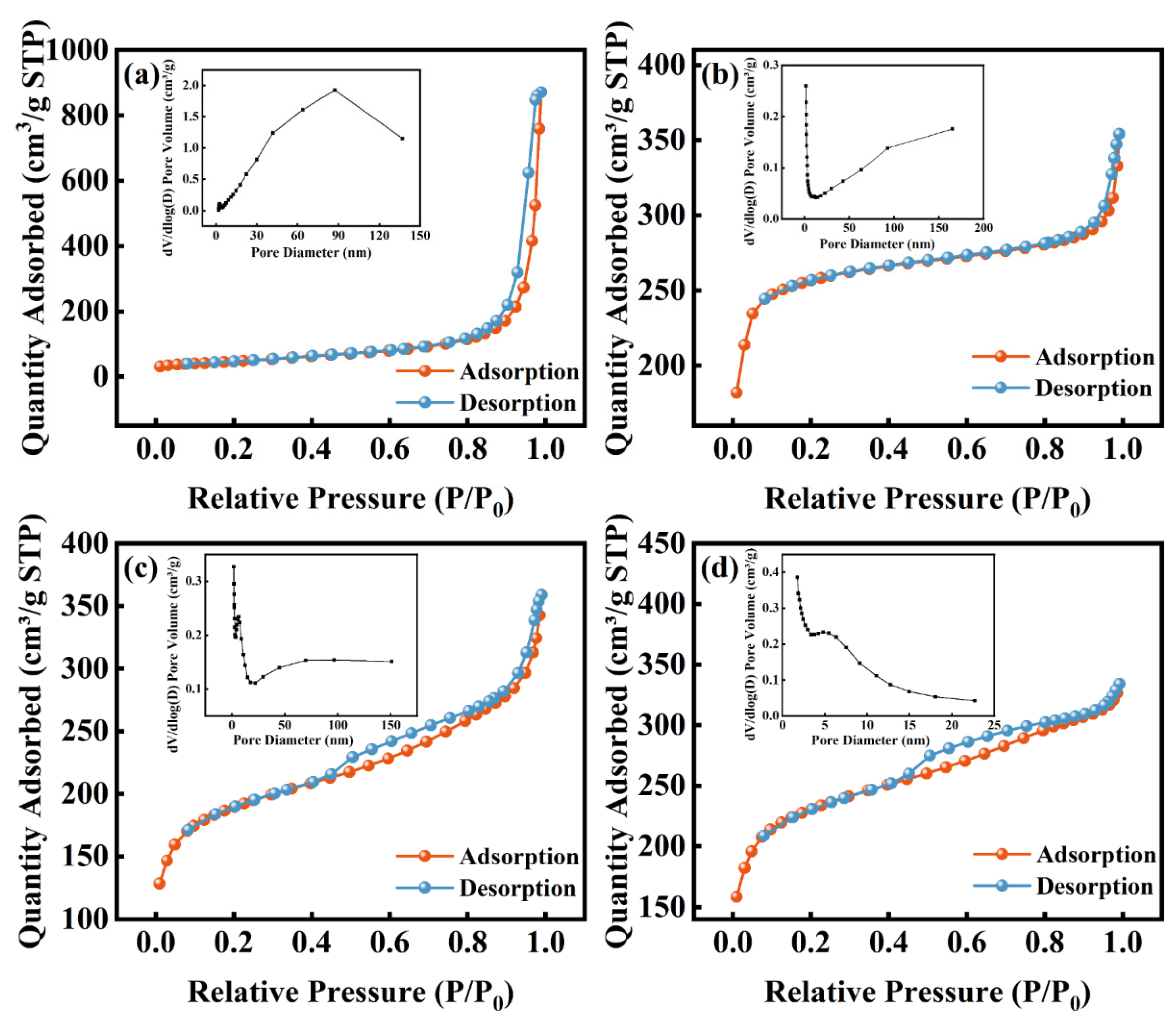
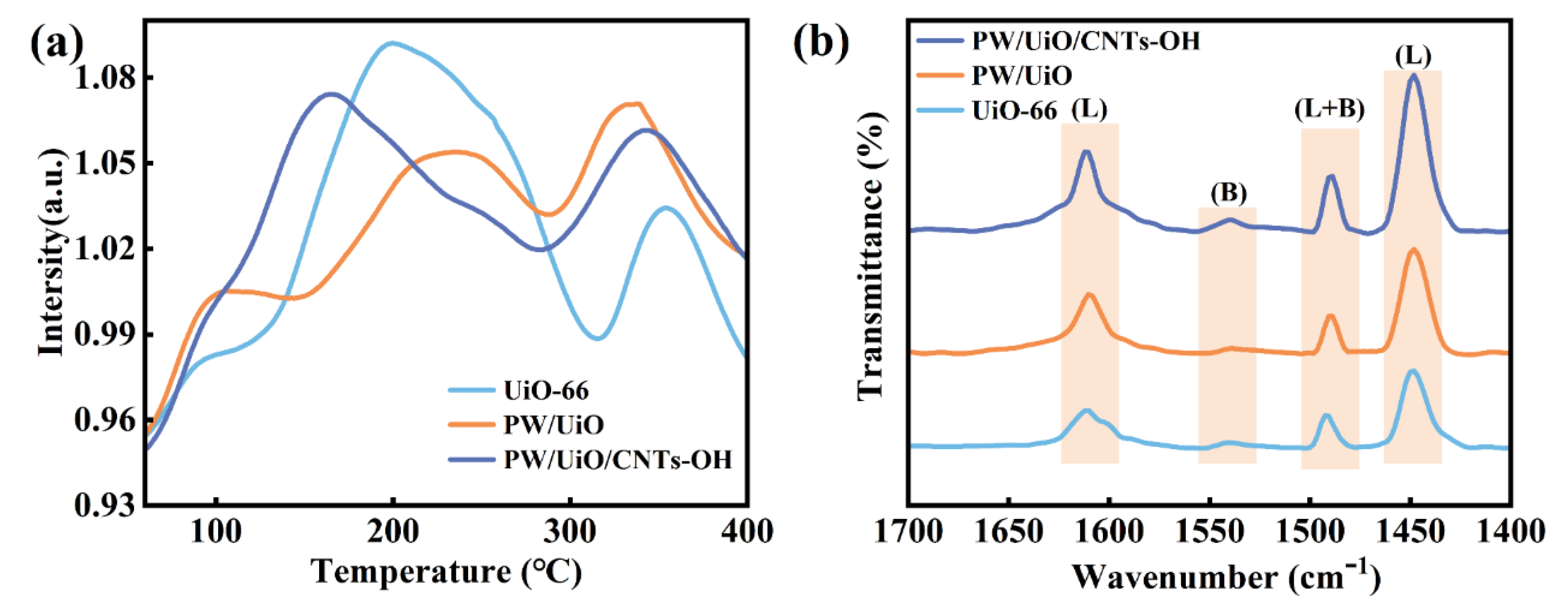
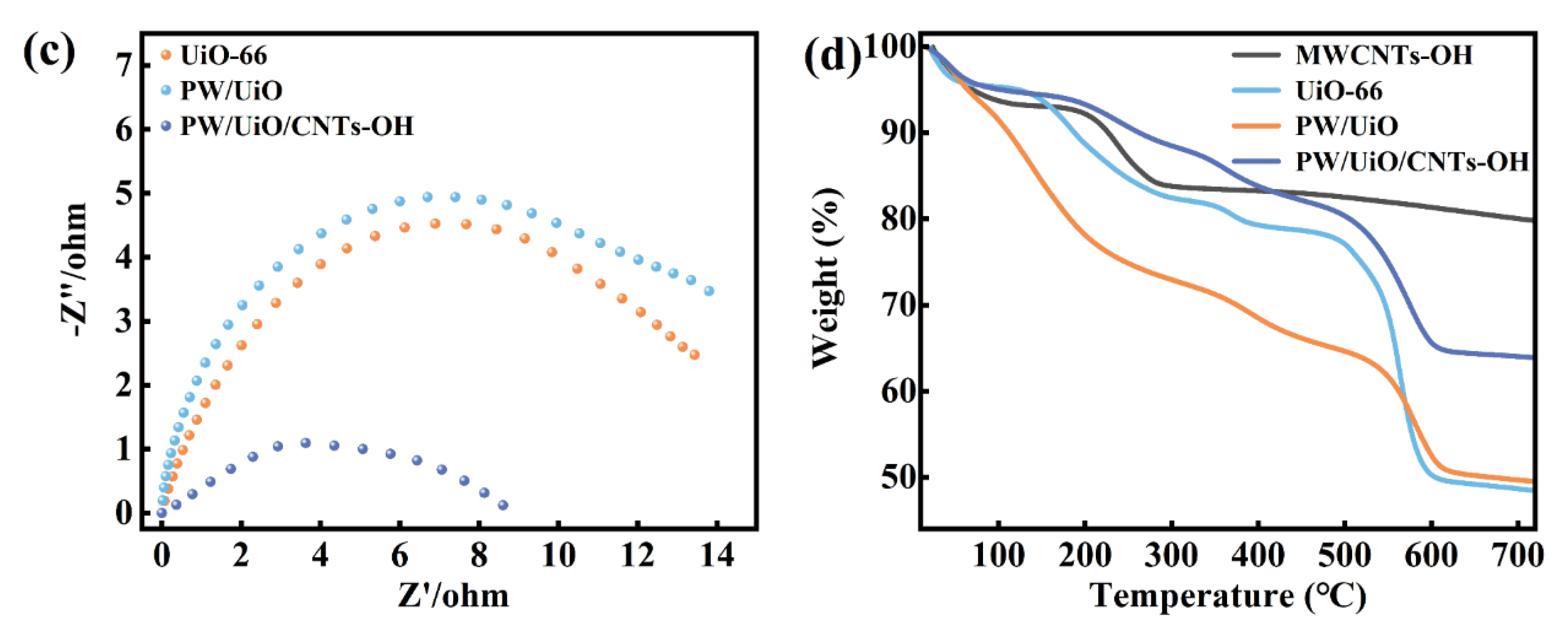
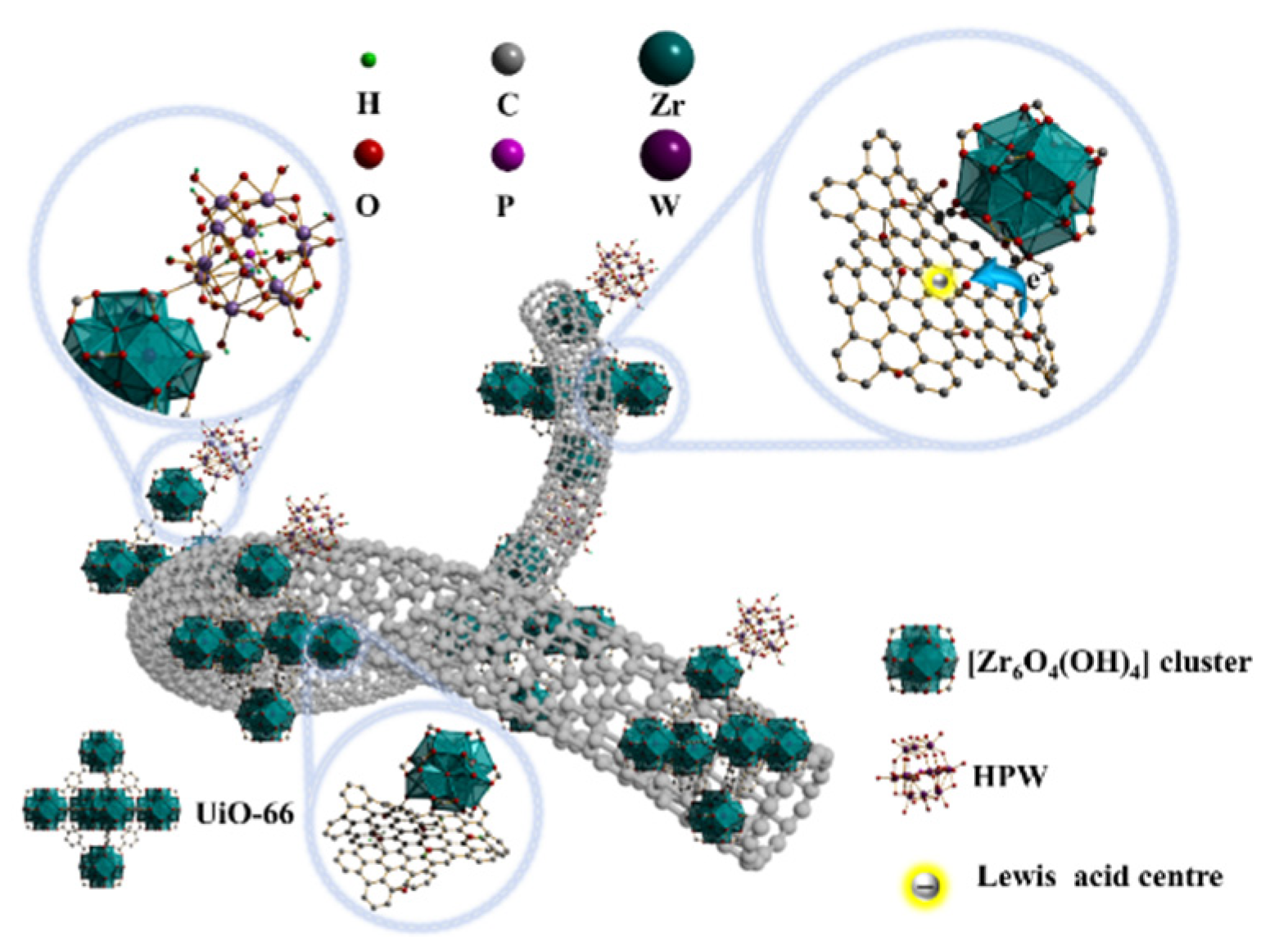
3.1. Catalyst Characterization
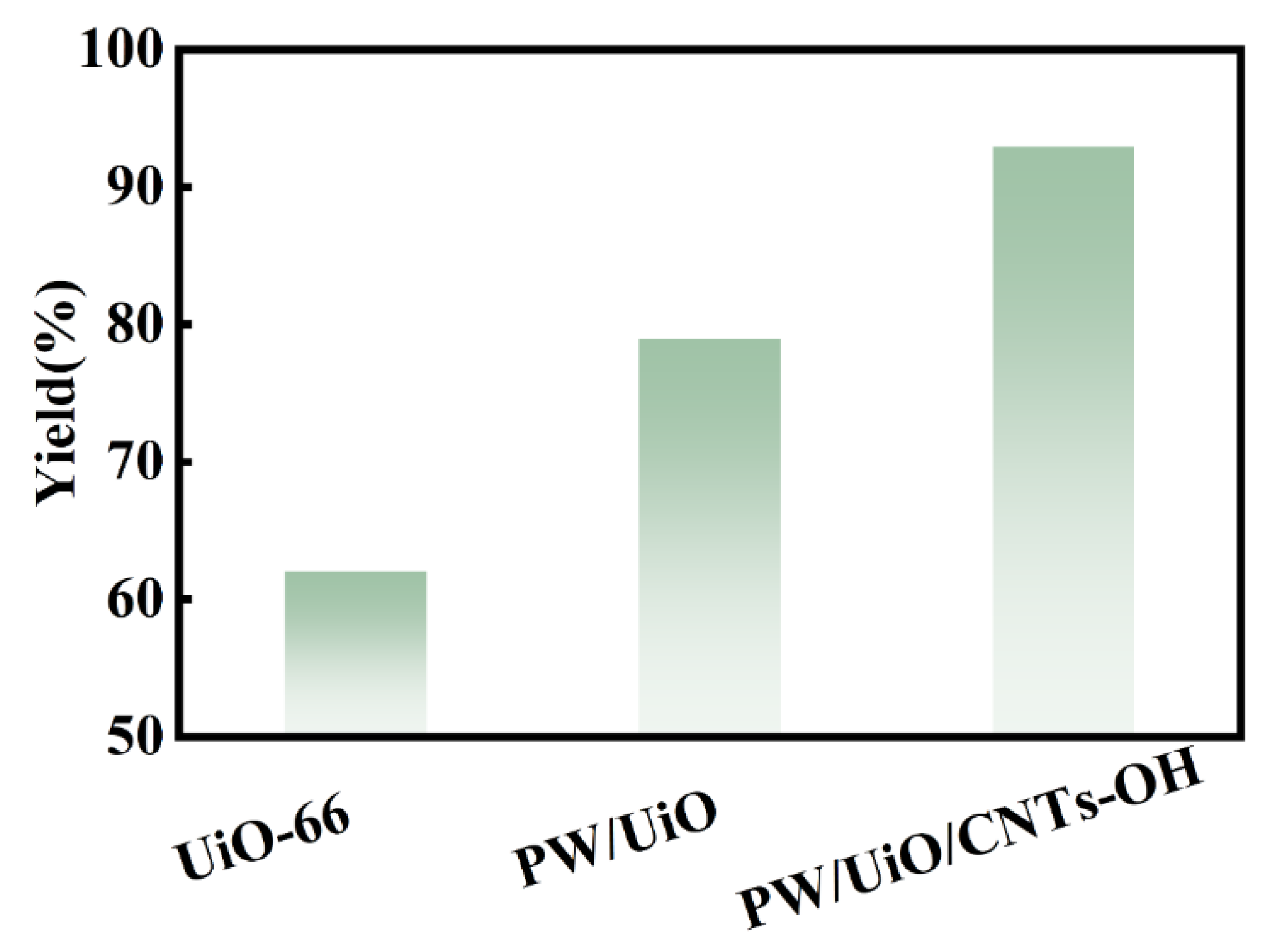
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A Review of Biomass-Derived Heterogeneous Catalyst for a Sustainable Biodiesel Production. Renewable and Sustainable Energy Reviews 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Bergmann, J.C.; Tupinambá, D.D.; Costa, O.Y.A.; Almeida, J.R.M.; Barreto, C.C.; Quirino, B.F. Biodiesel Production in Brazil and Alternative Biomass Feedstocks. Renewable and Sustainable Energy Reviews 2013, 21, 411–420. [Google Scholar] [CrossRef]
- Zabeti, M.; Wan Daud, W.M.A.; Aroua, M.K. Activity of Solid Catalysts for Biodiesel Production: a Review. Fuel Processing Technology 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to Value Addition: Utilization of Waste Brassica nigra Plant Derived Novel Green Heterogeneous Base Catalyst for Effective Synthesis of Biodiesel. Journal of Cleaner Production 2019, 239, 118112. [Google Scholar] [CrossRef]
- Frattini, L.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Kyriakou, G.; Lee, A.F. Support Enhanced α-pinene Isomerization over HPW/SBA-15. Applied Catalysis B: Environmental 2017, 200, 10–18. [Google Scholar] [CrossRef]
- You, X.; Yu, L.-l.; Xiao, F.-f.; Wu, S.-c.; Yang, C.; Cheng, J.-h. Synthesis of Phosphotungstic Acid-Supported Bimodal Mesoporous Silica-Based Catalyst for Defluorination of Aqueous Perfluorooctanoic Acid under Vacuum UV Irradiation. Chemical Engineering Journal 2018, 335, 812–821. [Google Scholar] [CrossRef]
- Bornhof, A.-B.; Bauzá, A.; Aster, A.; Pupier, M.; Frontera, A.; Vauthey, E.; Sakai, N.; Matile, S. Synergistic Anion–(π)n–π Catalysis on π-Stacked Foldamers. Journal of the American Chemical Society 2018, 140, 4884–4892. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Hedin, N.; Ding, X.; Zhang, S.; Yao, X.; Nie, Y.; Zhang, Y. Highly Efficient Adsorption of Benzothiophene from Model Fuel on a Metal-Organic Framework Modified with Dodeca-Tungstophosphoric Acid. Chemical Engineering Journal 2019, 362, 30–40. [Google Scholar] [CrossRef]
- Arrais Gonçalves, M.; Karine Lourenço Mares, E.; Roberto Zamian, J.; Narciso da Rocha Filho, G.; Rafael Vieira da Conceição, L. Statistical Optimization of Biodiesel Production from Waste Cooking Oil Using Magnetic Acid Heterogeneous Catalyst MoO3/SrFe2O4. Fuel 2021, 304. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: a Synergic Combination of Experiment and Theory. Chemistry of Materials 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Man, Z.; Meng, Y.; Lin, X.; Dai, X.; Wang, L.; Liu, D. Assembling UiO-66@TiO2 Nanocomposites for Efficient Photocatalytic Degradation of Dimethyl Sulfide. Chemical Engineering Journal 2022, 431, 133952. [Google Scholar] [CrossRef]
- Ma, Y.; Li, A.; Wang, C.; Ge, X. Preparation of HPW@UiO-66 Catalyst with Defects and its Application in Oxidative Desulfurization. Chemical Engineering Journal 2021, 404, 127062. [Google Scholar] [CrossRef]
- Zeng, Z.; Sorescu, D.C.; White, D.L.; Hwang, S.I.; Shao, W.; He, X.; Schulte, Z.M.; Rosi, N.L.; Star, A. Heterogeneous Growth of UiO-66-NH2 on Oxidized Single-Walled Carbon Nanotubes to Form “Beads-on-a-String” Composites. ACS Applied Materials & Interfaces 2021, 13, 15482–15489. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Bakkali, B.E.; Trautwein, G.; Reinoso, S. Zirconia-supported Tungstophosphoric Heteropolyacid as Heterogeneous Acid Catalyst for Biodiesel Production. Applied Catalysis B: Environmental 2018, 224, 194–203. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption Behaviors of Organic Micropollutants on Zirconium Metal–Organic Framework UiO-66: Analysis of Surface Interactions. ACS Applied Materials & Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef]
- Rao, K.N.; Sridhar, A.; Lee, A.F.; Tavener, S.J.; Young, N.A.; Wilson, K. Zirconium Phosphate Supported Tungsten Oxide Solid Acid Catalysts for the Esterification of Palmitic Acid. Green Chemistry 2006, 8. [Google Scholar] [CrossRef]
- Yan, L.; Duan, T.; Huang, T.; Zhao, B.; Fan, Y. Phosphotungstic Acid Immobilized on Mixed-Ligand-Directed UiO-66 for the Esterification of 1-butene with Acetic Acid to Produce High-Octane Gasoline. Fuel 2019, 245, 226–232. [Google Scholar] [CrossRef]
- Kar, A.K.; Sarkar, R.; Manal, A.K.; Kumar, R.; Chakraborty, S.; Ahuja, R.; Srivastava, R. Unveiling and Understanding the Remarkable Enhancement in the Catalytic Activity by the Defect Creation in UiO-66 during the Catalytic Transfer Hydrodeoxygenation of Vanillin with Isopropanol. Applied Catalysis B: Environmental 2023, 325, 122385. [Google Scholar] [CrossRef]
- Ma, T.; Liu, D.; Liu, Z.; Xu, J.; Dong, Y.; Chen, G.; Yun, Z. 12-Tungstophosphoric Acid-Encapsulated Metal-Mrganic Framework UiO-66: A Promising Catalyst for the Esterification of Acetic Acid with N-butanol. Journal of the Taiwan Institute of Chemical Engineers 2022, 133. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Y.; Zhou, Y.; Liu, H.; Cai, Y.; Lu, S.; Yao, Y. Homogeneously Dispersed HPW/Graphene for High Efficient Catalytic Oxidative Desulfurization Prepared by Electrochemical Deposition. Applied Surface Science 2019, 484, 917–924. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Liu, X.-L.; Bao, Z.-L.; Qian, P.-F.; Liu, K.; Shi, Y.; Ming, X.; Geng, H.-Z. Highly Stable Phosphotungstic Acid/Au Dual Doped Carbon Nanotube Transparent Conductive Films for Transparent Flexible Heaters. Carbon 2023, 207, 219–229. [Google Scholar] [CrossRef]
- Ellis, J.E.; Zeng, Z.; Hwang, S.I.; Li, S.; Luo, T.-Y.; Burkert, S.C.; White, David L. ; Rosi, N.L.; Gassensmith, J.J.; Star, A. Growth of ZIF-8 on Molecularly Ordered 2-Methylimidazole/Single-Walled Carbon Nanotubes to Form Highly Porous, Electrically Conductive Composites. Chemical Science 2019, 10, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. Journal of the American Chemical Society 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zheng, S.; Xiao, Q.; Zhong, Y.; Zhu, W.; Lin, A.; Samy El-Shall, M. Synergetic Catalysis of Palladium Nanoparticles Encaged within Amine-Functionalized UiO-66 in the Hydrodeoxygenation of Vanillin in Water. Green Chemistry 2016, 18, 2900–2908. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.-P.; Wang, X.-Y.; Liu, T.-T.; Wang, X.-S.; Huang, Y.-B.; Cao, R. Postsynthetic Ionization of an Imidazole-Containing Metal–Organic Framework for the Cycloaddition of Carbon Dioxide and Epoxides. Chemical Science 2017, 8, 1570–1575. [Google Scholar] [CrossRef]
- Freitas, E.F.; Araújo, Á.A.L.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Comparative Acidity of BEA and Y Zeolite Composites with 12-tungstophosphoric and 12-tungstosilicic Acids. Molecular Catalysis 2018, 458, 152–160. [Google Scholar] [CrossRef]
- Yang, X.-L.; Qiao, L.-M.; Dai, W.-L. Phosphotungstic Acid Encapsulated in Metal-Organic Framework UiO-66: An Effective Catalyst for the Selective Oxidation of Cyclopentene to Glutaraldehyde. Microporous and Mesoporous Materials 2015, 211, 73–81. [Google Scholar] [CrossRef]
- Niu, S.; Ning, Y.; Lu, C.; Han, K.; Yu, H.; Zhou, Y. Esterification of Oleic Acid to Produce Biodiesel Catalyzed by Sulfonated Activated Carbon from Bamboo. Energy Conversion and Management 2018, 163, 59–65. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, J.; Sun, K.; Ma, L.; Ding, J. Esterification of Oleic Acid with Ethanol Catalyzed by Sulfonated Cation Exchange Resin: Experimental and Kinetic Studies. Energy Conversion and Management 2013, 76, 980–985. [Google Scholar] [CrossRef]


| Factor | Code | Range and Levels | ||||
| -ɑ (-1.682) | -1 | 0 | 1 | ɑ (1.682) | ||
| the molar ratio of methanol to oleic acid (mol/mol) | X1 | 10.64 | 12 | 14 | 16 | 17.36 |
| Catalyst loading (wt%) | X2 | 2.64 | 4 | 6 | 8 | 9.36 |
| Reaction temperature (°C) | X3 | 53.18 | 60 | 70 | 80 | 86.82 |
| Catalyst | Average mass of W (g/g of catalyst) | The actual W loading (wt%) |
|---|---|---|
| PW/CNTs | 0.0149 | 1.49 |
| PW/CNTs-OH | 0.0167 | 1.67 |
| PW/UiO-66 | 0.0439 | 4.39 |
| PW/UiO/CNTs-OH | 0.1487 | 14.87 |
| Catalyst | Mean pore size (m2/g) | Pore volume (cm3/g) | Mean pore size (nm) |
|---|---|---|---|
| HPW | 2.830 | 0.005 | 7.178 |
| MWCNTs-OH | 163.590 | 1.347 | 32.939 |
| UiO-66 | 997.531 | 0.547 | 2.197 |
| PW/UiO | 700.046 | 0.555 | 3.173 |
| PW/UiO/CNTs-OH | 857.147 | 0.517 | 2.411 |
| Catalyst | Weak acidic site (mmol/g) | Moderate acidic site (mmol/g) | Brӧnsted acidity (μmol/g) | Lewis acidity (μmol/g) | Total acidity (mmol/g) | Brönsted/Lewis acidity ratio(B/L) |
|---|---|---|---|---|---|---|
| UiO-66 | 4.57 | 1.23 | 3.63 | 34.72 | 5.79 | 0.10 |
| PW/UiO | 3.64 | 2.58 | 3.65 | 44.54 | 6.21 | 0.08 |
| PW/UiO/CNTs-OH | 4.36 | 3.05 | 9.98 | 83.69 | 7.40 | 0.12 |
| Std. | X1 (mol/mol) | X2 (wt%) | X3 (°C) | Y (%) |
|---|---|---|---|---|
| 1 | 14 | 6 | 70 | 90.3 |
| 2 | 14 | 6 | 70 | 90.8 |
| 3 | 12 | 8 | 80 | 52.1 |
| 4 | 17.3636 | 6 | 70 | 62.4 |
| 5 | 14 | 2.6364 | 70 | 52.3 |
| 6 | 10.6364 | 6 | 70 | 55.8 |
| 7 | 16 | 8 | 60 | 73.8 |
| 8 | 12 | 4 | 60 | 50.3 |
| 9 | 12 | 4 | 80 | 59.9 |
| 10 | 14 | 6 | 70 | 90.8 |
| 11 | 16 | 4 | 60 | 51.1 |
| 12 | 14 | 9.3636 | 70 | 68.8 |
| 13 | 16 | 4 | 80 | 54.1 |
| 14 | 14 | 6 | 70 | 91.4 |
| 15 | 14 | 6 | 70 | 92.0 |
| 16 | 16 | 8 | 80 | 63.7 |
| 17 | 12 | 8 | 60 | 63.7 |
| 18 | 14 | 6 | 86.8179 | 57.2 |
| 19 | 14 | 6 | 53.1821 | 62.8 |
| 20 | 14 | 6 | 70 | 90.8 |
| Source | Sum of squares | Degree of freedom | Factor | F-value | p-value |
|---|---|---|---|---|---|
| Model | 4910.43 | 9 | 545.60 | 545.60 | < 0.0001 |
| X1 | 56.59 | 1 | 56.59 | 56.59 | < 0.0001 |
| X2 | 315.58 | 1 | 315.58 | 315.58 | < 0.0001 |
| X3 | 25.11 | 1 | 25.11 | 25.11 | 0.0011 |
| X1X2 | 89.11 | 1 | 89.11 | 89.11 | < 0.0001 |
| X1X3 | 3.25 | 1 | 3.25 | 3.25 | 0.1330 |
| X2X3 | 147.06 | 1 | 147.06 | 147.06 | < 0.0001 |
| X12 | 1794.60 | 1 | 1794.60 | 1794.60 | < 0.0001 |
| X22 | 1633.50 | 1 | 1633.50 | 1633.50 | < 0.0001 |
| X32 | 1693.71 | 1 | 1693.71 | 1693.71 | < 0.0001 |
| Residual | 12.16 | 10 | 1.22 | ||
| Lack of Fit | 10.39 | 5 | 2.08 | 5.88 | 0.0372 |
| Pure Error | 1.77 | 5 | 0.3537 | ||
| Total sum of squares | 4922.59 | 19 | |||
| Sted.Dev | 1.10 | R2 | 0.9975 | ||
| mean | 68.70 | Adj R2 | 0.9953 | ||
| C.V.% | 1.60 | Pred R2 | 0.9808 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





