1. Introduction
The field of molecular biology has undergone a paradigm shift with the elucidation of long noncoding RNAs (lncRNAs) [
1]. These enigmatic molecules, defined as RNA transcripts exceeding 200 nucleotides in length that do not encode proteins, have emerged as pivotal regulators in the intricate landscape of gene regulation and cellular function [
2,
3]. The significance of lncRNAs is profound, as they have been implicated in a diverse array of biological processes, from embryogenesis to pathogenesis, challenging the traditional central dogma of molecular biology. LncRNAs constitute a vast and heterogeneous class of transcripts, potentially outnumbering protein-coding genes in the human genome. In contrast to messenger RNAs (mRNAs), which serve as templates for protein synthesis, lncRNAs exert their functions through a multitude of mechanisms independent of translation. This functional versatility has captivated the scientific community and opened novel avenues for elucidating the intricacies of gene regulation and cellular homeostasis. The definition of lncRNAs, while ostensibly straightforward, belies the complexity and heterogeneity of this RNA class. The 200-nucleotide threshold, although somewhat arbitrary, serves to distinguish lncRNAs from other well-characterized short noncoding RNAs such as microRNAs and small interfering RNAs. However, it is crucial to note that length alone does not determine functionality, and some lncRNAs may indeed encode small peptides, blurring the distinction between coding and non-coding transcripts [
4,
5]. The importance of lncRNAs is underscored by their evolutionary conservation and tissue-specific expression patterns. While the primary sequence of many lncRNAs may not be highly conserved across species, their genomic loci, secondary structures, and expression profiles often exhibit significant conservation, suggesting functional relevance [
6,
7]. Moreover, the exquisite regulation of lncRNA expression in distinct cell types and developmental stages indicates their critical roles in maintaining cellular identity and orchestrating complex biological processes [
6].
One of the most intriguing aspects of lncRNA biology is the diverse array of functions these molecules can perform. Unlike proteins, which often possess well-defined structural domains that dictate their functions, lncRNAs exhibit remarkable functional plasticity [
8]. This versatility stems from their ability to form complex secondary and tertiary structures, enabling them to interact with a wide range of biomolecules, including DNA, RNA, and proteins. Despite significant progress in lncRNA research, numerous challenges and unanswered questions persist. The mechanisms by which lncRNAs achieve specificity in their interactions with other biomolecules remain incompletely elucidated. The evolutionary significance of lncRNAs and their contribution to species-specific traits are active areas of investigation [
6]. Furthermore, the potential redundancy and compensatory mechanisms among lncRNAs complicate functional studies and therapeutic interventions [
9].
This comprehensive review aims to synthesize the current state of knowledge regarding lncRNAs and their interactions with other biomolecules. I will explore the diverse mechanisms through which lncRNAs exert their biological functions, from transcriptional regulation [
10] to post-transcriptional control [
11] and cellular organization [
12]. By examining the interplay between lncRNAs and various cellular components, I seek to provide insights into the complex regulatory networks that govern cellular processes. This review will encompass the latest advancements in lncRNA research, including novel techniques for studying lncRNA structure and function [
13], emerging roles in development and disease [
14], and potential applications in diagnostics and therapeutics [
15]. I will critically evaluate the evidence supporting various lncRNA functions and discuss the challenges and controversies in the field. Additionally, I will highlight important areas for future research and the potential impact of lncRNA studies on our understanding of genome organization and gene regulation [
16]. By providing a comprehensive overview of lncRNA biology, this review seeks to serve as a valuable resource for researchers, clinicians, and students engaged in this rapidly evolving field. I aim to inspire new research directions and foster interdisciplinary collaborations that will further elucidate the mysteries of these fascinating RNA molecules and their roles in health and disease.
2. Interactions with LncRNAs
2.1. LncRNA-DNA Interactions
The intricate interplay between lncRNAs and DNA represents a fascinating aspect of molecular biology that has garnered significant attention in recent years. These interactions play crucial roles in various cellular processes, including gene regulation, chromatin organization [
16], and genome stability [
17]. This section will elucidate the complex world of lncRNA-DNA interactions, focusing on three key aspects: triplex structure formation, enhancer-associated lncRNAs, and chromatin structure regulation. Triplex structure formation is a remarkable phenomenon that occurs when lncRNAs interact with double-stranded DNA to form a three-stranded nucleic acid structure. This unique configuration, also known as an RNA-DNA triplex or R-loop, is characterized by the formation of Hoogsteen or reverse Hoogsteen base pairs between the RNA strand and the purine-rich strand of the DNA duplex [
18]. The resulting structure can have profound effects on DNA topology, accessibility, and function [
17].
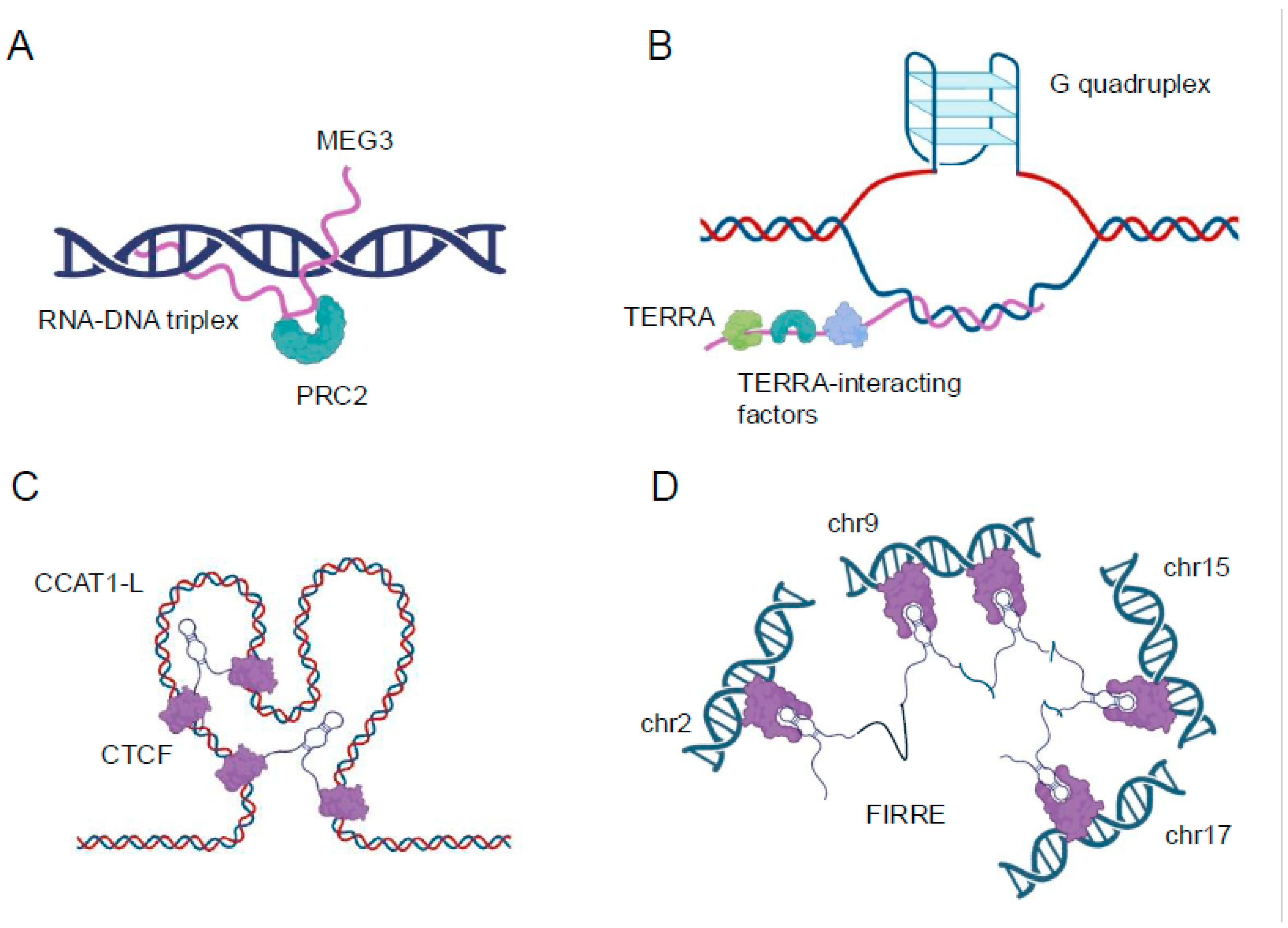
The formation of RNA-DNA triplexes is sequence-specific and depends on the presence of polypurine tracts in the target DNA. These structures are typically formed by lncRNAs that contain complementary sequences to the target DNA region. The stability of triplexes is influenced by various factors, including pH, ionic strength, and the presence of divalent cations such as magnesium. Recent studies have revealed that triplex formation can occur both in cis, where the lncRNA interacts with nearby genomic regions, and in trans, where the lncRNA targets distant genomic loci [
19,
20,
21]. The biological significance of RNA-DNA triplexes is multifaceted. These structures can serve as molecular anchors, guiding lncRNAs to specific genomic locations where they can recruit protein complexes or modulate local chromatin structure [
6]. For instance, the lncRNA MEG3 forms triplexes at the TGF-β pathway genes, recruiting chromatin remodeling complexes to regulate their expression [
22] (
Figure 1A). Triplex formation can also impact DNA replication and repair processes. The presence of R-loops can lead to replication fork stalling and genomic instability if not properly resolved [
23]. Conversely, some lncRNAs may use triplex formation as a mechanism to protect certain genomic regions from DNA damage or to facilitate DNA repair processes [
24]. The lncRNA TERRA, for example, forms triplexes at telomeric regions, potentially contributing to telomere maintenance and genome stability [
25] (
Figure 1B).
Recent technological advancements have greatly enhanced our ability to study RNA-DNA triplexes. Techniques such as Triplex-seq [
26] and DNA-RNA immunoprecipitation sequencing (DRIP-seq) [
27] allow for genome-wide identification of triplex-forming regions and R-loops, respectively. These approaches have revealed that triplex formation is more prevalent than previously thought and occurs at various genomic elements, including promoters, enhancers, and gene bodies. Enhancer-associated lncRNAs represent a subset of lncRNAs that are transcribed from enhancer regions and play critical roles in gene regulation. Enhancers are distal regulatory elements that can activate gene expression over long genomic distances [
28]. The discovery that many enhancers are themselves transcribed to produce lncRNAs has added a new layer of complexity to our understanding of gene regulation [
29]. Enhancer-associated lncRNAs can act as molecular scaffolds, recruiting transcription factors, coactivators, and chromatin modifiers to their sites of action. The lncRNA CCAT1-L, for instance, interacts with the CTCF protein to modulate chromatin looping at the MYC locus, influencing MYC expression in colorectal cancer cells [
30] (
Figure 1C). Similarly, the eRNA NRIP1 facilitates the recruitment of cohesin and mediator complexes to estrogen-responsive enhancers, promoting estrogen-dependent gene activation [
31,
32].
The specificity and functionality of eRNAs are areas of ongoing research. While some eRNAs appear to have sequence-specific functions, others may act more generally through the process of transcription itself. The stability and processing of eRNAs also vary, with some being rapidly degraded while others are more stable and can even be spliced [
33]. LncRNAs play a crucial role in shaping the three-dimensional organization of the genome, influencing everything from local chromatin accessibility to higher-order chromatin structures [
16]. This process is fundamental to gene expression control and cellular identity. At the local level, lncRNAs can recruit chromatin-modifying complexes to specific genomic loci, leading to changes in histone modifications and DNA methylation status. The classic example of this is the Xist lncRNA, which coats the inactive X chromosome in female mammals, recruiting repressive complexes that lead to chromosome-wide silencing [
16,
32]. Similarly, the HOTAIR lncRNA interacts with the PRC2 and LSD1 complexes to induce repressive chromatin states at the HOXD locus and other genomic regions.
LncRNAs also play crucial roles in the formation and maintenance of higher-order chromatin structures, such as topologically associating domains and chromatin loops. The FIRRE lncRNA, for instance, has been implicated in maintaining long-range chromosomal interactions between its site of transcription and other genomic loci, potentially contributing to the organization of nuclear architecture [
6,
32] (
Figure 1D). The advent of high-resolution chromosome conformation capture techniques, such as Hi-C and ChIA-PET, combined with RNA-centric approaches like CHART and RAP, has provided unprecedented insights into the roles of lncRNAs in chromatin organization [
32,
35]. These studies have revealed that many lncRNAs are associated with specific chromatin interactions and can influence the formation of regulatory hubs within the nucleus. The interactions between lncRNAs and DNA represent a complex and dynamic aspect of gene regulation and genome organization. From the formation of triplex structures to the modulation of enhancer activity and the shaping of chromatin architecture, lncRNAs demonstrate remarkable versatility in their ability to influence DNA-based processes. As our understanding of these interactions continues to grow, so too does the potential for harnessing this knowledge for therapeutic interventions and biotechnological applications. Future research in this field promises to uncover even more intricate mechanisms by which lncRNAs contribute to the regulation of genomic function and cellular identity.
2.2. LncRNA-RNA Interactions
The intricate world of RNA-RNA interactions has emerged as a crucial aspect of gene regulation, with lncRNAs playing pivotal roles in modulating various RNA-mediated processes [
36]. This section elucidates the multifaceted interactions between lncRNAs and other RNA species, focusing on three key areas: mRNA stability and translational control [
37], miRNA sponge function [
38], and splicing regulation [
39]. These interactions highlight the remarkable versatility of lncRNAs in fine-tuning gene expression at the post-transcriptional level.
The regulation of mRNA stability and translation by lncRNAs represents a sophisticated mechanism for controlling gene expression. LncRNAs can influence mRNA fate through direct base-pairing interactions or by modulating the activity of RNA-binding proteins that affect mRNA stability and translation efficiency [
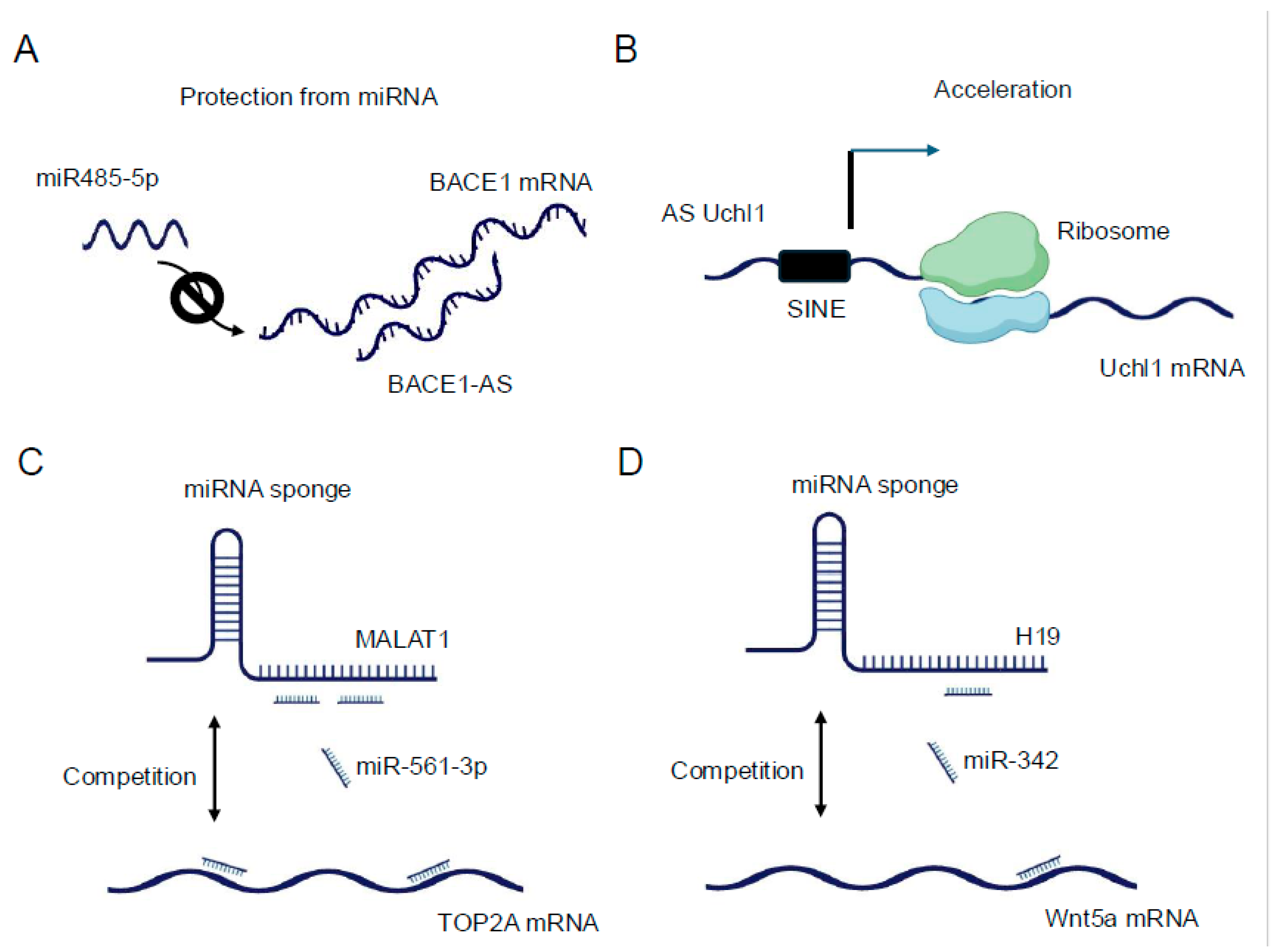
40]. A prominent example is the lncRNA BACE1-AS, which forms an RNA duplex with the BACE1 mRNA, increasing its stability and subsequently enhancing BACE1 protein levels [
41] (
Figure 2A). This interaction has implications in Alzheimer's disease pathogenesis, underscoring the physiological relevance of such lncRNA-mRNA interactions. LncRNAs can also regulate mRNA stability by competing with or facilitating the binding of stabilizing or destabilizing factors to target mRNAs. For instance, the lncRNA NEAT1 has been shown to interact with and sequester the RNA-destabilizing protein KSRP, thereby indirectly stabilizing a subset of mRNAs involved in cancer progression [
42]. Conversely, some lncRNAs can promote mRNA decay by recruiting deadenylation complexes or other decay-promoting factors to specific transcripts [
36].
In terms of translational control, lncRNAs exhibit diverse mechanisms to modulate protein synthesis. Some lncRNAs can directly interact with ribosomes or translation initiation factors, influencing the efficiency of translation. The lncRNA AS Uchl1, for example, enhances the translation of the UCHL1 mRNA by facilitating its association with polysomes [
43] (
Figure 2B). Other lncRNAs may act as scaffolds, bringing together translational regulators and their target mRNAs to fine-tune protein synthesis in response to cellular cues [
36]. Recent advances in high-throughput techniques, such as CLIP-seq [
44] and ribosome profiling [
45], have revealed the extensive involvement of lncRNAs in translational regulation. These studies have uncovered complex networks of lncRNA-mRNA interactions that respond to various cellular stresses and developmental signals, highlighting the dynamic nature of lncRNA-mediated translational control [
37].
The miRNA sponge function of lncRNAs, also known as the competing endogenous RNA (ceRNA) mechanism, represents another fascinating aspect of lncRNA-RNA interactions. In this paradigm, lncRNAs can modulate the availability of miRNAs by acting as molecular decoys, thereby influencing the expression of miRNA target genes [
46]. This mechanism adds an additional layer of complexity to the post-transcriptional regulatory network and has been implicated in various biological processes and disease states [
38]. The concept of ceRNA activity was first prominently described with the discovery of the PTENP1 pseudogene, which acts as a decoy for miRNAs targeting the tumor suppressor PTEN [
47]. Since then, numerous lncRNAs have been identified as miRNA sponges, with some capable of binding multiple miRNAs and thus potentially influencing entire gene expression programs (
Figure 2C). For example, the lncRNA H19 has been shown to sponge let-7 family miRNAs, affecting the expression of genes involved in muscle differentiation and cancer progression [
48,
49,
50] (
Figure 2D). The efficacy of lncRNAs as miRNA sponges depends on various factors, including the abundance of the lncRNA, the number and affinity of miRNA binding sites, and the cellular context. Recent studies have employed advanced bioinformatics approaches and experimental validations to identify bona fide ceRNA interactions. Techniques such as crosslinking, ligation, and sequencing of hybrids (CLASH) [
51] and CLEAR-CLIP [
52] have provided direct evidence for lncRNA-miRNA interactions in living cells. It is important to note that the physiological relevance of the ceRNA mechanism has been a subject of debate, with some studies suggesting that the abundance of most lncRNAs may be insufficient to significantly affect miRNA activity [
50]. However, emerging evidence indicates that ceRNA effects may be particularly relevant in specific cellular compartments or under certain physiological conditions where the local concentrations of lncRNAs and miRNAs are sufficiently high [
53].
The role of lncRNAs in splicing regulation represents yet another critical aspect of their RNA-RNA interactions. Alternative splicing is a key mechanism for generating proteome diversity and regulating gene expression, and lncRNAs have emerged as important modulators of this process [
54]. LncRNAs can influence splicing decisions through various mechanisms, including direct interactions with pre-mRNAs, modulation of splicing factor activity, and alteration of chromatin structure at splice sites [
55]. One well-characterized example of splicing regulation by lncRNAs is the MALAT1 lncRNA. MALAT1 localizes to nuclear speckles, where it interacts with and modulates the activity of several serine/arginine (SR) splicing factors. By influencing the phosphorylation status and localization of these factors, MALAT1 can affect the splicing patterns of numerous pre-mRNAs, particularly those involved in cell cycle regulation and cancer progression [
54]. Some lncRNAs can directly base pair with pre-mRNAs to mask or expose splice sites, thereby influencing splice site selection. The natural antisense transcript (NAT) Zeb2-AS, for instance, masks a 5' splice site in the Zeb2 pre-mRNA, promoting intron retention and enhancing Zeb2 protein production [
56]. This mechanism plays a crucial role in epithelial-mesenchymal transition, a process important in development and cancer metastasis. LncRNAs can also modulate splicing by altering the local chromatin environment around splice sites. The lncRNA NEAT1, in addition to its role in paraspeckle formation, has been shown to influence alternative splicing by modulating the kinetics of RNA polymerase II elongation [
57]. By promoting the formation of paraspeckles, NEAT1 can sequester certain splicing factors, indirectly affecting splicing decisions for a subset of genes.
Recent technological advancements, such as long-read sequencing [
58] and single-cell RNA-seq [
59], have provided unprecedented insights into the complexity of alternative splicing and the roles of lncRNAs in this process. These techniques have revealed extensive isoform diversity and cell-type-specific splicing patterns, many of which are influenced by lncRNAs. The diverse RNA-RNA interactions mediated by lncRNAs underscore their importance as key regulators of gene expression at the post-transcriptional level. From modulating mRNA stability and translation to acting as miRNA sponges and influencing splicing decisions, lncRNAs demonstrate remarkable versatility in fine-tuning RNA-based processes. As our understanding of these interactions continues to grow, so does the potential for developing RNA-based therapeutics and diagnostic tools. Future research in this field promises to uncover even more intricate mechanisms by which lncRNAs contribute to the complex tapestry of gene regulation and cellular function.
2.3. LncRNA-Protein Interactions
LncRNAs have emerged as crucial regulators of gene expression through their diverse interactions with proteins. These interactions are fundamental to understanding the molecular mechanisms by which lncRNAs exert their biological functions. This section explores three key aspects of lncRNA-protein interactions: their association with chromatin-modifying complexes, binding to transcription factors, and role in controlling cellular localization.
The interaction between lncRNAs and chromatin-modifying complexes represents a pivotal mechanism by which these noncoding transcripts influence gene expression at the epigenetic level [
6,
16]. LncRNAs can serve as molecular scaffolds, bringing together various components of chromatin-modifying complexes and guiding them to specific genomic loci. One of the most well-studied examples of this interaction is the association between lncRNAs and the Polycomb Repressive Complex 2 (PRC2) [
16]. PRC2 is responsible for the trimethylation of histone H3 at lysine 27 (H3K27me3), a repressive epigenetic mark. Numerous lncRNAs, such as HOTAIR, have been shown to interact directly with PRC2 components, particularly EZH2, the catalytic subunit of the complex [
16,
60]. These interactions facilitate the recruitment of PRC2 to specific genomic regions, leading to targeted gene silencing. The specificity of lncRNA-guided chromatin modification is often achieved through the formation of RNA-DNA triplex structures or through the recognition of specific DNA sequences by RNA-binding proteins associated with the lncRNA-chromatin modifier complex [
26,
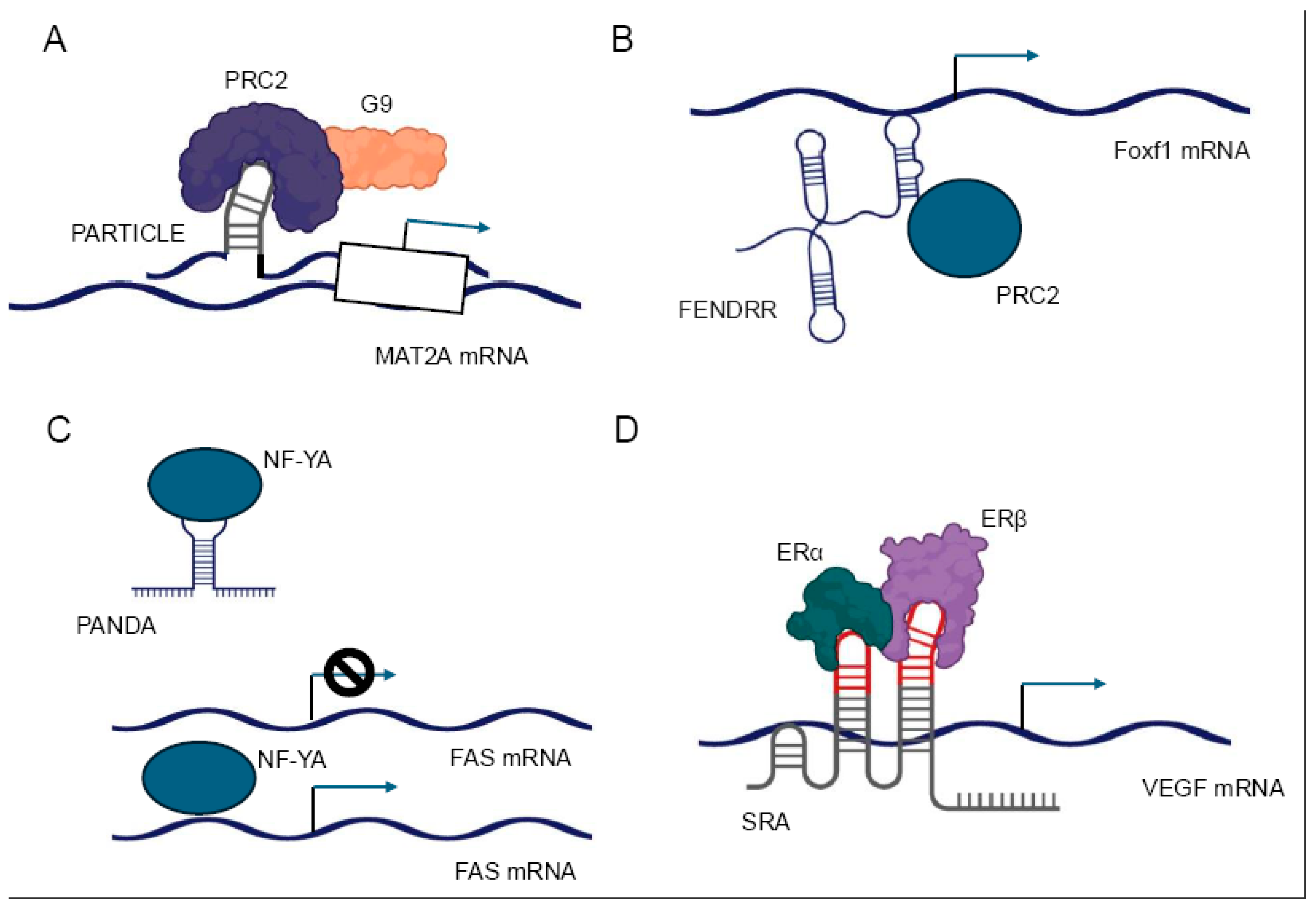
32]. For instance, the lncRNA PARTICLE has been demonstrated to form triplexes at promoter regions of certain genes, influencing their expression in response to environmental stimuli such as low-dose radiation [
17] (
Figure 3A).
Moreover, lncRNAs can interact with multiple chromatin-modifying complexes simultaneously, orchestrating complex patterns of histone modifications [
32]. The lncRNA FENDRR, for example, has been reported to interact with both the MLL complex (associated with activating H3K4 methylation) and PRC2, allowing for fine-tuned regulation of target genes during embryonic development [
61,
62] (
Figure 3B). Recent technological advancements, such as Capture hybridization analysis of RNA targets (CHART) [
63,
64] and chromatin isolation by RNA purification (ChIRP) [
65,
66], have greatly enhanced our ability to map lncRNA-chromatin interactions genome-wide. These techniques have revealed that many lncRNAs associate with specific chromatin regions, often correlating with sites of active chromatin modification.
LncRNAs can modulate the activity of transcription factors through various mechanisms, including direct binding, sequestration, and alteration of post-translational modifications. These interactions can either enhance or inhibit the transcriptional activity of their protein partners, providing a nuanced control over gene expression. One mechanism by which lncRNAs regulate transcription factors is by acting as molecular decoys. In this scenario, lncRNAs can bind to transcription factors and sequester them away from their DNA targets, effectively inhibiting their transcriptional activity. For example, the lncRNA PANDA has been shown to interact with the transcription factor NF-YA, preventing it from activating pro-apoptotic genes following DNA damage [
67] (
Figure 3C).
Conversely, lncRNAs can also function as co-activators or co-repressors of transcription factors. By binding to transcription factors, lncRNAs can modulate their affinity for DNA, alter their interaction with other regulatory proteins, or influence their stability. The lncRNA SRA, for instance, acts as a co-activator for several nuclear receptors, including the estrogen receptor, enhancing their transcriptional activity [
68] (
Figure 3D). Furthermore, lncRNAs can influence the post-translational modifications of transcription factors, thereby affecting their activity or stability. Some lncRNAs have been found to interact with kinases or phosphatases that modify transcription factors, altering their phosphorylation status and, consequently, their transcriptional activity [
69]. The subcellular localization of lncRNAs is a critical determinant of their function, and conversely, lncRNAs can influence the localization of their protein partners, affecting cellular processes and signaling pathways [
70]. LncRNAs exhibit diverse subcellular localizations, including nuclear, cytoplasmic, and even organelle-specific distributions. This localization is often dynamic and can change in response to cellular stimuli or during different stages of development. The mechanisms governing lncRNA localization are complex and involve interactions with various RNA-binding proteins that recognize specific sequence or structural motifs within the lncRNA [
64].
Nuclear retention of lncRNAs is often mediated by specific sequence elements or secondary structures that are recognized by nuclear retention factors. For example, the BORG lncRNA contains a SINE element that promotes its nuclear localization through interaction with the HNRNPK protein [
71,
72]. Conversely, lncRNAs can also influence the subcellular localization of proteins, particularly transcription factors and chromatin-modifying enzymes. By binding to these proteins, lncRNAs can either promote or inhibit their nuclear translocation, thereby regulating their access to genomic targets [
57]. The lncRNA NRON, for instance, interacts with members of the importin-beta superfamily and the nuclear factor of activated T cells (NFAT), regulating the nuclear translocation of NFAT and subsequent transcriptional activity [
73].
LncRNAs also play a role in the formation and maintenance of nuclear bodies, such as paraspeckles and nuclear speckles. The lncRNA NEAT1 is essential for the formation of paraspeckles, nuclear bodies involved in the regulation of gene expression under stress conditions. By interacting with various proteins, NEAT1 acts as a scaffold for the assembly of these structures, demonstrating how lncRNAs can organize higher-order nuclear architecture [
74]. The interactions between lncRNAs and proteins, particularly in the context of chromatin modification, transcription factor regulation, and cellular localization control, represent a complex and dynamic aspect of gene regulation. As our understanding of these interactions continues to grow, so does the potential for developing novel therapeutic strategies targeting lncRNA-protein interactions in various diseases, including cancer and neurodegenerative disorders. Future research in this field promises to uncover even more intricate mechanisms by which lncRNAs contribute to the regulation of cellular function and organismal development.
3. LncRNA in Biomolecular Condensates
The role of lncRNAs in the formation and regulation of biomolecular condensates has emerged as a compelling area of investigation in molecular biology. These condensates, also referred to as membrane-less organelles or phase-separated domains, are dynamic assemblies of proteins and nucleic acids that play pivotal roles in diverse cellular processes. LncRNAs have been identified as crucial mediators in the promotion of phase separation, the formation of nuclear bodies, and the construction of transcriptional control hubs within these condensates.
The capacity of lncRNAs to promote phase separation is rooted in their distinctive molecular characteristics. In contrast to their protein-coding counterparts, lncRNAs exhibit intrinsic structural flexibility and the ability to form complex secondary and tertiary structures [
75]. These attributes enable lncRNAs to interact simultaneously with multiple protein partners, often through low-complexity domains or intrinsically disordered regions. Such multivalent interactions are fundamental to the process of liquid-liquid phase separation (LLPS), which underlies the formation of biomolecular condensates [
76,
77]. Recent studies have revealed that some lncRNAs are involved in the formation of phase-separated nuclear condensates, which can compartmentalize chromatin and influence gene expression. The NEAT1 lncRNA, for example, is essential for the formation of paraspeckles, nuclear bodies that sequester certain RNA-binding proteins and modulate gene expression under stress conditions [
78,
79].
LncRNAs function as scaffolds or nucleators for phase separation, providing a platform for the assembly of proteins and other nucleic acids. Their length and ability to form specific structural motifs enable them to create a network of interactions that can lower the critical concentration required for phase separation. For instance, the lncRNA NEAT1 has also been demonstrated to be essential for the formation of paraspeckles, a type of nuclear body [
80]. NEAT1 acts as an architectural RNA, recruiting specific proteins and creating a scaffold upon which the paraspeckle structure is constructed [
81,
82]. The formation of nuclear bodies represents another critical aspect of lncRNA function in biomolecular condensates. Nuclear bodies are membrane-less structures within the nucleus that concentrate specific sets of proteins and RNAs to perform specialized functions. LncRNAs play pivotal roles in nucleating and maintaining these structures [
83]. In addition to paraspeckles, lncRNAs are involved in the formation of other nuclear bodies such as nuclear stress bodies and histone locus bodies [
84]. Nuclear stress bodies, for example, are formed in response to various cellular stresses and are nucleated by the lncRNA HSATIII [
85]. This lncRNA is rapidly transcribed upon heat shock and serves as a platform for the recruitment of specific splicing factors and other RNA-binding proteins. The formation of these bodies is hypothesized to regulate gene expression and RNA processing during stress conditions [
86,
87]. Recent research identified a novel nuclear structure called the Heat-inducible Noncoding RNA-containing nuclear body (HiNoCo body) [
88]. This structure forms in response to heat shock and contains the lncRNA MALAT1, which relocates from nuclear speckles. HiNoCo bodies are distinct from other known nuclear bodies, form independently of HSF1, and show reversible dynamics. Cells lacking MALAT1 exhibited reduced proliferation after heat shock, suggesting a crucial role for HiNoCo bodies in stress response.
The construction of transcriptional control hubs represents another crucial function of lncRNAs within biomolecular condensates. These hubs are specialized condensates that bring together various regulatory elements, transcription factors, and co-factors to control gene expression. LncRNAs can serve as organizational centers for these hubs, facilitating the assembly of complex regulatory networks [
26]. One mechanism by which lncRNAs construct these hubs is through their ability to interact with both DNA and proteins. Some lncRNAs can form RNA-DNA triplexes, allowing them to target specific genomic loci while simultaneously recruiting regulatory proteins [
17]. This bridging function enables lncRNAs to create three-dimensional regulatory landscapes within the nucleus. Furthermore, lncRNAs can act as molecular sponges or decoys within these transcriptional hubs, modulating the availability of transcription factors or other regulatory proteins. By sequestering these factors, lncRNAs can fine-tune gene expression patterns. The lncRNA NORAD, for instance, sequesters PUMILIO proteins in the cytoplasm, preventing them from repressing their target mRNAs and thereby maintaining genomic stability [
89,
90]. The involvement of lncRNAs in biomolecular condensates represents a paradigm shift in our understanding of gene regulation and cellular organization. Through their roles in promoting phase separation, forming nuclear bodies, and constructing transcriptional control hubs, lncRNAs emerge as master regulators of cellular processes. As research in this field progresses, it is becoming increasingly evident that the unique properties of lncRNAs make them ideally suited to orchestrate the complex, dynamic assemblies that underlie many aspects of cellular function. Future investigations will likely uncover even more intricate mechanisms by which lncRNAs shape the physical and functional landscape of the cell through their interactions within biomolecular condensates.
4. Structural Basis of LncRNA Interactions
The structural basis of lncRNA interactions is a critical aspect in elucidating their diverse functions in cellular processes. LncRNAs exhibit unique structural features that facilitate interactions with various biomolecules, including DNA, RNA, and proteins, thereby influencing gene regulation, chromatin organization, and cellular signaling pathways.
A prominent structural characteristic of lncRNAs is their capacity to form complex secondary and tertiary structures. In contrast to mRNAs, which primarily serve as templates for protein synthesis, lncRNAs rely extensively on their structural properties to exert their biological functions. Recent investigations have revealed that lncRNAs generally possess a higher degree of structure compared to mRNAs yet exhibit less structural complexity than ribosomal RNAs [
91,
92,
93]. This intermediate level of structural organization enables lncRNAs to maintain an equilibrium between flexibility and stability, which is crucial for their diverse functional roles. LncRNAs frequently possess distinct structural motifs that contribute to their overall architecture. These motifs encompass stem-loops, hairpins, bulges, internal loops, and pseudoknots [
94] The combination and arrangement of these structural elements create a unique three-dimensional landscape that facilitates specific interactions with other molecules. For instance, the lncRNA HOTAIR contains multiple stem-loop structures that are essential for its interaction with the PRC2 complex, a key player in epigenetic regulation [
95]. Chemical probing experiments, such as Selective 2'-Hydroxyl Acylation analyzed by Primer Extension (SHAPE), have provided valuable insights into the secondary structure of lncRNAs [
96]. These studies have demonstrated that many lncRNAs form hierarchical structures with distinct subdomains, each containing multiple secondary structure motifs. This hierarchical organization allows lncRNAs to interact with multiple partners simultaneously and perform complex regulatory functions [
97,
98].
The modular structure of lncRNAs is a fundamental aspect of their functionality. Many lncRNAs are composed of discrete functional domains that can operate independently or in concert to achieve specific biological outcomes [
99]. These modules often correspond to distinct secondary structure elements or clusters of motifs that serve as interaction platforms for proteins or other nucleic acids [
95,
100]. The modular nature of lncRNAs confers functional versatility and evolutionary plasticity. Different modules can be acquired, lost, or rearranged over evolutionary time, leading to the diversification of lncRNA functions across species [
7]. This modular architecture also enables lncRNAs to act as molecular scaffolds, bringing together multiple protein complexes to form functional ribonucleoprotein assemblies [
90,
101]. Functional domains in lncRNAs can range from a few nucleotides to hundreds of bases in length. These domains can mediate specific interactions with proteins, such as chromatin modifiers or transcription factors, or facilitate base-pairing with other nucleic acids [
33]. For example, the lncRNA NEAT1 contains distinct domains that are responsible for its role in paraspeckle formation and its interaction with specific RNA-binding proteins [
57].
The significance of repetitive sequences in lncRNA structure and function cannot be overstated. Repetitive elements, once considered "junk DNA," are now recognized as critical components of many lncRNAs. These repeats can be derived from transposable elements, simple sequence repeats, or tandem duplications of functional motifs [
102]. Repetitive sequences in lncRNAs serve multiple purposes. They can act as structural building blocks, creating regular patterns that contribute to the overall architecture of the RNA. For instance, the MALAT1 lncRNA contains a triple helix structure at its 3' end, formed by repetitive sequences, which protects it from degradation and influences its cellular localization [
103]. Moreover, repeats can function as protein-binding platforms, allowing for the recruitment of multiple copies of the same protein or different proteins to a single lncRNA molecule. This property is particularly important for lncRNAs that act as molecular scaffolds or participate in the formation of membrane-less organelles through phase separation [
104,
105]. The evolutionary conservation of repetitive elements in lncRNAs across species suggests their functional importance. While the primary sequence of lncRNAs often evolves rapidly, the presence and arrangement of certain repetitive motifs can be conserved, indicating selective pressure to maintain these structural features [
102].
The structural complexity imparted by repetitive sequences allows lncRNAs to fine-tune their interactions with various cellular components PMID: 36035193. Some repeats can serve as spacers between functional domains, providing the necessary flexibility for lncRNAs to adopt different conformations in response to cellular conditions or binding partners [
106,
107]. The structural basis of lncRNA interactions is a multifaceted aspect of their biology, encompassing unique structural features, modular organization, and the critical role of repetitive sequences. The complex secondary and tertiary structures formed by lncRNAs provide a diverse landscape for molecular interactions, enabling these versatile molecules to perform a wide array of cellular functions. As our understanding of lncRNA structure continues to advance, so does our appreciation for the intricate relationship between their architecture and their biological roles. Future research in this field promises to elucidate even more sophisticated mechanisms by which lncRNAs leverage their structural properties to regulate gene expression, modulate chromatin states, and orchestrate cellular processes.
5. Methods to Study LncRNA Interactions
The investigation of lncRNA interactions has become increasingly crucial in elucidating their diverse roles in cellular processes. This section explores the various methodologies employed to analyze RNA structure, identify interaction partners, and elucidate the functions of lncRNAs.
RNA structure analysis techniques have advanced significantly, providing researchers with powerful tools to investigate the complex architectures of lncRNAs. Chemical and enzymatic probing approaches, such as SHAPE and Dimethyl Sulfate sequencing (DMS-seq), utilize chemical modifications of RNA nucleotides to reveal structural information [
93,
108,
109]. These techniques have been adapted for high-throughput analysis, enabling transcriptome-wide structural studies. A particularly innovative method in RNA structure analysis is in vivo click SHAPE with mutational profiling (icSHAPE-MaP) [
110]. This technique combines the advantages of icSHAPE reagents with mutational profiling in reverse transcription, allowing for the probing of intact RNA structures in living cells. icSHAPE-MaP has been successfully applied to determine the complete structural information for cellular small RNAs and can be combined with RNA immunoprecipitation to study the structural landscape of specific RNA-protein complexes [
111].
Another cutting-edge approach is fragmentation sequencing (Frag-Seq), which employs nuclease P1 to specifically degrade single-stranded nucleic acids. This method, followed by high-throughput sequencing and bioinformatic analysis, provides valuable insights into RNA secondary structures on a genome-wide scale [
112]. The development of long-read sequencing technologies has further enhanced the ability to capture complex RNA structures and isoforms. Computational methods play a crucial role in complementing experimental approaches for RNA structure analysis. Advanced algorithms and machine learning techniques are being developed to predict RNA secondary and tertiary structures based on sequence information and experimental data [
113]. These computational tools are particularly valuable for studying large lncRNAs, where experimental methods may have limitations. Identifying interaction partners of lncRNAs is essential for understanding their molecular functions. A variety of techniques have been developed to study lncRNA-protein, lncRNA-DNA, and lncRNA-RNA interactions. For lncRNA-protein interactions, methods such as RNA immunoprecipitation (RIP) followed by sequencing (RIP-seq) have been widely used [
114,
115]. These techniques allow for the identification of proteins associated with specific lncRNAs in vivo.
Cross-linking and immunoprecipitation (CLIP) and its variants such as HITS-CLIP [
44] and PAR-CLIP [
116] provide higher resolution by identifying the precise binding sites of proteins on lncRNAs. These techniques involve UV crosslinking to stabilize RNA-protein interactions, followed by immunoprecipitation and high-throughput sequencing. Moreover, RNA And DNA interacting complexes ligated, and sequence (RADICL-seq) involves cross-linking RNA, proteins, and DNA in nuclei using formaldehyde, then linking RNA and DNA via adapters. After removing proteins, RNA-DNA complexes are sequenced to map interactions genome-wide, revealing both known and novel RNA-chromatin structures [
117]. RADIP extends RADICL-seq by incorporating immunoprecipitation to specifically enrich RNA-DNA complexes associated with target proteins. This involves cross-linking, adapter-mediated ligation, and antibody-based purification, followed by sequencing to comprehensively map protein-mediated DNA-RNA interactions genome-wide [
118].
For studying lncRNA-DNA interactions, techniques such as CHART and ChIRP have been developed [
65,
119] (
Table 1). These methods use biotinylated oligonucleotides complementary to the lncRNA of interest to pull down associated chromatin, allowing for the identification of genomic binding sites. To investigate lncRNA-RNA interactions, methods like CLASH and psoralen analysis of RNA interactions and structures (PARIS) have been employed [
51,
120] (
Table 2). These techniques can reveal direct base-pairing interactions between lncRNAs and other RNA molecules, providing insights into potential regulatory mechanisms. Recent advancements in proximity ligation-based methods, such as mapping RNA interactome in vivo (MARIO), have enabled the study of RNA-RNA interactions on a genome-wide scale [
120]. These techniques can capture both direct and indirect interactions, offering a comprehensive view of the RNA interactome.
Functional analysis approaches for lncRNAs have become increasingly sophisticated, allowing researchers to elucidate their roles in various biological processes. The CRISPR-Cas9 system and its derivatives, such as CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa), have been adapted for lncRNA studies, enabling targeted repression or activation of lncRNA expression from their endogenous loci [
121,
122]. High-throughput CRISPR screens have been developed specifically for lncRNAs, allowing for the simultanfeous interrogation of hundreds or thousands of lncRNAs [
123,
124]. These screens can be designed to identify lncRNAs involved in specific cellular processes, such as proliferation, drug resistance, or differentiation [
122]. Antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) continue to be valuable tools for lncRNA knockdown studies, especially for lncRNAs that are difficult to target with CRISPR-based approaches [
125]. LNA GapmeRs, a type of ASO, have shown promise in targeting nuclear lncRNAs, which are often resistant to siRNA-mediated knockdown. For gain-of-function studies, overexpression of lncRNAs using traditional plasmid-based systems or more advanced methods like CRISPRa can reveal phenotypes associated with increased lncRNA levels [
126]. The choice between plasmid-based overexpression and CRISPRa depends on the specific research question, as each method has its advantages and limitations. Emerging technologies like RNA-guided RNA-targeting CRISPR effectors (e.g., Cas13) are opening new avenues for lncRNA functional studies. These systems offer the potential for highly specific RNA targeting in both the nucleus and cytoplasm, although their utility is still being fully explored.
The field of lncRNA research is rapidly evolving, with new methods continually being developed to study their structure, interactions, and functions. The integration of multiple approaches, including high-throughput sequencing, advanced imaging techniques, and computational analysis, is crucial for gaining a comprehensive understanding of lncRNA biology. As these methods continue to improve and new technologies emerge, our knowledge of lncRNA functions and their roles in health and disease will undoubtedly expand, potentially leading to new therapeutic strategies and diagnostic tools.
6. Biological Significance and Disease Relevance
In the development and differentiation, lncRNAs exhibit intricate and stage-specific expression patterns that are essential for proper organismal development. During embryogenesis, numerous lncRNAs are expressed in a highly regulated manner, contributing to the complex gene regulatory networks that guide cell fate decisions and tissue patterning. For instance, the lncRNA Braveheart has been demonstrated to play a critical role in cardiovascular lineage commitment [
127]. This lncRNA interacts with the PRC2 complex to epigenetically regulate key cardiac genes, illustrating how lncRNAs can influence cell fate through chromatin modification. In the nervous system, lncRNAs such as Evf2 and Pnky are involved in neuronal differentiation and the maintenance of neural stem cell populations [
128,
129]. Evf2 functions as a co-activator for the Dlx transcription factors, which are crucial for GABAergic interneuron development, while Pnky regulates neural stem cell differentiation through modulation of alternative splicing. These examples highlight the diverse mechanisms by which lncRNAs can influence developmental processes.
LncRNAs also play significant roles in maintaining stem cell pluripotency and directing lineage-specific differentiation. The lncRNA RMST, for example, is essential for neuronal differentiation of human embryonic stem cells [
130]. It interacts with SOX2, a key pluripotency factor, to co-regulate neurogenic genes [
131]. In the hematopoietic system, lncRNAs such as HOTAIRM1 are involved in myeloid differentiation, demonstrating the importance of lncRNAs in blood cell development [
132]. The involvement of lncRNAs in disease processes, particularly in cancer, has been a subject of intense research. Numerous lncRNAs have been implicated in various aspects of cancer biology, including tumor initiation, progression, metastasis, and drug resistance. The lncRNA MALAT1, for instance, is overexpressed in multiple cancer types and promotes tumor growth and metastasis by regulating gene expression and alternative splicing [
133]. Another well-studied oncogenic lncRNA, HOTAIR, is associated with increased metastasis and poor prognosis in several cancers [
134]. It functions by recruiting chromatin-modifying complexes to alter the epigenetic landscape of cancer cells. Hypoxia-induced HIF-1α also upregulates lncRNA STEAP3-AS1, which competitively interacts with YTHDF2 to enhance STEAP3 mRNA stability and protein expression. This STEAP3-AS1/STEAP3/Wnt/β-catenin axis promotes colorectal cancer (CRC) progression, offering potential diagnostic biomarkers and therapeutic targets for CRC treatment [
135]. Conversely, some lncRNAs act as tumor suppressors. The lncRNA MEG3, for example, inhibits tumor growth by activating p53 and repressing the MDM2 oncogene [
136]. The loss of MEG3 expression has been observed in various cancer types, underscoring its importance in maintaining cellular homeostasis. These examples illustrate how lncRNAs can function as both oncogenes and tumor suppressors, depending on their specific molecular interactions and cellular context.
Beyond cancer, lncRNAs have been implicated in a wide range of other diseases, including neurodegenerative diseases. In neurodegenerative disorders such as Alzheimer's disease, the lncRNA BACE1-AS regulate the expression of genes involved in amyloid-β production, potentially contributing to disease pathogenesis [
41]. The involvement of lncRNAs in various diseases has naturally led to interest in their potential as therapeutic targets. The unique properties of lncRNAs, including their tissue-specific expression and diverse regulatory mechanisms, make them attractive candidates for targeted therapies. Several approaches are being explored to modulate lncRNA function for therapeutic purposes [
137]. One promising strategy is the use of ASOs to target specific lncRNAs. ASOs can be designed to bind to lncRNAs and induce their degradation or alter their secondary structure, thereby modulating their function. This approach has shown promise in preclinical models for targeting oncogenic lncRNAs like MALAT1 [
138]. Another method involves the use of siRNAs to knockdown lncRNA expression. While this approach has been successful in vitro, delivery challenges remain for in vivo applications. CRISPR-Cas9 technology is also being explored for lncRNA-based therapies. This approach allows for precise genomic editing to modulate lncRNA expression or function. Small molecule inhibitors that target specific lncRNA-protein interactions are another avenue being explored for therapeutic intervention. These molecules can disrupt the binding of lncRNAs to their protein partners, potentially altering disease-associated pathways [
139]. However, identifying small molecules that specifically target RNA-protein interactions remains challenging and is an active area of research (
Table 3).
LncRNAs also hold promise as diagnostic and prognostic biomarkers [
140,
141]. Their tissue-specific expression patterns and presence in bodily fluids make them attractive candidates for non-invasive diagnostics. For instance, the lncRNA PCA3 is already used clinically as a biomarker for prostate cancer detection [
142]. Similarly, circulating lncRNAs in blood or other biofluids are being investigated as potential biomarkers for various cancers and other diseases [
143]. The biological significance of lncRNAs in development, differentiation, and disease processes is becoming increasingly evident. Their diverse roles in regulating gene expression, chromatin structure, and cellular signaling pathways position them as important players in both normal physiology and pathological conditions. As our understanding of lncRNA biology continues to expand, so does the potential for developing lncRNA-based therapies and diagnostic tools [
144]. However, significant challenges remain, including improving our understanding of lncRNA structure-function relationships, developing effective delivery methods for RNA-based therapeutics, and elucidating the complex regulatory networks in which lncRNAs participate. Overcoming these challenges will be crucial for translating our knowledge of lncRNA biology into effective clinical applications.
7. Conclusion and Future Perspectives
Recent years have seen remarkable advancements in the field of lncRNA research, revolutionizing our understanding of gene regulation and cellular function. This comprehensive review has explored the multifaceted nature of lncRNAs, from their structural characteristics to their diverse roles in biological processes and disease pathogenesis. As I conclude, it is essential to synthesize key findings, address unresolved questions, and outline future research directions in this rapidly evolving field. One of the most significant insights from lncRNA studies is their versatility in regulating gene expression. LncRNAs function at multiple levels, including transcriptional regulation, post-transcriptional processing, and epigenetic modification. Their ability to interact with DNA, RNA, and proteins enables them to serve as molecular scaffolds, guides, and decoys, orchestrating complex regulatory networks within cells. The discovery of lncRNAs involved in phase separation and biomolecular condensate formation has expanded our understanding of their role in cellular organization and function. Recent research has focused on the structural basis of lncRNA interactions. Advanced RNA structure analysis techniques have revealed that lncRNAs possess complex secondary and tertiary structures crucial for their function. The modular nature of many lncRNAs, with distinct functional domains and repetitive elements, provides a flexible platform for diverse molecular interactions. This structural complexity underlies their ability to engage in specific interactions with various biomolecules and respond dynamically to cellular conditions. In development and differentiation, lncRNAs are critical regulators of cell fate decisions and tissue-specific gene expression programs. From embryonic stem cell maintenance to lineage-specific differentiation, lncRNAs play essential roles in fine-tuning gene expression networks. Their involvement in processes such as X chromosome inactivation and genomic imprinting highlights their importance in fundamental biological phenomena. The implication of lncRNAs in various diseases, particularly cancer, has driven the field forward. Numerous lncRNAs have been identified as oncogenes or tumor suppressors, influencing key hallmarks of cancer such as proliferation, metastasis, and drug resistance. Beyond cancer, lncRNAs have been implicated in cardiovascular diseases, neurodegenerative disorders, and autoimmune conditions, underscoring their broad relevance to human health. Despite these advances, several challenges remain. One major challenge is the functional characterization of the vast number of lncRNAs identified through high-throughput sequencing. Developing efficient strategies to systematically investigate their functions is a pressing need. Another challenge lies in understanding how lncRNAs achieve specificity in their interactions with other biomolecules. Elucidating the structural and sequence determinants governing these interactions will be crucial for predicting lncRNA functions and designing targeted therapies.
The evolutionary conservation of lncRNAs remains a topic of debate. While some lncRNAs show high sequence conservation across species, many others exhibit poor sequence conservation but maintain structural or functional conservation. Developing better computational and experimental approaches to assess lncRNA conservation will be important for understanding their biological roles and translating findings from model organisms to humans. In therapeutics, significant hurdles remain in translating our knowledge of lncRNAs into effective clinical interventions. Challenges include developing efficient delivery methods for RNA-based therapeutics, ensuring specificity of action, and minimizing off-target effects. A deeper understanding of their tissue-specific roles is necessary before they can be safely targeted in a therapeutic context. Looking to the future, several promising research directions are emerging. One area of interest is the further exploration of lncRNA roles in phase separation and biomolecular condensate formation. Understanding how lncRNAs contribute to the organization of these membrane-less organelles could provide new insights into both normal physiology and disease processes. The integration of lncRNA research with emerging fields like single-cell genomics and spatial transcriptomics holds great promise. These technologies will allow for a nuanced understanding of lncRNA expression and function at the individual cell level and within complex tissues. Such approaches could reveal cell type-specific functions of lncRNAs and their roles in maintaining cellular heterogeneity within tissues. Advances in structural biology techniques, including cryo-electron microscopy and nuclear magnetic resonance spectroscopy, will provide unprecedented insights into lncRNA structure and interactions. Combining these structural data with functional studies will be crucial for understanding the molecular mechanisms underlying lncRNA function and designing targeted therapeutic interventions. The development of sophisticated CRISPR-based tools for lncRNA manipulation will enable more precise functional studies. Techniques allowing for the modulation of lncRNA expression or structure without altering the underlying DNA sequence will be particularly valuable for dissecting lncRNA functions in their native genomic context.
Finally, translating lncRNA research into clinical applications remains a key goal. This includes not only developing lncRNA-based therapeutics but also utilizing lncRNAs as diagnostic and prognostic biomarkers. The tissue-specific expression patterns of many lncRNAs make them attractive candidates for non-invasive diagnostics, and further research could lead to novel clinical tools. The study of lncRNAs has dramatically expanded our understanding of gene regulation and cellular function. As we continue to unravel their complex roles in development, disease, and cellular organization, new opportunities for therapeutic intervention and diagnostic applications are likely to emerge. The future of lncRNA research promises to be both challenging and exciting, with the potential to fundamentally reshape our understanding of biology and medicine.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments). Where GenAI has been used for purposes such as generating text, data, or graphics, or for study design, data collection, analysis, or interpretation of data, please add “During the preparation of this manuscript/study, the author(s) used [tool name, version information] for the purposes of [description of use]. The authors have reviewed and edited the output and take full responsibility for the content of this publication.”
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mattick, J. S.; Amaral, P. P.; Carninci, P.; Carpenter, S.; Chang, H. Y.; Chen, L. L.; Chen, R.; Dean, C.; Dinger, M. E.; Fitzgerald, K. A.; et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol 2023, 24(6), 430-447. [CrossRef]
- Obuse, C.; Hirose, T. Functional domains of nuclear long noncoding RNAs: Insights into gene regulation and intracellular architecture. Curr Opin Cell Biol 2023, 85, 102250. [CrossRef]
- Onoguchi-Mizutani, R.; Akimitsu, N. Long noncoding RNA and phase separation in cellular stress response. J Biochem 2022, 171(3), 269-276. [CrossRef]
- Agarwal, S.; Macfarlan, T. S.; Sartor, M. A.; Iwase, S. Sequencing of first-strand cDNA library reveals full-length transcriptomes. Nat Commun 2015, 6, 6002. [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K. I.; Clohessy, J. G.; Pandolfi, P. P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017, 541(7636), 228-232. [CrossRef]
- Hacisuleyman, E.; Goff, L. A.; Trapnell, C.; Williams, A.; Henao-Mejia, J.; Sun, L.; McClanahan, P.; Hendrickson, D. G.; Sauvageau, M.; Kelley, D. R.; et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol 2014, 21(2), 198-206. [CrossRef]
- Pauli, A.; Valen, E.; Lin, M. F.; Garber, M.; Vastenhouw, N. L.; Levin, J. Z.; Fan, L.; Sandelin, A.; Rinn, J. L.; Regev, A.; et al. Systematic identification of long noncoding RNAs expressed during zebrafish embryogenesis. Genome Res 2012, 22(3), 577-591. [CrossRef]
- Khalil, A. M.; Guttman, M.; Huarte, M.; Garber, M.; Raj, A.; Morales, D. R.; Thomas, K.; Presser, A.; Bernstein, B. E.; van Oudenaarden, A.; et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 2009, 106(28), 11667-11672. [CrossRef]
- Calo, E.; Flynn, R. A.; Martin, L.; Spitale, R. C.; Chang, H. Y.; Wysocka, J. RNA helicase DDX21 coordinates transcription and ribosomal RNA processing. Nature 2015, 518(7538), 249-253. [CrossRef]
- McLane, L. M.; Corbett, A. H. Nuclear localization signals and human disease. IUBMB Life 2009, 61(7), 697-706. [CrossRef]
- Goodarzi, H.; Najafabadi, H. S.; Oikonomou, P.; Greco, T. M.; Fish, L.; Salavati, R.; Cristea, I. M.; Tavazoie, S. Systematic discovery of structural elements governing stability of mammalian messenger RNAs. Nature 2012, 485(7397), 264-268. [CrossRef]
- Gnirke, A.; Melnikov, A.; Maguire, J.; Rogov, P.; LeProust, E. M.; Brockman, W.; Fennell, T.; Giannoukos, G.; Fisher, S.; Russ, C.; et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol 2009, 27(2), 182-189. [CrossRef]
- Treutlein, B.; Lee, Q. Y.; Camp, J. G.; Mall, M.; Koh, W.; Shariati, S. A.; Sim, S.; Neff, N. F.; Skotheim, J. M.; Wernig, M.; et al. Dissecting direct reprogramming from fibroblast to neuron using single-cell RNA-seq. Nature 2016, 534(7607), 391-395. [CrossRef]
- Ernst, T.; Chase, A. J.; Score, J.; Hidalgo-Curtis, C. E.; Bryant, C.; Jones, A. V.; Waghorn, K.; Zoi, K.; Ross, F. M.; Reiter, A.; et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 2010, 42(8), 722-726. [CrossRef]
- González-Gómez, P. L.; Razeto-Barry, P.; Araya-Salas, M.; Estades, C. F. Does Environmental Heterogeneity Promote Cognitive Abilities? Integr Comp Biol 2015, 55(3), 432-443. [CrossRef]
- Engreitz, J. M.; Ollikainen, N.; Guttman, M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol 2016, 17(12), 756-770. [CrossRef]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P. K.; Del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 2016, 165(3), 566-579. [CrossRef]
- Buske, F. A.; Bauer, D. C.; Mattick, J. S.; Bailey, T. L. Triplexator: detecting nucleic acid triple helices in genomic and transcriptomic data. Genome Res 2012, 22(7), 1372-1381. [CrossRef]
- Ogunleye, A. J.; Romanova, E.; Medvedeva, Y. A. Genome-wide regulation of CpG methylation by ecCEBPα in acute myeloid leukemia. F1000Res 2021, 10, 204. [CrossRef]
- O'Leary, V. B.; Ovsepian, S. V.; Smida, J.; Atkinson, M. J. PARTICLE - The RNA podium for genomic silencers. J Cell Physiol 2019, 234(11), 19464-19470. [CrossRef]
- Sentürk Cetin, N.; Kuo, C. C.; Ribarska, T.; Li, R.; Costa, I. G.; Grummt, I. Isolation and genome-wide characterization of cellular DNA:RNA triplex structures. Nucleic Acids Res 2019, 47(5), 2306-2321. [CrossRef]
- Mondal, T.; Subhash, S.; Vaid, R.; Enroth, S.; Uday, S.; Reinius, B.; Mitra, S.; Mohammed, A.; James, A. R.; Hoberg, E.; et al. MEG3 long noncoding RNA regulates the TGF-β pathway genes through formation of RNA-DNA triplex structures. Nat Commun 2015, 6, 7743. [CrossRef]
- Aguilera, A.; García-Muse, T. R loops: from transcription byproducts to threats to genome stability. Mol Cell 2012, 46(2), 115-124. [CrossRef]
- Boque-Sastre, R.; Soler, M.; Oliveira-Mateos, C.; Portela, A.; Moutinho, C.; Sayols, S.; Villanueva, A.; Esteller, M.; Guil, S. Head-to-head antisense transcription and R-loop formation promotes transcriptional activation. Proc Natl Acad Sci U S A 2015, 112(18), 5785-5790. [CrossRef]
- Chu, H. P.; Cifuentes-Rojas, C.; Kesner, B.; Aeby, E.; Lee, H. G.; Wei, C.; Oh, H. J.; Boukhali, M.; Haas, W.; Lee, J. T. TERRA RNA Antagonizes ATRX and Protects Telomeres. Cell 2017, 170(1), 86-101.e16. [CrossRef]
- Kuo, C. C.; Hänzelmann, S.; Sentürk Cetin, N.; Frank, S.; Zajzon, B.; Derks, J. P.; Akhade, V. S.; Ahuja, G.; Kanduri, C.; Grummt, I.; et al. Detection of RNA-DNA binding sites in long noncoding RNAs. Nucleic Acids Res 2019, 47(6), e32. [CrossRef]
- Wahba, L.; Costantino, L.; Tan, F. J.; Zimmer, A.; Koshland, D. S1-DRIP-seq identifies high expression and polyA tracts as major contributors to R-loop formation. Genes Dev 2016, 30(11), 1327-1338. [CrossRef]
- Hou, Y.; Zhang, R.; Sun, X. Enhancer LncRNAs Influence Chromatin Interactions in Different Ways. Front Genet 2019, 10, 936. [CrossRef]
- Kim, T. K.; Hemberg, M.; Gray, J. M.; Costa, A. M.; Bear, D. M.; Wu, J.; Harmin, D. A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465(7295), 182-187. [CrossRef]
- Xiang, J. F.; Yin, Q. F.; Chen, T.; Zhang, Y.; Zhang, X. O.; Wu, Z.; Zhang, S.; Wang, H. B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res 2014, 24(5), 513-531. [CrossRef]
- Li, W.; Notani, D.; Ma, Q.; Tanasa, B.; Nunez, E.; Chen, A. Y.; Merkurjev, D.; Zhang, J.; Ohgi, K.; Song, X.; et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 2013, 498(7455), 516-520. [CrossRef]
- Statello, L.; Guo, C. J.; Chen, L. L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol 2021, 22(2), 96-118. [CrossRef]
- Lam, M. T.; Cho, H.; Lesch, H. P.; Gosselin, D.; Heinz, S.; Tanaka-Oishi, Y.; Benner, C.; Kaikkonen, M. U.; Kim, A. S.; Kosaka, M.; et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 2013, 498(7455), 511-515. [CrossRef]
- Han, P.; Chang, C. P. Long non-coding RNA and chromatin remodeling. RNA Biol 2015, 12(10), 1094-1098. [CrossRef]
- Zhao, T.; Cai, M.; Liu, M.; Su, G.; An, D.; Moon, B.; Lyu, G.; Si, Y.; Chen, L.; Lu, W. lncRNA 5430416N02Rik Promotes the Proliferation of Mouse Embryonic Stem Cells by Activating Mid1 Expression through 3D Chromatin Architecture. Stem Cell Reports 2020, 14(3), 493-505. [CrossRef]
- Yoon, J. H.; Abdelmohsen, K.; Gorospe, M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol 2013, 425(19), 3723-3730. [CrossRef]
- Geisler, S.; Coller, J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 2013, 14(11), 699-712. [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P. P. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014, 505(7483), 344-352. [CrossRef]
- Romero-Barrios, N.; Legascue, M. F.; Benhamed, M.; Ariel, F.; Crespi, M. Splicing regulation by long noncoding RNAs. Nucleic Acids Res 2018, 46(5), 2169-2184. [CrossRef]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Noncoding RNA 2021, 7(1), 3. [CrossRef]
- Faghihi, M. A.; Modarresi, F.; Khalil, A. M.; Wood, D. E.; Sahagan, B. G.; Morgan, T. E.; Finch, C. E.; St Laurent, G. 3rd.; Kenny, P. J.; Wahlestedt, C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 2008, 14(7), 723-730. [CrossRef]
- Yamazaki, T.; Souquere, S.; Chujo, T.; Kobelke, S.; Chong, Y. S.; Fox, A. H.; Bond, C. S.; Nakagawa, S.; Pierron, G.; Hirose, T. Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly through Phase Separation. Mol Cell 2018, 70(6), 1038-1053.e7. [CrossRef]
- Carrieri, C.; Cimatti, L.; Biagioli, M.; Beugnet, A.; Zucchelli, S.; Fedele, S.; Pesce, E.; Ferrer, I.; Collavin, L.; Santoro, C.; et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 2012, 491(7424), 454-457. [CrossRef]
- Licatalosi, D. D.; Mele, A.; Fak, J. J.; Ule, J.; Kayikci, M.; Chi, S. W.; Clark, T. A.; Schweitzer, A. C.; Blume, J. E.; Wang, X.; et al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 2008, 456(7221), 464-469. [CrossRef]
- Ingolia, N. T.; Ghaemmaghami, S.; Newman, J. R.; Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009, 324(5924), 218-223. [CrossRef]
- Li, R.; Fang, L.; Pu, Q.; Bu, H.; Zhu, P.; Chen, Z.; Yu, M.; Li, X.; Weiland, T.; Bansal, A.; et al. MEG3-4 is a miRNA decoy that regulates IL-1β abundance to initiate and then limit inflammation to prevent sepsis during lung infection. Sci Signal 2018, 11(536), eaao2387. [CrossRef]
- Zhang, R.; Guo, Y.; Ma, Z.; Ma, G.; Xue, Q.; Li, F.; Liu, L. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget 2017, 8(16), 26079-26089. [CrossRef]
- Kallen, A. N.; Zhou, X. B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J. S.; Zhang, H.; Min, W.; et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell 2013, 52(1), 101-112. [CrossRef]
- Peng, F.; Li, T. T.; Wang, K. L.; Xiao, G. Q.; Wang, J. H.; Zhao, H. D.; Kang, Z. J.; Fan, W. J.; Zhu, L. L.; Li, M.; et al. H19/let-7/LIN28 reciprocal negative regulatory circuit promotes breast cancer stem cell maintenance. Cell Death Dis 2017, 8(1), e2569. [CrossRef]
- Denzler, R.; Agarwal, V.; Stefano, J.; Bartel, D. P.; Stoffel, M. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol Cell 2014, 54(5), 766–776. [CrossRef]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153(3), 654–665. [CrossRef]
- Moore, M. J.; Scheel, T. K.; Luna, J. M.; Park, C. Y.; Fak, J. J.; Nishiuchi, E.; Rice, C. M.; Darnell, R. B. miRNA-target chimeras reveal miRNA 3'-end pairing as a major determinant of Argonaute target specificity. Nat Commun 2015, 6, 8864. [CrossRef]
- Bosson, A. D.; Zamudio, J. R.; Sharp, P. A. Endogenous miRNA and target concentrations determine susceptibility to potential ceRNA competition. Mol Cell 2014, 56(3), 347–359. [CrossRef]
- Tripathi, V.; Ellis, J. D.; Shen, Z.; Song, D. Y.; Pan, Q.; Watt, A. T.; Freier, S. M.; Bennett, C. F.; Sharma, A.; Bubulya, P. A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010, 39(6), 925–938. [CrossRef]
- Ouyang, J.; Zhong, Y.; Zhang, Y.; Yang, L.; Wu, P.; Hou, X.; Xiong, F.; Li, X.; Zhang, S.; Gong, Z.; et al. Long non-coding RNAs are involved in alternative splicing and promote cancer progression. Br J Cancer 2022, 126(8), 1113–1124. [CrossRef]
- Beltran, M.; Puig, I.; Peña, C.; García, J. M.; Alvarez, A. B.; Peña, R.; Bonilla, F.; de Herreros, A. G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev 2008, 22(6), 756–769. [CrossRef]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Bénard, M.; Fox, A. H.; et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol Biol Cell 2014, 25(1), 169–183. [CrossRef]
- Tilgner, H.; Jahanbani, F.; Blauwkamp, T.; Moshrefi, A.; Jaeger, E.; Chen, F.; Harel, I.; Bustamante, C. D.; Rasmussen, M.; Snyder, M. P. Comprehensive transcriptome analysis using synthetic long-read sequencing reveals molecular co-association of distant splicing events. Nat Biotechnol 2015, 33(7), 736–742. [CrossRef]
- Shalek, A. K.; Satija, R.; Shuga, J.; Trombetta, J. J.; Gennert, D.; Lu, D.; Chen, P.; Gertner, R. S.; Gaublomme, J. T.; Yosef, N.; S et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 2014, 510(7505), 363–369. [CrossRef]
- Rinn, J. L.; Kertesz, M.; Wang, J. K.; Squazzo, S. L.; Xu, X.; Brugmann, S. A.; Goodnough, L. H.; Helms, J. A.; Farnham, P. J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129(7), 1311–1323. [CrossRef]
- Grote, P.; Wittler, L.; Hendrix, D.; Koch, F.; Währisch, S.; Beisaw, A.; Macura, K.; Bläss, G.; Kellis, M.; Werber, M.; et al. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 2013, 24(2), 206–214. [CrossRef]
- Grote, P.; Herrmann, B. G. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol 2013, 10(10), 1579–1585. [CrossRef]
- Simon, M. D.; Wang, C. I.; Kharchenko, P. V.; West, J. A.; Chapman, B. A.; Alekseyenko, A. A.; Borowsky, M. L.; Kuroda, M. I.; Kingston, R. E. The genomic binding sites of a noncoding RNA. Proc Natl Acad Sci U S A 2011, 108(51), 20497–20502. [CrossRef]
- Engreitz, J. M.; Pandya-Jones, A.; McDonel, P.; Shishkin, A.; Sirokman, K.; Surka, C.; Kadri, S.; Xing, J.; Goren, A.; Lander, E. S.; et al. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science 2013, 341(6147), 1237973. [CrossRef]
- Chu, C.; Qu, K.; Zhong, F. L.; Artandi, S. E.; Chang, H. Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell 2011, 44(4), 667–678. [CrossRef]
- Quinn, J. J.; Ilik, I. A.; Qu, K.; Georgiev, P.; Chu, C.; Akhtar, A.; Chang, H. Y. Revealing long noncoding RNA architecture and functions using domain-specific chromatin isolation by RNA purification. Nat Biotechnol 2014, 32(9), 933–940. [CrossRef]
- Hung, T.; Wang, Y.; Lin, M. F.; Koegel, A. K.; Kotake, Y.; Grant, G. D.; Horlings, H. M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 2011, 43(7), 621–629. [CrossRef]
- Lanz, R. B.; McKenna, N. J.; Onate, S. A.; Albrecht, U.; Wong, J.; Tsai, S. Y.; Tsai, M. J.; O'Malley, B. W. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 1999, 97(1), 17–27. [CrossRef]
- Yang, L.; Lin, C.; Liu, W.; Zhang, J.; Ohgi, K. A.; Grinstein, J. D.; Dorrestein, P. C.; Rosenfeld, M. G. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 2011, 147(4), 773–788. [CrossRef]
- Willingham, A. T.; Orth, A. P.; Batalov, S.; Peters, E. C.; Wen, B. G.; Aza-Blanc, P.; Hogenesch, J. B.; Schultz, P. G. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 2005, 309(5740), 1570–1573. [CrossRef]
- Zhang, B.; Gunawardane, L.; Niazi, F.; Jahanbani, F.; Chen, X.; Valadkhan, S. A novel RNA motif mediates the strict nuclear localization of a long noncoding RNA. Mol Cell Biol 2014, 34(12), 2318–2329. [CrossRef]
- Lubelsky, Y.; Ulitsky, I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature 2018, 555(7694), 107–111. [CrossRef]
- Lee, Y.; Shen, Y.; Francey, L. J.; Ramanathan, C.; Sehgal, A.; Liu, A. C.; Hogenesch, J. B. The NRON complex controls circadian clock function through regulated PER and CRY nuclear translocation. Sci Rep 2019, 9(1), 11883. [CrossRef]
- Naganuma, T.; Nakagawa, S.; Tanigawa, A.; Sasaki, Y. F.; Goshima, N.; Hirose, T. Alternative 3'-end processing of long noncoding RNA initiates construction of nuclear paraspeckles. EMBO J 2012, 31(20), 4020–4034. [CrossRef]
- Yamazaki, T.; Souquere, S.; Chujo, T.; Kobelke, S.; Chong, Y. S.; Fox, A. H.; Bond, C. S.; Nakagawa, S.; Pierron, G.; Hirose, T. Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly through Phase Separation. Mol Cell 2018, 70(6), 1038–1053.e7. [CrossRef]
- Guo, Q.; Shi, X.; Wang, X. RNA and liquid-liquid phase separation. Noncoding RNA Res 2021, 6(2), 92–99. [CrossRef]
- Yamazaki, T.; Yamamoto, T.; Hirose, T. Micellization: A new principle in the formation of biomolecular condensates. Front Mol Biosci 2022, 9, 974772. [CrossRef]
- Adriaens, C.; Standaert, L.; Barra, J.; Latil, M.; Verfaillie, A.; Kalev, P.; Boeckx, B.; Wijnhoven, P. W.; Radaelli, E.; Vermi, W.; et al. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat Med 2016, 22(8), 861–868. [CrossRef]
- Shen, W.; Liang, X. H.; Sun, H.; De Hoyos, C. L.; Crooke, S. T. Depletion of NEAT1 lncRNA attenuates nucleolar stress by releasing sequestered P54nrb and PSF to facilitate c-Myc translation. PLoS One 2017, 12(3), e0173494. [CrossRef]
- Ninomiya, K.; Hirose, T. Short Tandem Repeat-Enriched Architectural RNAs in Nuclear Bodies: Functions and Associated Diseases. Noncoding RNA 2020, 6(1), 6. [CrossRef]
- Fox, A. H.; Nakagawa, S.; Hirose, T.; Bond, C. S. Paraspeckles: Where Long Noncoding RNA Meets Phase Separation. Trends Biochem Sci 2018, 43(2), 124–135. [CrossRef]
- Nakagawa, S.; Yamazaki, T.; Mannen, T.; Hirose, T. ArcRNAs and the formation of nuclear bodies. Mamm Genome 2022, 33(2), 382–401. [CrossRef]
- Chujo, T.; Yamazaki, T.; Kawaguchi, T.; Kurosaka, S.; Takumi, T.; Nakagawa, S.; Hirose, T. Unusual semi-extractability as a hallmark of nuclear body-associated architectural noncoding RNAs. EMBO J 2017, 36(10), 1447–1462. [CrossRef]
- Shevtsov, S. P.; Dundr, M. Nucleation of nuclear bodies by RNA. Nat Cell Biol 2011, 13(2), 167–173. [CrossRef]
- Ninomiya, K.; Adachi, S.; Natsume, T.; Iwakiri, J.; Terai, G.; Asai, K.; Hirose, T. LncRNA-dependent nuclear stress bodies promote intron retention through SR protein phosphorylation. EMBO J 2020, 39(3), e102729. [CrossRef]
- Aly, M. K.; Ninomiya, K.; Adachi, S.; Natsume, T.; Hirose, T. Two distinct nuclear stress bodies containing different sets of RNA-binding proteins are formed with HSATIII architectural noncoding RNAs upon thermal stress exposure. Biochem Biophys Res Commun 2019, 516(2), 419–423. [CrossRef]
- Ninomiya, K.; Iwakiri, J.; Aly, M. K.; Sakaguchi, Y.; Adachi, S.; Natsume, T.; Terai, G.; Asai, K.; Suzuki, T.; Hirose, T. m6 A modification of HSATIII lncRNAs regulates temperature-dependent splicing. EMBO J 2021, 40(15), e107976. [CrossRef]
- Onoguchi-Mizutani, R.; Kirikae, Y.; Ogura, Y.; Gutschner, T.; Diederichs, S.; Akimitsu, N. Identification of a heat-inducible novel nuclear body containing the long noncoding RNA MALAT1. J Cell Sci 2021, 134(10), jcs253559. [CrossRef]
- Lee, S.; Kopp, F.; Chang, T. C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J. T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164(1–2), 69–80. [CrossRef]
- Munschauer, M.; Nguyen, C. T.; Sirokman, K.; Hartigan, C. R.; Hogstrom, L.; Engreitz, J. M.; Ulirsch, J. C.; Fulco, C. P.; Subramanian, V.; Chen, J.; et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature 2018, 561(7721), 132–136. [CrossRef]
- Wan, Y.; Qu, K.; Zhang, Q. C.; Flynn, R. A.; Manor, O.; Ouyang, Z.; Zhang, J.; Spitale, R. C.; Snyder, M. P.; Segal, E.; Chang, H. Y. Landscape and variation of RNA secondary structure across the human transcriptome. Nature 2014, 505(7485), 706–709. [CrossRef]
- Ding, Y.; Tang, Y.; Kwok, C. K.; Zhang, Y.; Bevilacqua, P. C.; Assmann, S. M. In vivo genome-wide profiling of RNA secondary structure reveals novel regulatory features. Nature 2014, 505(7485), 696–700. [CrossRef]
- Rouskin, S.; Zubradt, M.; Washietl, S.; Kellis, M.; Weissman, J. S. Genome-wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 2014, 505(7485), 701–705. [CrossRef]
- Przanowska, R. K.; Weidmann, C. A.; Saha, S.; Cichewicz, M. A.; Jensen, K. N.; Przanowski, P.; Irving, P. S.; Janes, K. A.; Guertin, M. J.; Weeks, K. M.; et al. Distinct MUNC lncRNA structural domains regulate transcription of different promyogenic factors. Cell Rep 2022, 38(7), 110361. [CrossRef]
- Balas, M. M.; Hartwick, E. W.; Barrington, C.; Roberts, J. T.; Wu, S. K.; Bettcher, R.; Griffin, A. M.; Kieft, J. S.; Johnson, A. M. Establishing RNA-RNA interactions remodels lncRNA structure and promotes PRC2 activity. Sci Adv 2021, 7(16), eabc9191. [CrossRef]
- Wilkinson, K. A.; Merino, E. J.; Weeks, K. M. Selective 2'-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat Protoc 2006, 1(3), 1610–1616. [CrossRef]
- Smola, M. J.; Rice, G. M.; Busan, S.; Siegfried, N. A.; Weeks, K. M. Selective 2'-hydroxyl acylation analyzed by primer extension and mutational profiling (SHAPE-MaP) for direct, versatile and accurate RNA structure analysis. Nat Protoc 2015, 10(11), 1643–1669. [CrossRef]
- Yankey, A.; Clark, S. C.; Owens, M. C.; Somarowthu, S. Purification and Structural Characterization of the Long Noncoding RNAs. Methods Mol Biol 2021, 2372, 93–110. [CrossRef]
- Chu, C.; Quinn, J.; Chang, H. Y. Chromatin isolation by RNA purification (ChIRP). J Vis Exp 2012, 61, 3912. [CrossRef]
- Owens, M. C.; Clark, S. C.; Yankey, A.; Somarowthu, S. Identifying Structural Domains and Conserved Regions in the Long Non-Coding RNA lncTCF7. Int J Mol Sci 2019, 20(19), 4770. [CrossRef]
- Andric, V.; Nevers, A.; Hazra, D.; Auxilien, S.; Menant, A.; Graille, M.; Palancade, B.; Rougemaille, M. A scaffold lncRNA shapes the mitosis to meiosis switch. Nat Commun 2021, 12(1), 770. [CrossRef]
- Kapranov, P.; Cheng, J.; Dike, S.; Nix, D. A.; Duttagupta, R.; Willingham, A. T.; Stadler, P. F.; Hertel, J.; Hackermüller, J.; Hofacker, I. L.; et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007, 316(5830), 1484–1488. [CrossRef]
- Ageeli, A. A.; McGovern-Gooch, K. R.; Kaminska, M. M.; Baird, N. J. Finely tuned conformational dynamics regulate the protective function of the lncRNA MALAT1 triple helix. Nucleic Acids Res 2019, 47(3), 1468–1481. [CrossRef]
- Simko, E. A. J.; Liu, H.; Zhang, T.; Velasquez, A.; Teli, S.; Haeusler, A. R.; Wang, J. G-quadruplexes offer a conserved structural motif for NONO recruitment to NEAT1 architectural lncRNA. Nucleic Acids Res 2020, 48(13), 7421–7438. [CrossRef]
- Yamazaki, T.; Nakagawa, S.; Hirose, T. Architectural RNAs for Membraneless Nuclear Body Formation. Cold Spring Harb Symp Quant Biol 2019, 84, 227–237. [CrossRef]
- Somasundaram, K.; Gupta, B.; Jain, N.; Jana, S. LncRNAs divide and rule: The master regulators of phase separation. Front Genet 2022, 13, 930792. [CrossRef]
- Duvignaud, J. B.; Bédard, M.; Nagata, T.; Muto, Y.; Yokoyama, S.; Gagné, S. M.; Vincent, M. Structure, Dynamics, and Interaction of p54(nrb)/NonO RRM1 with 5' Splice Site RNA Sequence. Biochemistry 2016, 55(18), 2553–2566. [CrossRef]
- Smola, M. J.; Christy, T. W.; Inoue, K.; Nicholson, C. O.; Friedersdorf, M.; Keene, J. D.; Lee, D. M.; Calabrese, J. M.; Weeks, K. M. SHAPE reveals transcript-wide interactions, complex structural domains, and protein interactions across the Xist lncRNA in living cells. Proc Natl Acad Sci U S A 2016, 113(37), 10322–10327. [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E.; et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat Biotechnol 2015, 33(5), 445–449. [CrossRef]
- Smola, M. J.; Calabrese, J. M.; Weeks, K. M. Detection of RNA-Protein Interactions in Living Cells with SHAPE. Biochemistry 2015, 54(46), 6867–6875. [CrossRef]
- Luo, Q. J.; Zhang, J.; Li, P.; Wang, Q.; Zhang, Y.; Roy-Chaudhuri, B.; Xu, J.; Kay, M. A.; Zhang, Q. C. RNA structure probing reveals the structural basis of Dicer binding and cleavage. Nat Commun 2021, 12(1), 3397. [CrossRef]
- Underwood, J. G.; Uzilov, A. V.; Katzman, S.; Onodera, C. S.; Mainzer, J. E.; Mathews, D. H.; Lowe, T. M.; Salama, S. R.; Haussler, D. FragSeq: transcriptome-wide RNA structure probing using high-throughput sequencing. Nat Methods 2010, 7(12), 995–1001. [CrossRef]
- Washietl, S.; Hofacker, I. L.; Stadler, P. F.; Kellis, M. RNA folding with soft constraints: reconciliation of probing data and thermodynamic secondary structure prediction. Nucleic Acids Res 2012, 40(10), 4261–4272. [CrossRef]
- Xing, Z.; Lin, C.; Yang, L. LncRNA Pulldown Combined with Mass Spectrometry to Identify the Novel LncRNA-Associated Proteins. Methods Mol Biol 2016, 1402, 1–9. [CrossRef]
- Bierhoff, H. Analysis of lncRNA-Protein Interactions by RNA-Protein Pull-Down Assays and RNA Immunoprecipitation (RIP). Methods Mol Biol 2018, 1686, 241–250. [CrossRef]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M. Jr.; Jungkamp, A. C.; Munschauer, M.; et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141(1), 129–141. [CrossRef]
- Bonetti, A.; Agostini, F.; Suzuki, A. M.; Hashimoto, K.; Pascarella, G.; Gimenez, J.; Roos, L.; Nash, A. J.; Ghilotti, M.; Cameron, C. J. F.; et al. RADICL-seq identifies general and cell type-specific principles of genome-wide RNA-chromatin interactions. Nat Commun 2020, 11(1), 1018. [CrossRef]
- Shu, X.; Kato, M.; Takizawa, S.; Suzuki, Y.; Carninci, P. RADIP technology comprehensively identifies H3K27me3-associated RNA-chromatin interactions. Nucleic Acids Res 2024, 52(22), e104. [CrossRef]
- Davis, C. P.; West, J. A. Purification of specific chromatin regions using oligonucleotides: capture hybridization analysis of RNA targets (CHART). Methods Mol Biol 2015, 1262, 167–182. [CrossRef]
- Schäfer, R. A.; Voß, B. RNAnue: efficient data analysis for RNA-RNA interactomics. Nucleic Acids Res 2021, 49(10), 5493–5501. [CrossRef]
- Ghosh, S.; Tibbit, C.; Liu, J. L. Effective knockdown of Drosophila long non-coding RNAs by CRISPR interference. Nucleic Acids Res 2016, 44(9), e84. [CrossRef]
- Joung, J.; Engreitz, J. M.; Konermann, S.; Abudayyeh, O. O.; Verdine, V. K.; Aguet, F.; Gootenberg, J. S.; Sanjana, N. E.; Wright, J. B.; Fulco, C. P.; et al. Genome-scale activation screen identifies a lncRNA locus regulating a gene neighbourhood. Nature 2017, 548(7667), 343–346. [CrossRef]
- Liu, S. J.; Horlbeck, M. A.; Cho, S. W.; Birk, H. S.; Malatesta, M.; He, D.; Attenello, F. J.; Villalta, J. E.; Cho, M. Y.; Chen, Y.; et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017, 355(6320), aah7111. [CrossRef]
- Bester, A. C.; Lee, J. D.; Chavez, A.; Lee, Y. R.; Nachmani, D.; Vora, S.; Victor, J.; Sauvageau, M.; Monteleone, E.; Rinn, J. L.; et al. An Integrated Genome-wide CRISPRa Approach to Functionalize lncRNAs in Drug Resistance. Cell 2018, 173(3), 649–664.e20. [CrossRef]
- Tani, H. Recent Advances and Prospects in RNA Drug Development. Int. J. Mol. Sci. 2024, 25(22), 12284. [CrossRef]
- Shi, P.; Wu, X. Programmable RNA targeting with CRISPR-Cas13. RNA Biol 2024, 21(1), 1–9. [CrossRef]
- Klattenhoff, C. A.; Scheuermann, J. C.; Surface, L. E.; Bradley, R. K.; Fields, P. A.; Steinhauser, M. L.; Ding, H.; Butty, V. L.; Torrey, L.; Haas, S.; et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 2013, 152(3), 570–583. [CrossRef]
- Bond, A. M.; Vangompel, M. J.; Sametsky, E. A.; Clark, M. F.; Savage, J. C.; Disterhoft, J. F.; Kohtz, J. D. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci 2009, 12(8), 1020–1027. [CrossRef]
- Ramos, A. D.; Andersen, R. E.; Liu, S. J.; Nowakowski, T. J.; Hong, S. J.; Gertz, C.; Salinas, R. D.; Zarabi, H.; Kriegstein, A. R.; et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell 2015, 16(4), 439–447. [CrossRef]
- Ng, S. Y.; Bogu, G. K.; Soh, B. S.; Stanton, L. W. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell 2013, 51(3), 349–359. [CrossRef]
- Tani, H. RMST: a long noncoding RNA involved in cancer and disease. J Biochem 2025, 177(2), 73–78. [CrossRef]
- Chen, Z. H.; Wang, W. T.; Huang, W.; Fang, K.; Sun, Y. M.; Liu, S. R.; Luo, X. Q.; Chen, Y. Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ 2017, 24(2), 212–224. [CrossRef]
- Gutschner, T.; Hämmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 2013, 73(3), 1180–1189. [CrossRef]
- Wu, Y.; Zhang, L.; Zhang, L.; Wang, Y.; Li, H.; Ren, X.; Wei, F.; Yu, W.; Liu, T.; Wang, X.; et al. Long non-coding RNA HOTAIR promotes tumor cell invasion and metastasis by recruiting EZH2 and repressing E-cadherin in oral squamous cell carcinoma. Int J Oncol 2015, 46(6), 2586–2594. [CrossRef]
- Zhou, L.; Jiang, J.; Huang, Z.; Jin, P.; Peng, L.; Luo, M.; Zhang, Z.; Chen, Y.; Xie, N.; Gao, W.; et al. Hypoxia-induced lncRNA STEAP3-AS1 activates Wnt/β-catenin signaling to promote colorectal cancer progression by preventing m6A-mediated degradation of STEAP3 mRNA. Mol Cancer 2022, 21(1), 168. [CrossRef]
- Liu, L. X.; Deng, W.; Zhou, X. T.; Chen, R. P.; Xiang, M. Q.; Guo, Y. T.; Pu, Z. J.; Li, R.; Wang, G. F.; Wu, L. F. The mechanism of adenosine-mediated activation of lncRNA MEG3 and its antitumor effects in human hepatoma cells. Int J Oncol 2016, 48(1), 421–429. [CrossRef]
- Pierce, J. B.; Zhou, H.; Simion, V.; Feinberg, M. W. Long Noncoding RNAs as Therapeutic Targets. Adv Exp Med Biol 2022, 1363, 161–175. [CrossRef]
- Parasramka, M. A.; Maji, S.; Matsuda, A.; Yan, I. K.; Patel, T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol Ther 2016, 161, 67–78. [CrossRef]
- Chang, J. Y.; Neugebauer, C.; Mues Genannt Koers, A.; 't Hart, P. Small molecule WDR5 inhibitors down-regulate lncRNA expression. RSC Med Chem 2024, 15(2), 636–640. [CrossRef]
- Yagi, Y.; Abe, R.; Tani, H. Exploring IDI2-AS1, OIP5-AS1, and LITATS1: Changes in Long Non-coding RNAs Induced by the Poly I:C Stimulation. Biol. Pharm. Bull. 2024, 47(6), 1144-1147. [CrossRef]
- Yokoyama, S.; Muto, H.; Honda, T.; Kurokawa, Y.; Ogawa, H.; Nakajima, R.; Kawashima, H.; Tani, H. Identification of Two Long Noncoding RNAs, Kcnq1ot1 and Rmst, as Biomarkers in Chronic Liver Diseases in Mice. Int. J. Mol. Sci. 2024, 25(16), 8927. [CrossRef]
- Lemos, A. E. G.; Matos, A. D. R.; Ferreira, L. B.; Gimba, E. R. P. The long non-coding RNA PCA3: an update of its functions and clinical applications as a biomarker in prostate cancer. Oncotarget 2019, 10(61), 6589–6603. [CrossRef]
- Chen, M.; Li, C.; Luo, Q.; Tan, A. LncRNA LINC02257: A Potential Biomarker for Diagnosis and Prognosis of Colorectal Cancer. J Oncol 2022, 2022, 4330630. [CrossRef]
- Tani, H. Metabolic labeling of RNA using ribonucleoside analogs enables the evaluation of RNA synthesis and degradation rates. Anal. Sci. 2025, 41(4), 345-351. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).