Submitted:
28 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
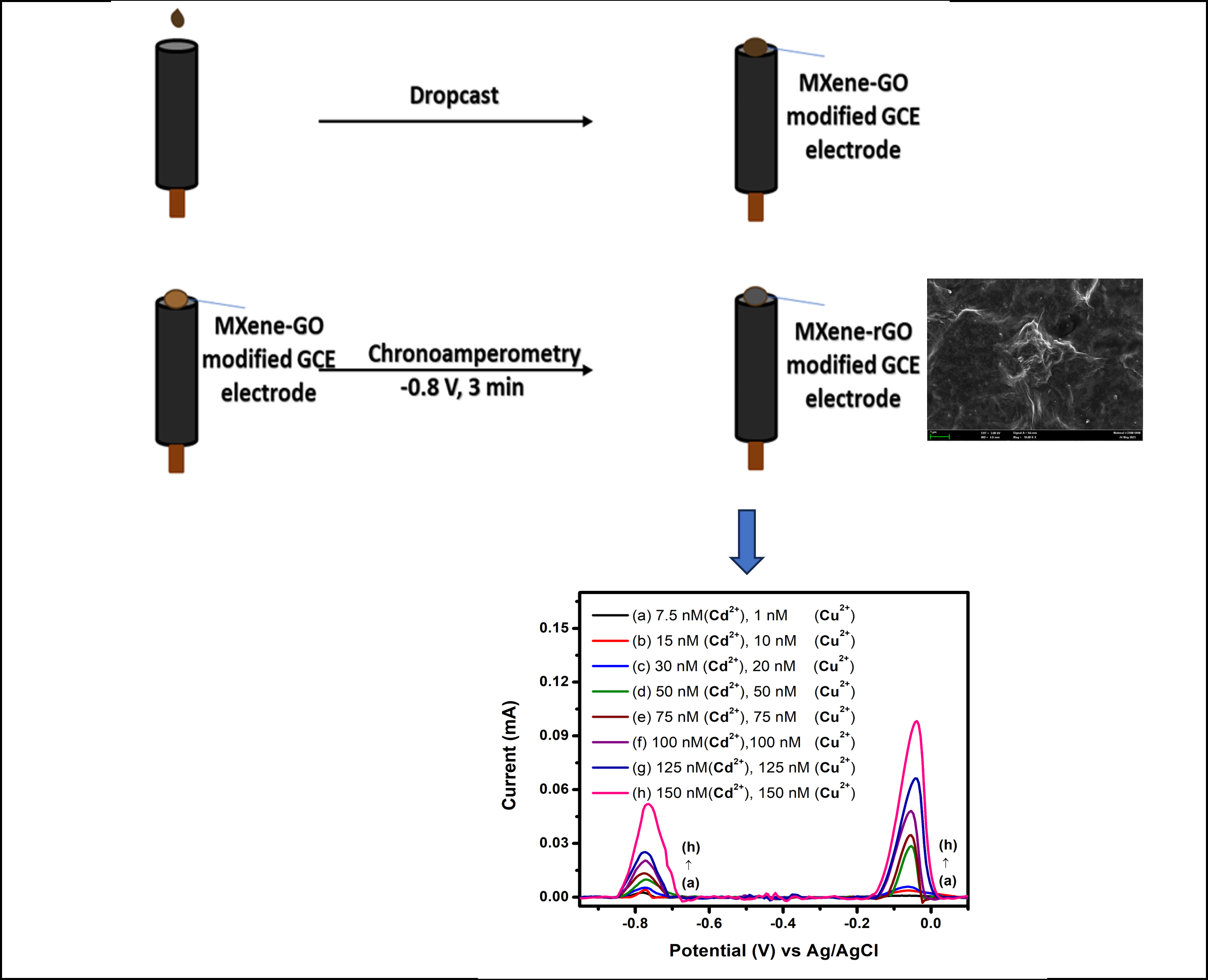
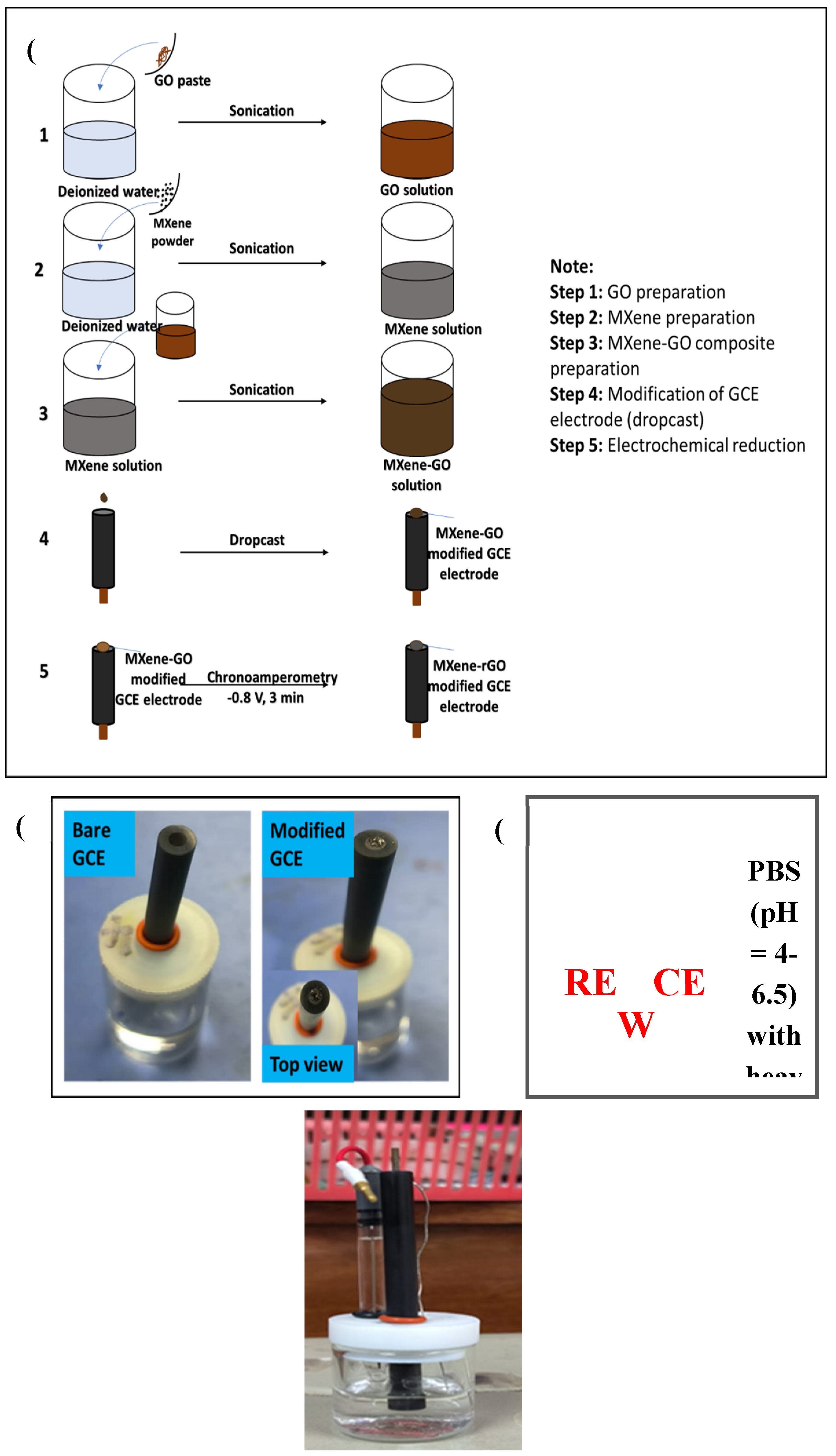
2.2. Preparation of Ti3C2Tx-rGO Nanocomposite
2.3. Ti3C2Tx-rGO Nanocomposite Characterization
2.4. Electrochemical Detection of Heavy Metals
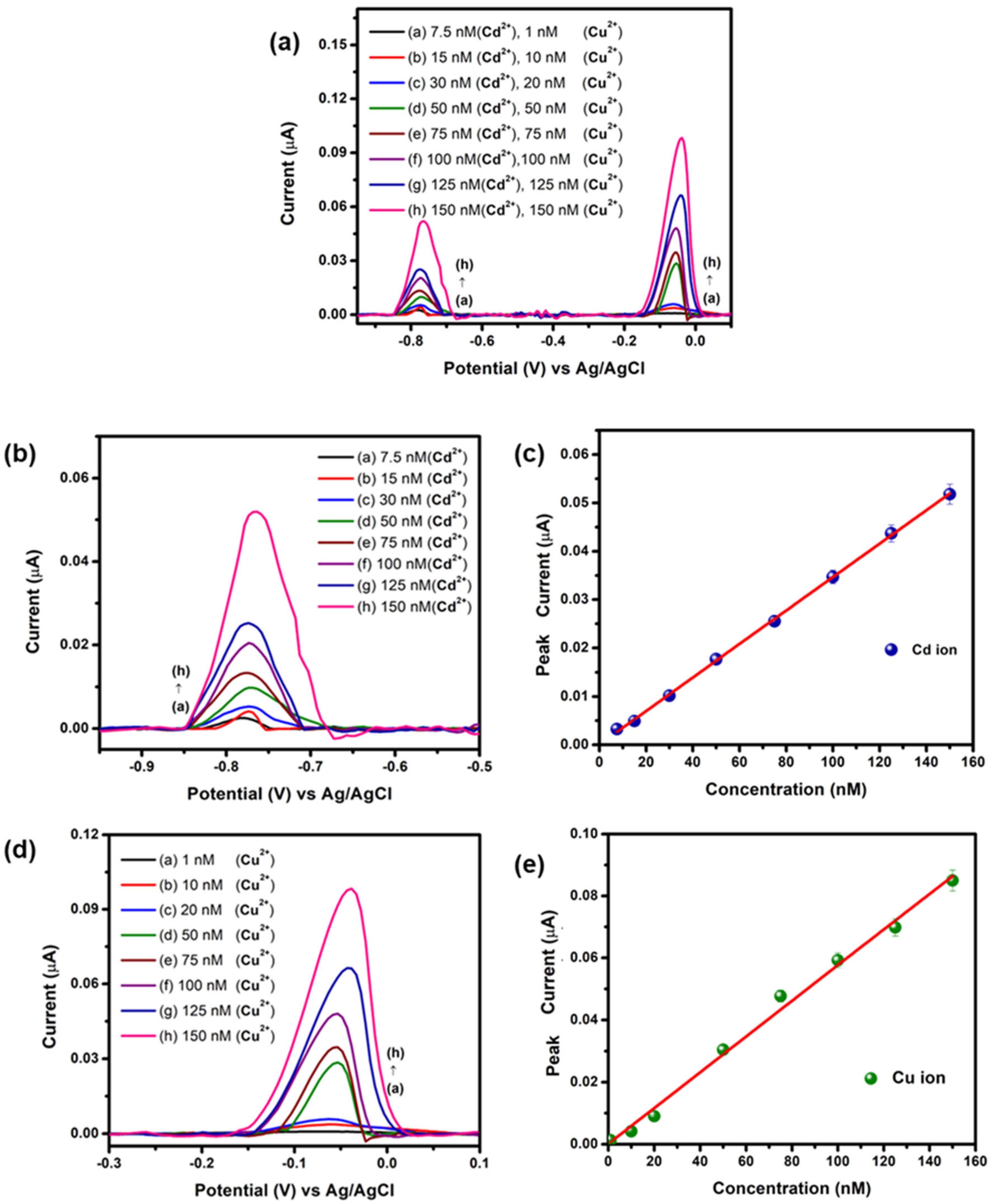
3. Result and Discussion
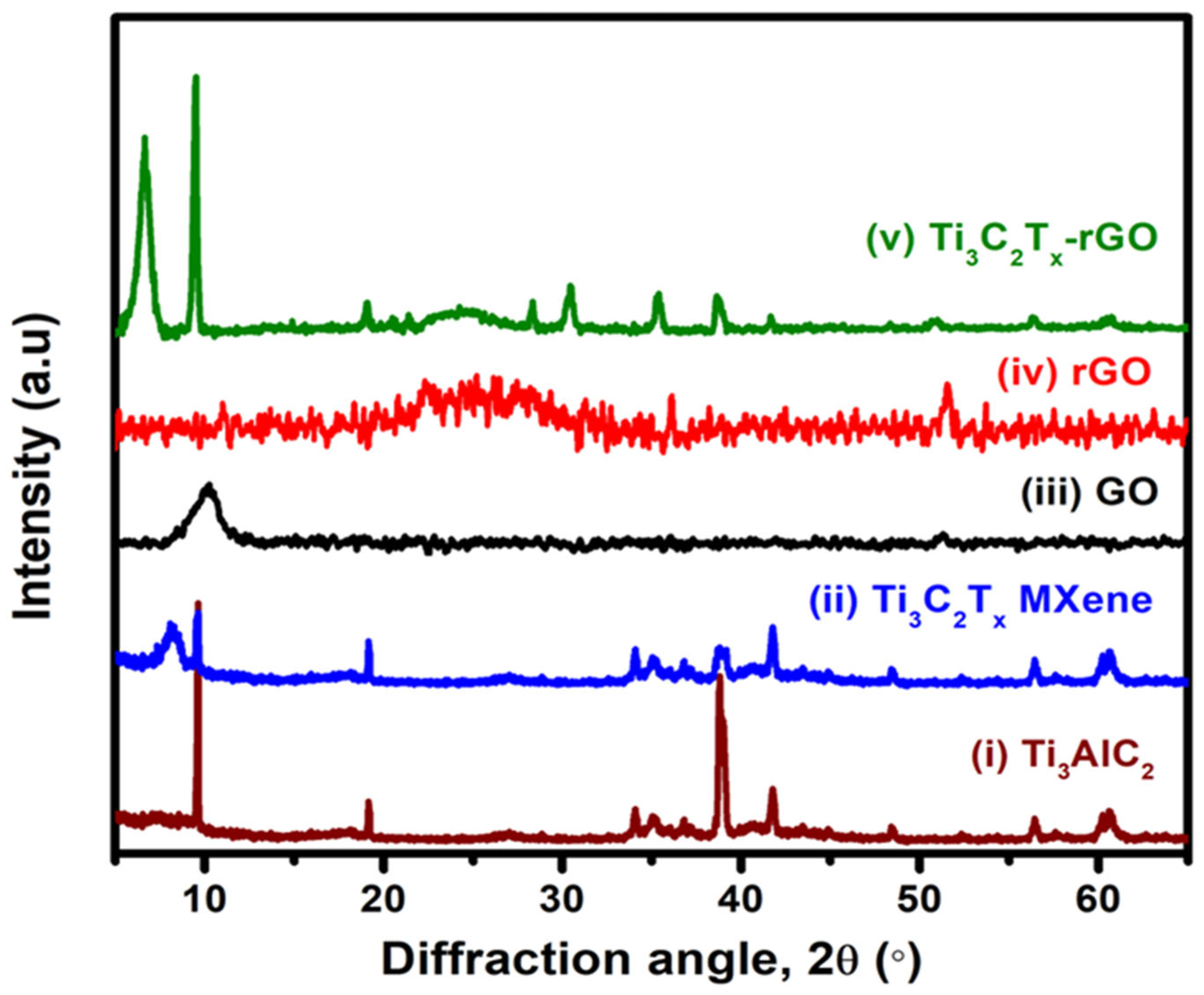
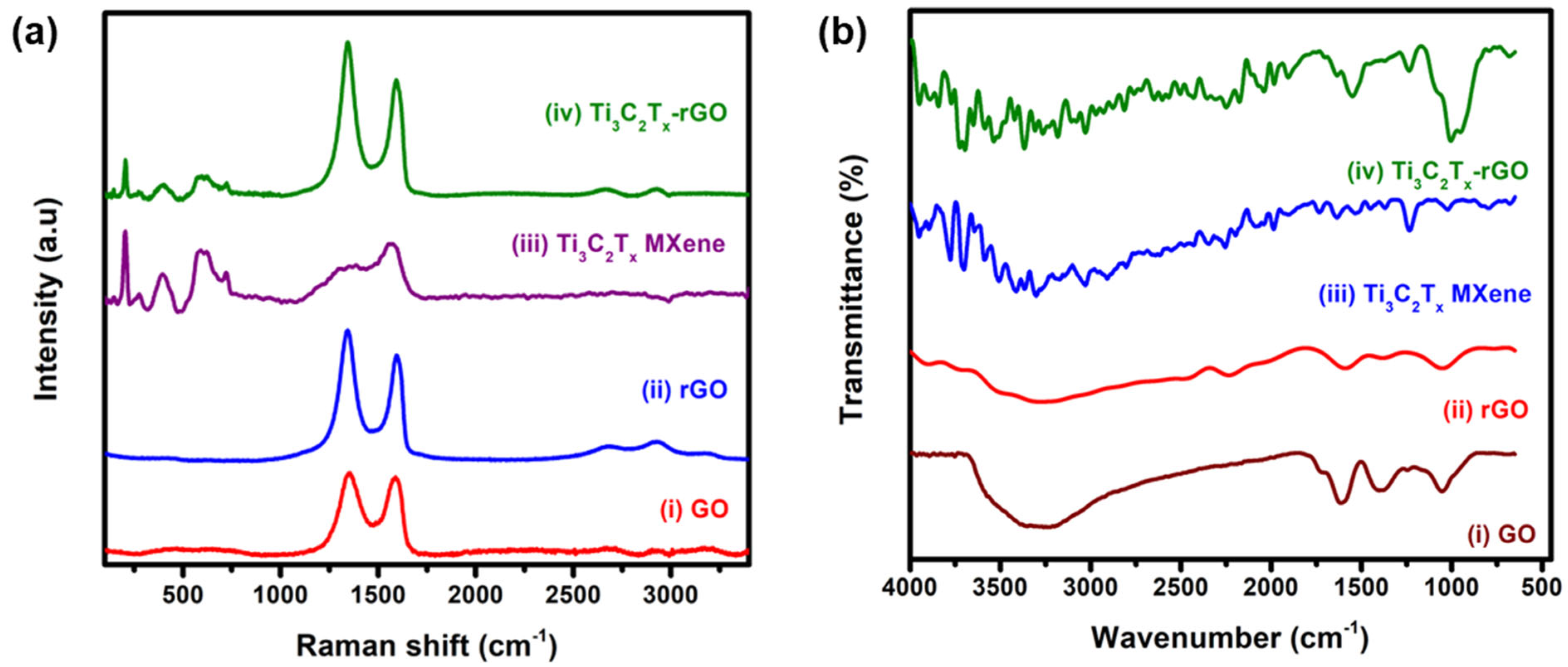
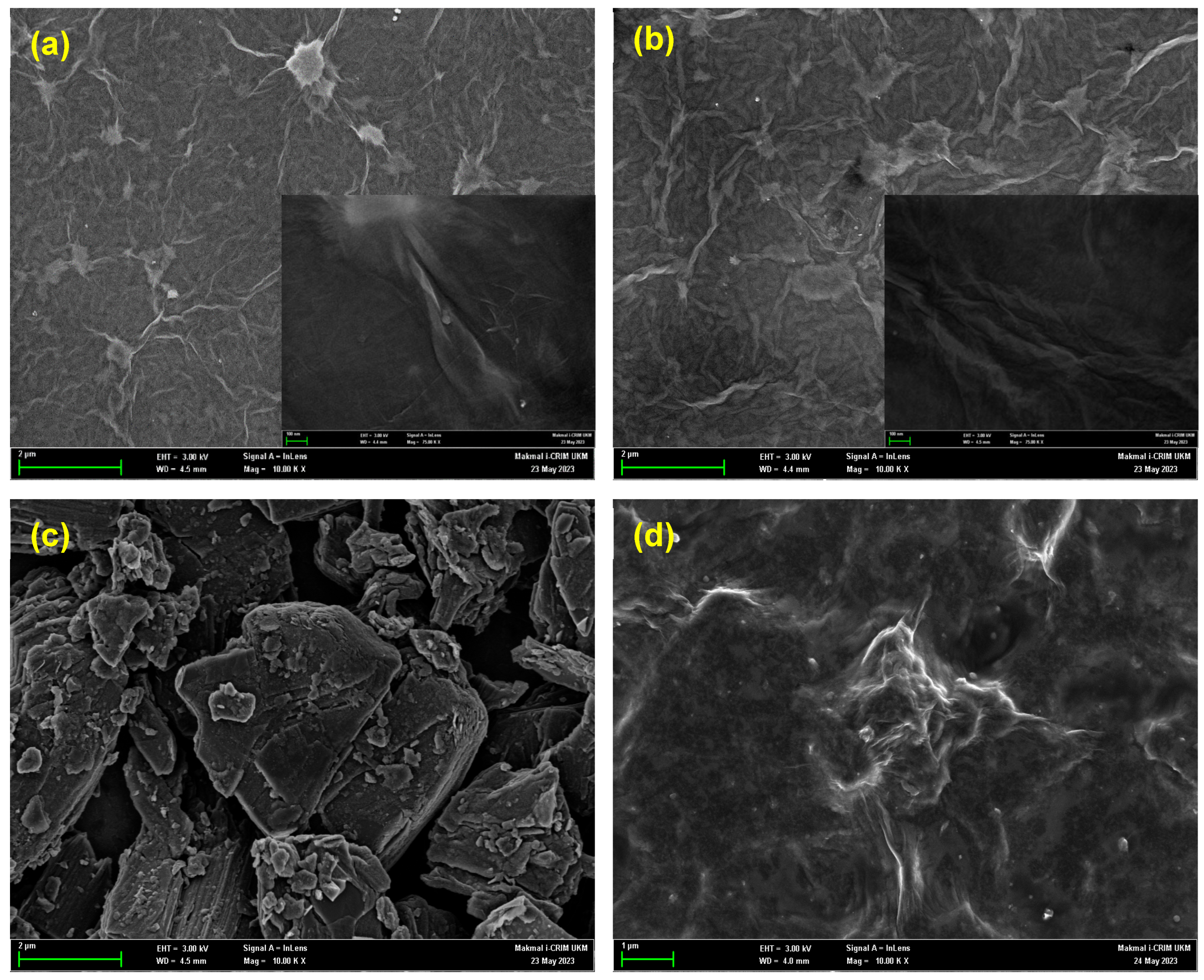
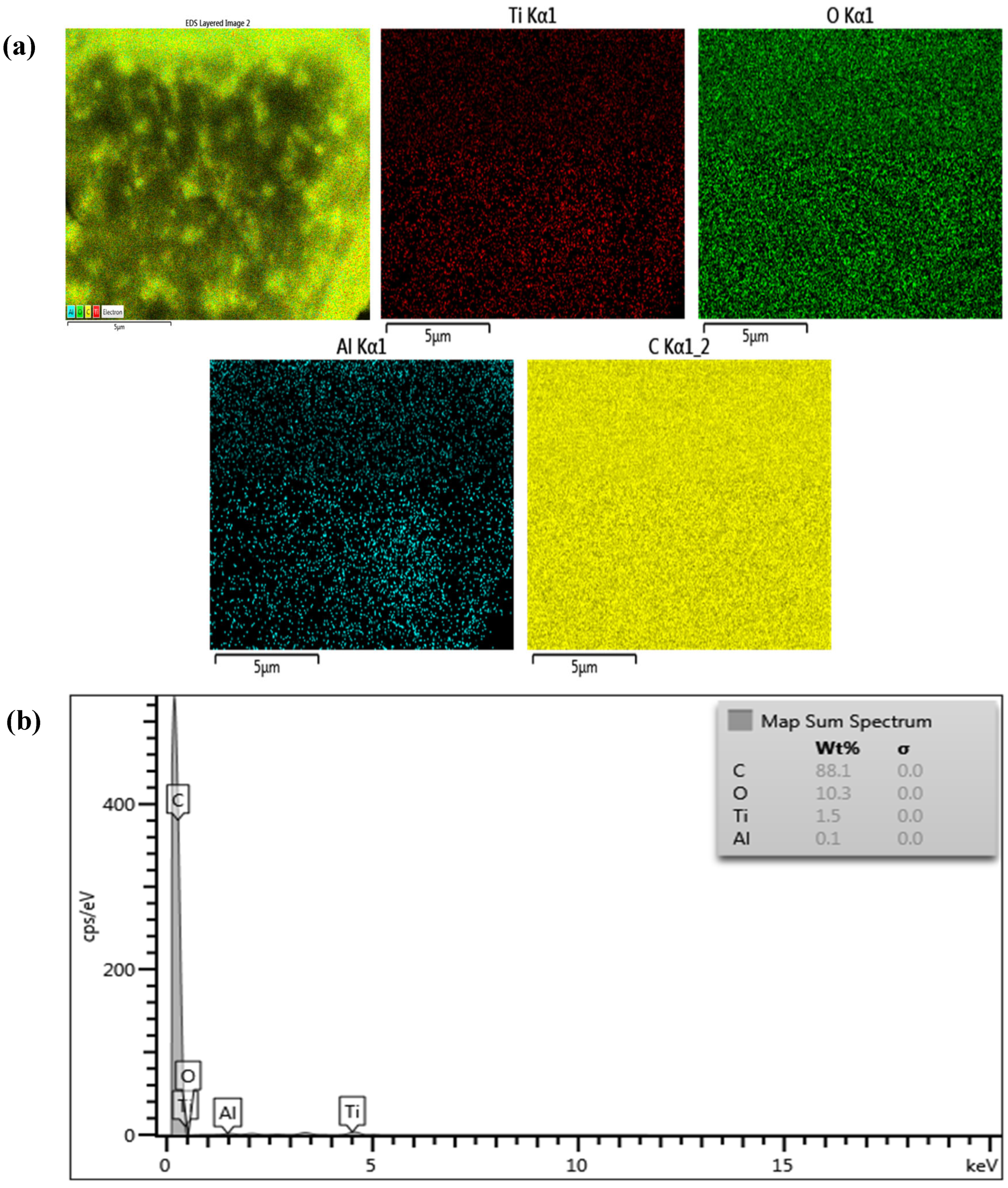
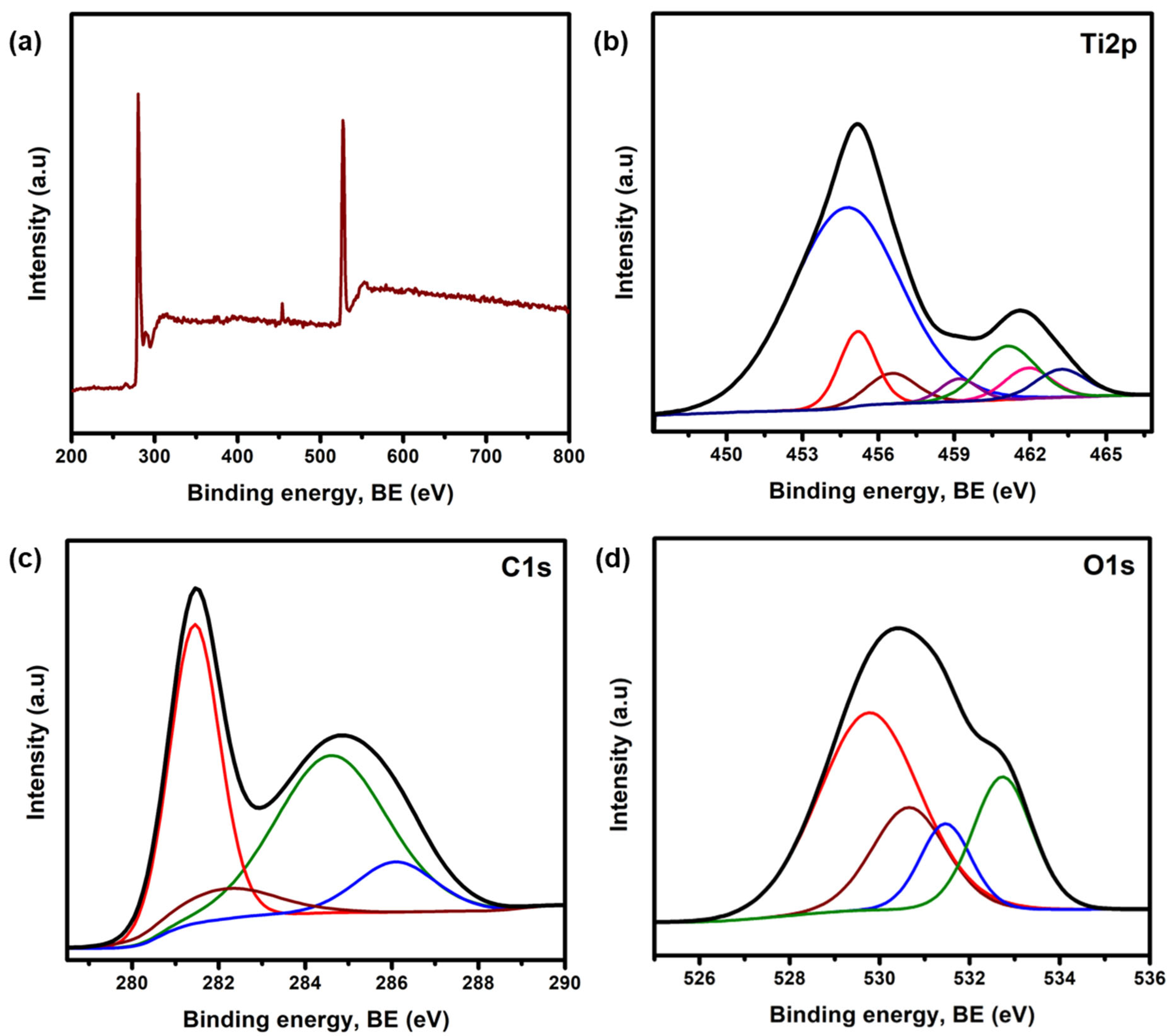
3.1. Characterization
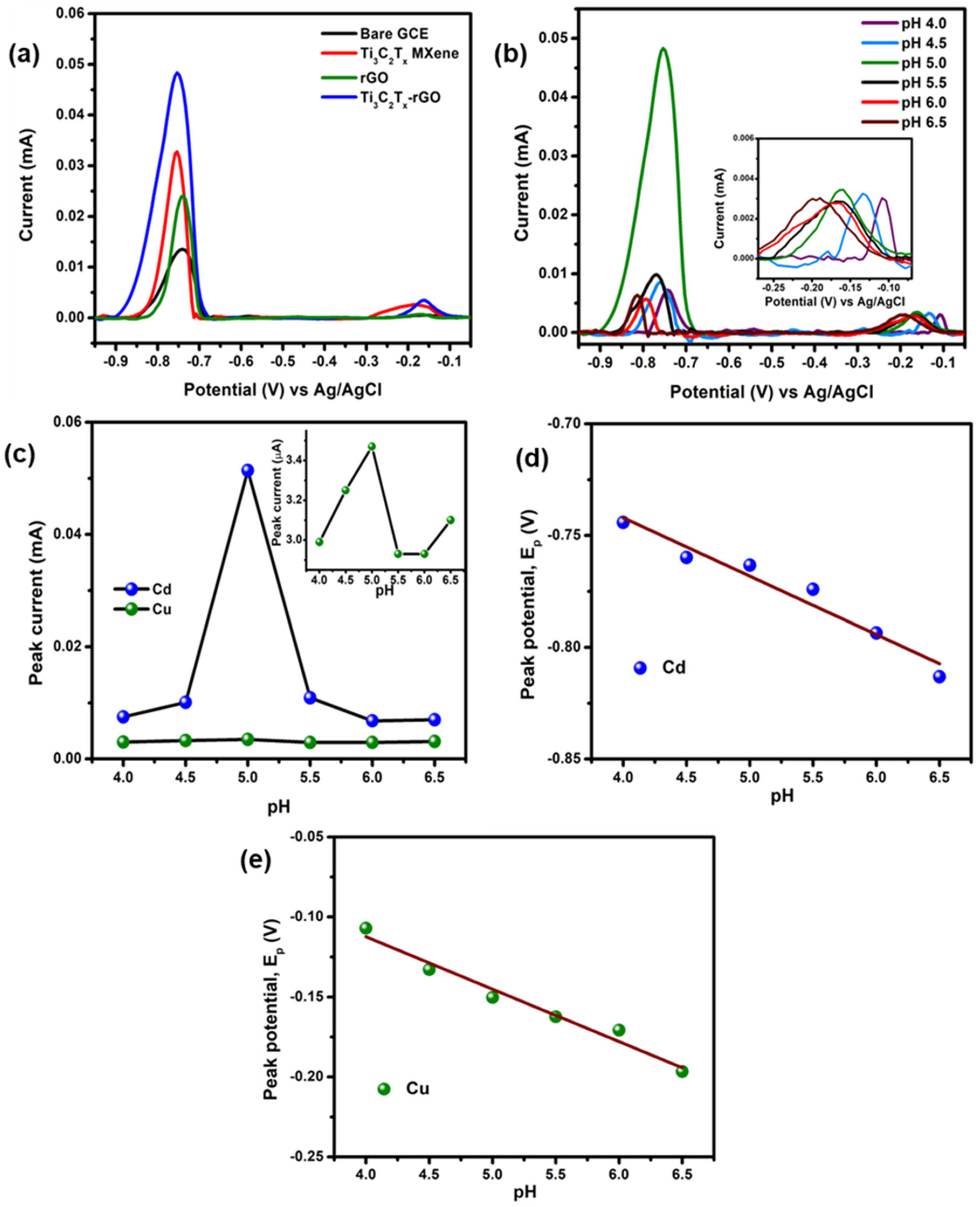
3.2. Electrochemical Detection
4. Conclusions
Acknowledgments
References
- M.B. Gumpu, S. Sethuraman, U.M. Krishnan, J.B.B. Rayappan, A review on detection of heavy metal ions in water–an electrochemical approach, Sensors and actuators B: chemical 213 (2015) 515-533. [CrossRef]
- H. Yin, Q. Zhang, Y. Zhou, Q. Ma, L. Zhu, S. Ai, Electrochemical behavior of catechol, resorcinol and hydroquinone at graphene-chitosan composite film modified glassy carbon electrode and their simultaneous determination in water samples, Electrochim. Acta 56 (2011) 2748-2753.
- A.J.S. Ahammad, M.M. Rahman, G.-R. Xu, S. Kim, J.-J. Lee, Highly sensitive and simultaneous determination of hydroquinone and catechol at poly (thionine) modified glassy carbon electrode, Electrochimica Acta 56 (2011) 5266-5271. [CrossRef]
- Y. Yi, Y. Zhao, Z. Zhang, Y. Wu, G. Zhu, Recent developments in electrochemical detection of cadmium, Trends in Environmental Analytical Chemistry 33 (2022) e00152.
- A. Shahzad, K. Rasool, W. Miran, M. Nawaz, J. Jang, K.A. Mahmoud, D.S. Lee, Two-dimensional Ti3C2Tx MXene nanosheets for efficient copper removal from water, ACS Sustainable Chemistry & Engineering 5 (2017) 11481-11488.
- Y. Yi, Y. Ma, F. Ai, Y. Xia, H. Lin, G. Zhu, Novel methodology for anodic stripping voltammetric sensing of heavy-metal ions using Ti 3 C 2 T x nanoribbons, Chemical Communications 57 (2021) 7790-7793.
- Q. Peng, J. Guo, Q. Zhang, J. Xiang, B. Liu, A. Zhou, R. Liu, Y. Tian, Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide, J. Am. Chem. Soc. 136 (2014) 4113-4116. [CrossRef]
- Y. Xia, Y. Ma, Y. Wu, Y. Yi, H. Lin, G. Zhu, Free-electrodeposited anodic stripping voltammetry sensing of Cu (II) based on Ti 3 C 2 T x MXene/carbon black, Microchimica Acta 188 (2021) 1-9. [CrossRef]
- Y. Xia, Y. Zhao, F. Ai, Y. Yi, T. Liu, H. Lin, G. Zhu, N and P co-doped MXenes nanoribbons for electrodeposition-free stripping analysis of Cu (II) and Hg (II), Journal of Hazardous Materials 425 (2022) 127974. [CrossRef]
- A. Maity, X. Sui, C.R. Tarman, H. Pu, J. Chang, G. Zhou, R. Ren, S. Mao, J. Chen, Pulse-driven capacitive lead ion detection with reduced graphene oxide field-effect transistor integrated with an analyzing device for rapid water quality monitoring, ACS sensors 2 (2017) 1653-1661. [CrossRef]
- K. Hamsawahini, P. Sathishkumar, R. Ahamad, A.R.M. Yusoff, A sensitive, selective and rapid determination of lead (II) ions in real-life samples using an electrochemically reduced graphene oxide-graphite reinforced carbon electrode, Talanta 144 (2015) 969-976. [CrossRef]
- X. Xuan, M. Hossain, J.Y. Park, A fully integrated and miniaturized heavy-metal-detection sensor based on micro-patterned reduced graphene oxide, Scientific reports 6 (2016) 1-8. [CrossRef]
- S. Wu, K. Zhang, X. Wang, Y. Jia, B. Sun, T. Luo, F. Meng, Z. Jin, D. Lin, W. Shen, Enhanced adsorption of cadmium ions by 3D sulfonated reduced graphene oxide, Chem. Eng. J. 262 (2015) 1292-1302. [CrossRef]
- D. Mohanadas, N.H.N. Azman, J. Abdullah, N.A. Endot, Y. Sulaiman, Bifunctional ternary manganese oxide/vanadium oxide/reduced graphene oxide as electrochromic asymmetric supercapacitor, Ceram. Int. 47 (2021) 34529-34537. [CrossRef]
- D. Mohanadas, N.H.N. Azman, Y. Sulaiman, A bifunctional asymmetric electrochromic supercapacitor with multicolor property based on nickel oxide/vanadium oxide/reduced graphene oxide, Journal of Energy Storage 48 (2022) 103954. [CrossRef]
- A. Sengupta, B.B. Rao, N. Sharma, S. Parmar, V. Chavan, S.K. Singh, S. Kale, S. Ogale, Comparative evaluation of MAX, MXene, NanoMAX, and NanoMAX-derived-MXene for microwave absorption and Li ion battery anode applications, Nanoscale 12 (2020) 8466-8476. [CrossRef]
- X. Li, Y. Qian, T. Liu, F. Cao, Z. Zang, X. Sun, S. Sun, Q. Niu, J. Wu, Enhanced lithium and electron diffusion of LiFePO4 cathode with two-dimensional Ti3C2 MXene nanosheets, Journal of Materials Science 53 (2018) 11078-11090. [CrossRef]
- H. Fang, Y. Pan, M. Yin, C. Pan, Enhanced visible light photocatalytic activity of CdS with alkalized Ti3C2 nano-sheets as co-catalyst for degradation of rhodamine B, Journal of Materials Science: Materials in Electronics 30 (2019) 14954-14966. [CrossRef]
- D. Mohanadas, M.A.A.M. Abdah, N.H.N. Azman, T.B. Ravoof, Y. Sulaiman, Facile synthesis of PEDOT-rGO/HKUST-1 for high performance symmetrical supercapacitor device, Scientific reports 11 (2021) 1-13. [CrossRef]
- S. Bai, X. Shen, X. Zhong, Y. Liu, G. Zhu, X. Xu, K. Chen, One-pot solvothermal preparation of magnetic reduced graphene oxide-ferrite hybrids for organic dye removal, Carbon 50 (2012) 2337-2346. [CrossRef]
- S.-X. Wang, H. Maimaiti, B. Xu, A. Awati, G.-B. Zhou, Y.-d. Cui, Synthesis and visible-light photocatalytic N2/H2O to ammonia of ZnS nanoparticles supported by petroleum pitch-based graphene oxide, Appl. Surf. Sci. 493 (2019) 514-524.
- Z. Gohari-Bajestani, O. Akhlaghi, Y. Yürüm, A. Yürüm, Synthesis of anatase TiO2 with exposed (001) facets grown on N-doped reduced graphene oxide for enhanced hydrogen storage, Int. J. Hydrogen Energy 42 (2017) 6096-6103. [CrossRef]
- M. Huang, J. Yu, Q. Hu, W. Su, M. Fan, B. Li, L. Dong, Preparation and enhanced photocatalytic activity of carbon nitride/titania (001 vs 101 facets)/reduced graphene oxide (g-C3N4/TiO2/rGO) hybrids under visible light, Appl. Surf. Sci. 389 (2016) 1084-1093. [CrossRef]
- J. Xu, Q. Liang, Z. Li, V.Y. Osipov, Y. Lin, B. Ge, Q. Xu, J. Zhu, H. Bi, Rational Synthesis of Solid-State Ultraviolet B Emitting Carbon Dots via Acetic Acid-Promoted Fractions of sp3 Bonding Strategy, Adv. Mater. 34 (2022) 2200011.
- Y. Chen, S. Yang, J. Zhang, The chemical composition and bonding structure of B–C–N–H thin films deposited by reactive magnetron sputtering, Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 41 (2009) 865-871.
- D. Mohanadas, M.A.A.M. Abdah, N.H.N. Azman, J. Abdullah, Y. Sulaiman, A promising negative electrode of asymmetric supercapacitor fabricated by incorporating copper-based metal-organic framework and reduced graphene oxide, Int. J. Hydrogen Energy 46 (2021) 35385-35396. [CrossRef]
- S.L. Rebelo, A. Guedes, M.E. Szefczyk, A.M. Pereira, J.P. Araújo, C. Freire, Progress in the raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: unraveling disorder in graphitic materials, PCCP 18 (2016) 12784-12796. [CrossRef]
- L. Lorencova, T. Bertok, E. Dosekova, A. Holazova, D. Paprckova, A. Vikartovska, V. Sasinkova, J. Filip, P. Kasak, M. Jerigova, Electrochemical performance of Ti3C2Tx MXene in aqueous media: towards ultrasensitive H2O2 sensing, Electrochim. Acta 235 (2017) 471-479. [CrossRef]
- Y.-Y. Yang, W.-T. Zhou, W.-L. Song, Q.-Q. Zhu, H.-J. Xiong, Y. Zhang, S. Cheng, P.-F. Luo, Y.-W. Lu, Terminal Groups-Dependent Near-Field Enhancement Effect of Ti3C2Tx Nanosheets, Nanoscale Research Letters 16 (2021) 1-7. [CrossRef]
- S. Khasim, A. Pasha, N. Badi, M. Lakshmi, Y.K. Mishra, High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices, RSC Advances 10 (2020) 10526-10539. [CrossRef]
- B. Genorio, K.L. Harrison, J.G. Connell, G. Dražić, K.R. Zavadil, N.M. Markovic, D. Strmcnik, Tuning the selectivity and activity of electrochemical interfaces with defective graphene oxide and reduced graphene oxide, ACS applied materials & interfaces 11 (2019) 34517-34525. [CrossRef]
- M. Gusain, R. Nagpal, MXene for solar cells, Solar Energy Harvesting, Conversion, and Storage, Elsevier2023, pp. 171-200.
- Y. Zhou, Y. Wang, Y. Wang, X. Li, Humidity-enabled ionic conductive trace carbon dioxide sensing of nitrogen-doped Ti3C2Tx MXene/polyethyleneimine composite films decorated with reduced graphene oxide nanosheets, Anal. Chem. 92 (2020) 16033-16042.
- A. Pazniak, P. Bazhin, N. Shplis, E. Kolesnikov, I. Shchetinin, A. Komissarov, J. Polcak, A. Stolin, D. Kuznetsov, Ti3C2Tx MXene characterization produced from SHS-ground Ti3AlC2, Materials & Design 183 (2019) 108143. [CrossRef]
- Q.T.H. Ta, N.M. Tran, J.-S. Noh, Rice crust-like ZnO/Ti3C2Tx MXene hybrid structures for improved photocatalytic activity, Catalysts 10 (2020) 1140.
- L. Yao, X. Tian, X. Cui, R. Zhao, X. Xiao, Y. Wang, Partially oxidized Ti3C2Tx MXene-sensitive material-based ammonia gas sensor with high-sensing performances for room temperature application, Journal of Materials Science: Materials in Electronics 32 (2021) 27837-27848. [CrossRef]
- X. Ding, Y. Huang, S. Li, N. Zhang, J. Wang, FeNi3 nanoalloy decorated on 3D architecture composite of reduced graphene oxide/molybdenum disulfide giving excellent electromagnetic wave absorption properties, J. Alloys Compd. 689 (2016) 208-217. [CrossRef]
- L. Jin, L. Chai, W. Yang, H. Wang, L. Zhang, Two-dimensional titanium carbides (Ti3C2Tx) functionalized by poly (m-phenylenediamine) for efficient adsorption and reduction of hexavalent chromium, International Journal of Environmental Research and Public Health 17 (2020) 167.
- W. Ma, X. Yao, D. Sun, Simultaneous electrochemical determination of dopamine, epinephrine and uric acid at silver doped poly-l-cysteine film electrode, Asian J. Chem 25 (2013) 6625-6634. [CrossRef]
- D. Mohanadas, N. Tukimin, Y. Sulaiman, Simultaneous electrochemical detection of hydroquinone and catechol using poly (3, 4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide, Synth. Met. 252 (2019) 76-81.
- Y. El Hamdouni, S. El Hajjaji, T. Szabo, L. Trif, I. Felhősi, K. Abbi, N. Labjar, L. Harmouche, A. Shaban, Biomass valorization of walnut shell into biochar as a resource for electrochemical simultaneous detection of heavy metal ions in water and soil samples: Preparation, characterization, and applications, Arabian Journal of Chemistry 15 (2022) 104252. [CrossRef]
- H. Huang, T. Chen, X. Liu, H. Ma, Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials, Anal. Chim. Acta 852 (2014) 45-54. [CrossRef]
- Y.-Z. Tang, K.Y. Gin, M. Aziz, The relationship between pH and heavy metal ion sorption by algal biomass, Adsorption Science & Technology 21 (2003) 525-537. [CrossRef]
- N. Qu, D. Zhu, K.C. Chan, W. Lei, Pulse electrodeposition of nanocrystalline nickel using ultra narrow pulse width and high peak current density, Surf. Coat. Technol. 168 (2003) 123-128. [CrossRef]
- X. Zhu, B. Liu, H. Hou, Z. Huang, K.M. Zeinu, L. Huang, X. Yuan, D. Guo, J. Hu, J. Yang, Alkaline intercalation of Ti3C2 MXene for simultaneous electrochemical detection of Cd (II), Pb (II), Cu (II) and Hg (II), Electrochim. Acta 248 (2017) 46-57.
- X. Lv, F. Pei, S. Feng, Y. Wu, S.-M. Chen, Q. Hao, W. Lei, Facile synthesis of protonated carbon nitride/Ti3C2Tx nanocomposite for simultaneous detection of Pb2+ and Cd2+, J. Electrochem. Soc. 167 (2020) 067509.
- X. Zhang, D. An, Z. Bi, W. Shan, B. Zhu, L. Zhou, L. Yu, H. Zhang, S. Xia, M. Qiu, Ti3C2-MXene@ N-doped carbon heterostructure-based electrochemical sensor for simultaneous detection of heavy metals, J. Electroanal. Chem. 911 (2022) 116239. [CrossRef]
- Y. He, L. Ma, L. Zhou, G. Liu, Y. Jiang, J. Gao, Preparation and application of bismuth/MXene nano-composite as electrochemical sensor for heavy metal ions detection, Nanomaterials 10 (2020) 866. [CrossRef]
| No. | Material | Heavy metal detected |
LOD (nM) | Linear range of detection (μM) | Reference |
|---|---|---|---|---|---|
| 1 | alk-Ti3C2 | Cu2+ Cd2+ |
39.00 82.00 |
0.1-1.4 μM 0.1-1.4 μM |
[45] |
| 2 | H–C3N4/Ti3C2Tx | Cd2+ Pb2+ |
1.00 0.60 |
0.5-1.5 μM 0.5-1.5 μM |
[46] |
| 3 | Ti3C2@N-C | Cd2+ Pb2+ |
2.25 1.10 |
0.1-4 μM 0.05-2 μM |
[47] |
| 4 | BiNPs/Ti3C2Tx | Cd2+ Pb2+ |
12.4 10.8 |
0.08-0.8 μM 0.06-0.6 μM |
[48] |
| 5 | Ti3C2Tx-rGO |
Cd2+ Cu2+ |
0.31 0.18 |
7.5-150 nM 1-150 nM |
This work |
| Stability period | Peak current retention (%) | |
|---|---|---|
| Cd2+ | Cu2+ | |
| 1 Week | 98.19% | 99.81% |
| 2 Week | 98.61% | 99.48% |
| 3 Week | 99.89% | 98.49% |
| 4 Week | 97.86% | 98.01% |
| Sample | Added (nM) | Obtained (nM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Cd2+ | Cu2+ | Cd2+ | Cu2+ | Cd2+ | Cu2+ | |
| 1 | 60 | 60 | 58.4 | 58.9 | 97.3% | 98.2% |
| 2 | 80 | 80 | 78.1 | 79.3 | 97.6% | 99.1% |
| 3 | 100 | 100 | 98.9 | 99.5 | 98.9% | 99.5% |
| Sample | Added (nM) | Obtained (nM) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Cd2+ | Cu2+ | Cd2+ | Cu2+ | Cd2+ | Cu2+ | |
| 1 | 60 | 60 | 58.7 | 57.6 | 97.8% | 96.0% |
| 2 | 80 | 80 | 78.2 | 78.3 | 97.8% | 97.9% |
| 3 | 100 | 100 | 99 | 98.8 | 99.0% | 98.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





