Submitted:
14 April 2025
Posted:
15 April 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Neuroinflammation: A Core Driver of Severe Depression
3. Oxidative Stress: A Silent Driver of Depression
4. Depression’s Mitochondrial Roots and Repair Strategies
5. Nutrition, Inflammation, and Depressive Spirals
6. Rethinking Depression Care: Drugs and Natural Options
7. Plant-Based Therapies: Natural Allies Against Depression
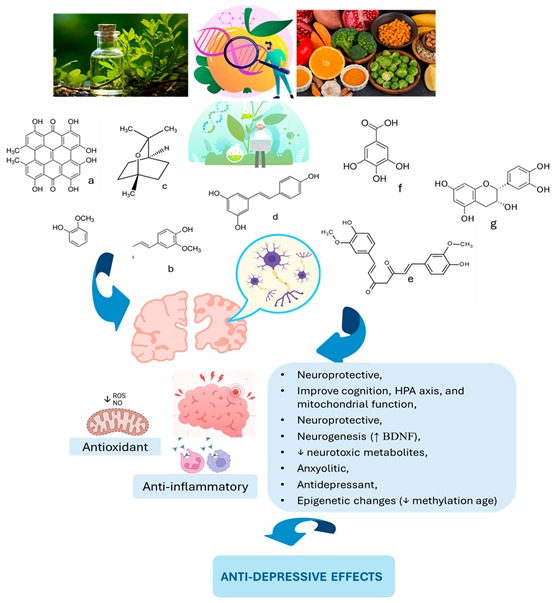
8. From Curcumin to Cocoa: Plant-Based Mood Solutions
| Study [ref.] | Country | Population | Intervention | Outcomes | Side Effects |
| Soltani et al. [262] | Iran | 42 patients (CSFP) aged 35–70 | Nano-curcumin 80 mg/day, 12 weeks | Improved depression & quality of life | Nausea, headache, diarrhea |
| Dehghani et al. [263] | Iran | 76 individuals aged 35–65 | Quercetin 500 mg/day, 8 weeks | Marginal depression improvement | Headache, joint pain, abdominal discomfort |
| Hajiluian et al. [264] | Iran | 50 MS patients with depression, aged 18-55 | Ellagic acid 180 mg/day, 12 weeks | Reduced inflammation & depression | None significant |
| Choi et al. [268] | South Korea | 40 young adults aged 18-29 with MDD | Flavonoid-rich orange juice, 8 weeks | Significant improvement in depressive scores | None significant |
| Maeda-Yamamoto et al. [269] | Japan | 15 older adults aged 50-70 | Anthocyanin-rich potatoes, 8 weeks | Improved psychological stress response | None significant |
| Parilli-Moser et al. [270] | UK | 38 new mothers | High-flavonoid diet, 2 weeks | Reduced anxiety, improved social relationships | None significant |
| Barfoot et al. [271] | Spain | 63 young overweight adults | Roasted peanuts & peanut butter, 6-7 months | Significant depression reduction | Digestive symptoms, softer stools |
| Bourdel-Marchasson et al. [272] | Europe | 125 elderly adults aged ~70 | Antioxidant-rich diet, 2 months | Reduced depressive symptoms | None serious |
| Kontogianni et al. [265] | UK | 99 mildly hypertensive adults aged 40-65 | High vs low polyphenol diet, 8 weeks | Improved depressive symptoms | Not reported |
| Park et al. [273] | South Korea | 40 healthy adults (20–30 y) | Flavonoid-rich (FR) or Flavonoid-low (FL) drinks, 190 mL twice daily for 8 week | ↓ Depressive symptoms (CES-D <20) | None significant |
| Smetanka et al. [274] | Slovakia | 67 adults with MDD, aged 18-65 | Pycnogenol with escitalopram, 12 weeks | No additional benefit compared to escitalopram alone | None significant |
| Kanchanatawan et al. [275] | Multinational | 61 adults aged 18-63 with MDD history | Curcumin 500-1500 mg/day, 12-16 weeks | Improved depression scores (MADRS) | Dizziness, nausea, insomnia, diarrhea |
| Terauchi et al. [266] | Italy | 60 menopausal women aged 50-55 | Equol & resveratrol, 12 weeks | Reduced depressive symptoms (HAM-D) | Mild diarrhea |
| Khalid et al. [276] | UK | Young adults & children | Wild blueberry drink (flavonoids), acute administration | Improved positive affect shortly after consumption | None significant |
| Hirose et al. [267] | Japan | 87 menopausal women aged 40-60 | Isoflavone aglycones, 8 weeks | Improved anxiety, modest depression changes | None significant |
| Sathyapalan et al. [277] | UK, Iran | 30 obese adults | Curcumin 1 g/day, 30 days | Reduced anxiety, no significant depression improvement | None significant |
| Terauch et al. [278] | Japan | 91 menopausal women (40–60 y) | GSPE (100 or 200 mg/day) vs placebo, 8 weeks | ↓ Anxiety (dose-dependent); no effect on depression | None significant |
| Pase et al. [10] | Australia | Adults aged 40-65 | Cocoa polyphenols 250-500 mg/day, 30 days | Improved mood, calmness | None |
| Clinical pilot [279] | UK | 10 adults | High-polyphenol chocolate, crossover | Improved anxiety and depression scores | None |
8. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| DNA | deoxyribonucleic acid |
| HAM-D | Hamilton depression rating scale |
| IL | Interleukin |
| MMA | methylmalonic acid |
| MDD | major depressive disorder |
| mtDNA | mitochondrial deoxyribonucleic acid |
| ROS | reactive oxygen species |
| SSRI | selective serotonin reuptake inhibitor |
| TCA | tricyclic antidepressant |
References
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Réus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre- and Clinical Studies. CNS Neurol Disord Drug Targets 2023, 22, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Suzuki, K.; Kavalali, E.T.; Monteggia, L.M. Ketamine: Mechanisms and Relevance to Treatment of Depression. Annu Rev Med 2024, 75, 129–143. [Google Scholar] [CrossRef]
- Schoeller, F.; Jain, A.; Adrien, V.; Maes, P.; Reggente, N. Aesthetic chills mitigate maladaptive cognition in depression. BMC Psychiatry 2024, 24, 40. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Bagby, S.P.; Martin, D.; Chung, S.T.; Rajapakse, N. From the outside in: biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. American journal of public health 2019, 109, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Tantardini, M.; De Simone, S.; Ramella-Cravaro, V.; Oliver, D.; Kingdon, J.; Kotlicka-Antczak, M.; Valmaggia, L.; Lee, J.; Millan, M. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. European Psychiatry 2017, 40, 65–75. [Google Scholar] [CrossRef]
- Lesch, K.-P. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. Molecular and functional models in neuropsychiatry 2011, 251–280. [Google Scholar]
- Simons, R.L.; Lei, M.K.; Stewart, E.A.; Beach, S.R.; Brody, G.H.; Philibert, R.A.; Gibbons, F.X. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth violence and juvenile justice 2012, 10, 3–24. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther 2024, 9, 30. [Google Scholar] [CrossRef]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. Journal of psychopharmacology (Oxford, England) 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Jia, S.; Hou, Y.; Wang, D.; Zhao, X. Flavonoids for depression and anxiety: a systematic review and meta-analysis. Critical reviews in food science and nutrition 2023, 63, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, T.; Jiang, P.; Dang, R. The interaction between autophagy and neuroinflammation in major depressive disorder: from pathophysiology to therapeutic implications. Pharmacological research 2021, 168, 105586. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, A.G.; Mingoti, M.E.D.; Ignácio, Z.M. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Reviews in the Neurosciences 2025, 36, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, X.; Ma, Y.; Yang, Y.; Pan, C.W.; Zhu, X.; Ke, C. Metabolomics on depression: A comparison of clinical and animal research. J Affect Disord 2024, 349, 559–568. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Pearson, K.; Beier, K.; Mardis, T.; Munoz, B.; Zaidi, A. The Neurochemistry of Depression: The Good, The Bad and The Ugly. Mo Med 2024, 121, 68–75. [Google Scholar]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural antioxidant anthocyanins—A hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- Jin, W.; Xu, X.; Chen, X.; Qi, W.; Lu, J.; Yan, X.; Zhao, D.; Cong, D.; Li, X.; Sun, L. Protective effect of pig brain polypeptides against corticosterone-induced oxidative stress, inflammatory response, and apoptosis in PC12 cells. Biomedicine & Pharmacotherapy 2019, 115, 108890. [Google Scholar]
- Wang, J.; Song, Y.; Chen, Z.; Leng, S.X. Connection between systemic inflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxidative medicine and cellular longevity 2018, 2018, 1972714. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural correlates and molecular mechanisms of memory and learning. 2024, 25, 2724. [Google Scholar] [CrossRef] [PubMed]
- Yoder, R.; Michaud, A.; Feagans, A.; Hinton-Froese, K.E.; Meyer, A.; Powers, V.A.; Stalnaker, L.; Hord, M.K. Family-Based Treatment for Anxiety, Depression, and ADHD for a Parent and Child. Int J Environ Res Public Health 2024, 21. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharmaceutica Sinica B 2023, 13, 3208–3237. [Google Scholar] [CrossRef] [PubMed]
- Ajao, A.A.-n.; Sabiu, S.; Balogun, F.O.; Adekomi, D.A.; Saheed, S.A. The Ambit of Phytotherapy in Psychotic Care. In Psychosis-Biopsychosocial and Relational Perspectives; IntechOpen, 2018. [Google Scholar]
- Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Kumar, H.M.S.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects. Curr Top Med Chem 2024, 24, v–vi. [Google Scholar] [CrossRef]
- Kumar, H.M.S.; Rajpal, V.R.; Koul, H.K.; Raina, S.N.; Qazi, G.N. Phytochemicals for Human Health: The Emerging Trends and Prospects, Part-3. Curr Top Med Chem 2024, 24, 1011–1012. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Critical reviews in food science and nutrition 2019, 59, 827–829. [Google Scholar] [CrossRef]
- Fais, A.; Era, B. Phytochemical Composition and Biological Activity. Plants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Cao, G.; Zhao, D.; Li, G.; Zhang, H.; Yan, M. Ethnic, Botanic, Phytochemistry and Pharmacology of the. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Reddy, K.; Stafford, G.I.; Makunga, N.P. Skeletons in the closet? Using a bibliometric lens to visualise phytochemical and pharmacological activities linked to. Front Plant Sci 2024, 15, 1268101. [Google Scholar] [CrossRef]
- Gong, G.; Ganesan, K.; Wang, Y.; Zhang, Z.; Liu, Y.; Wang, J.; Yang, F.; Zheng, Y. Ononin ameliorates depression-like behaviors by regulating BDNF-TrkB-CREB signaling in vitro and in vivo. J Ethnopharmacol 2024, 320, 117375. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef]
- Seung, H.-B.; Kwon, H.-J.; Kwon, C.-Y.; Kim, S.-H. Neuroendocrine Biomarkers of Herbal Medicine for Major Depressive Disorder: A Systematic Review and Meta-Analysis. Pharmaceuticals 2023, 16, 1176. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, K.R.; Alamdari-Palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut-Brain Axis: Therapeutic Implication. Curr Dev Nutr 2024, 8, 103785. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neuroscience & Biobehavioral Reviews 2024, 105791. [Google Scholar]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr Biol 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Kakarla, R.; Karuturi, P.; Siakabinga, Q.; Kasi Viswanath, M.; Dumala, N.; Guntupalli, C.; Nalluri, B.N.; Venkateswarlu, K.; Prasanna, V.S.; Gutti, G.; et al. Current understanding and future directions of cruciferous vegetables and their phytochemicals to combat neurological diseases. Phytother Res 2024, 38, 1381–1399. [Google Scholar] [CrossRef]
- Chandel, P.; Thapa, K.; Kanojia, N.; Rani, L.; Singh, T.G.; Rohilla, P. Exploring Therapeutic Potential of Phytoconstituents as a Gut Microbiota Modulator in the Management of Neurological and Psychological Disorders. Neuroscience 2024, 551, 69–78. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Advances in Clinical and Experimental Medicine 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Cordeiro, M.L.d.S.; Martins, V.G.d.Q.A.; Silva, A.P.d.; Rocha, H.A.O.; Rachetti, V.d.P.S.; Scortecci, K.C. Phenolic acids as antidepressant agents. Nutrients 2022, 14, 4309. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer’s disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Fazilat, S.; Tahmasbi, F.; Mirzaei, M.R.; Sanaie, S.; Yousefi, Z.; Asnaashari, S.; Yaqoubi, S.; Mohammadi, A.B.; Araj-khodaei, M. A systematic review on the use of phytotherapy in managing clinical depression. BioImpacts 2024, 15, 30532–30532. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Frontiers in psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C. Neuroinflammation and depression: A review. European journal of neuroscience 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. J Neuroinflammation 2023, 20, 283. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Neuroinflammation in Cerebral Ischemia and Ischemia/Reperfusion Injuries: From Pathophysiology to Therapeutic Strategies. Int J Mol Sci 2021, 23. [Google Scholar] [CrossRef]
- Fornari Laurindo, L.; Aparecido Dias, J.; Cressoni Araújo, A.; Torres Pomini, K.; Machado Galhardi, C.; Rucco Penteado Detregiachi, C.; Santos de Argollo Haber, L.; Donizeti Roque, D.; Dib Bechara, M.; Vialogo Marques de Castro, M.; et al. Immunological dimensions of neuroinflammation and microglial activation: exploring innovative immunomodulatory approaches to mitigate neuroinflammatory progression. Front Immunol 2023, 14, 1305933. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Barbalho, S.M.; Araújo, A.C.; Guiguer, E.L.; Mondal, A.; Bachtel, G.; Bishayee, A. Açaí (Euterpe oleracea Mart.) in Health and Disease: A Critical Review. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Simili, O.A.G.; Araújo, A.C.; Guiguer, E.L.; Direito, R.; Valenti, V.E.; de Oliveira, V.; de Oliveira, J.S.; Yanaguizawa Junior, J.L.; Dias, J.A.; et al. Melatonin from Plants: Going Beyond Traditional Central Nervous System Targeting-A Comprehensive Review of Its Unusual Health Benefits. Biology (Basel) 2025, 14. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From pathways to potential therapies. 2024, 13, 1098. [Google Scholar] [CrossRef]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G. Tumor necrosis factor and interleukin-1β modulate synaptic plasticity during neuroinflammation. Neural plasticity 2018, 2018, 8430123. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.V.; Prehar, S.; Kennedy, A.R.; Little, R.A.; Hopkins, S.J. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology 2003, 144, 1894–1906. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Zhang, L.; Cheng, J.-K.; Ji, R.-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. Journal of neuroscience 2008, 28, 5189–5194. [Google Scholar] [CrossRef]
- De Simoni, M.; Terreni, L.; Chiesa, R.; Mangiarotti, F.; Forloni, G. Interferon-γ potentiates interleukin (IL)-6 and tumor necrosis factor-α but not IL-1β induced by endotoxin in the brain. Endocrinology 1997, 138, 5220–5226. [Google Scholar] [CrossRef]
- Chaves, F.M.; Mansano, N.S.; Frazão, R.; Donato Jr, J. Tumor necrosis factor α and interleukin-1β acutely inhibit AgRP neurons in the arcuate nucleus of the hypothalamus. International Journal of Molecular Sciences 2020, 21, 8928. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress induced microglial activation contributes to depression. Pharmacological research 2022, 179, 106145. [Google Scholar] [CrossRef]
- Jia, X.; Gao, Z.; Hu, H. Microglia in depression: current perspectives. Science China Life Sciences 2021, 64, 911–925. [Google Scholar] [CrossRef]
- Turkin, A.; Tuchina, O.; Klempin, F. Microglia function on precursor cells in the adult hippocampus and their responsiveness to serotonin signaling. Frontiers in Cell and Developmental Biology 2021, 9, 665739. [Google Scholar] [CrossRef]
- Zanella, I. Neuroinflammation: From Molecular Basis to Therapy. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Poletti, S.; Mazza, M.G.; Benedetti, F. Inflammatory mediators in major depression and bipolar disorder. Transl Psychiatry 2024, 14, 247. [Google Scholar] [CrossRef]
- Xia, C.Y.; Guo, Y.X.; Lian, W.W.; Yan, Y.; Ma, B.Z.; Cheng, Y.C.; Xu, J.K.; He, J.; Zhang, W.K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol Res 2023, 187, 106625. [Google Scholar] [CrossRef]
- Han, Q.; Li, W.; Chen, P.; Wang, L.; Bao, X.; Huang, R.; Liu, G.; Chen, X. Microglial NLRP3 inflammasome-mediated neuroinflammation and therapeutic strategies in depression. Neural Regen Res 2024, 19, 1890–1898. [Google Scholar] [CrossRef]
- Bearoff, F.; Dhavale, D.; Kotzbauer, P.; Kortagere, S. Aggregated Alpha-Synuclein Activates Pro-Inflammatory NFKB Signaling Pathways Through TLR-Dependent and Independent Mechanisms in Peripheral Monocytic Cells. The FASEB Journal 2022, 36. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS neuroscience & therapeutics 2021, 27, 36–47. [Google Scholar]
- Wang, X.; Antony, V.; Wang, Y.; Wu, G.; Liang, G. Pattern recognition receptor-mediated inflammation in diabetic vascular complications. Medicinal Research Reviews 2020, 40, 2466–2484. [Google Scholar] [CrossRef]
- Barrera, M.-J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; González, S.; González, M.-J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjögren’s syndrome. Autoimmunity reviews 2021, 20, 102867. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, H.; Zhang, T.; Wu, J.; Tang, H.; Liang, X.; Ren, D.; Huang, J.; Li, W. Mitochondria of intestinal epithelial cells in depression: Are they at a crossroads of gut-brain communication? Neuroscience & Biobehavioral Reviews 2023, 153, 105403. [Google Scholar]
- Sipahi, H.; Mat, A.F.; Ozhan, Y.; Aydin, A. The interrelation between oxidative stress, depression and inflammation through the kynurenine pathway. Current Topics in Medicinal Chemistry 2023, 23, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, D.; Kondo, S.; Ferdousi, F.; Mizuno, S.; Yada, A.; Tominaga, K.; Takahashi, S.; Isoda, H. A rare olive compound oleacein functions as a TrkB agonist and mitigates neuroinflammation both in vitro and in vivo. Cell Commun Signal 2024, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y. Progress of plant polyphenol extracts in treating depression by anti-neuroinflammatory mechanism: A review. Medicine (Baltimore) 2024, 103, e37151. [Google Scholar] [CrossRef]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Corrigendum: Inflammation-Associated Synaptic Alterations as Shared Threads in Depression and Multiple Sclerosis. Front Cell Neurosci 2020, 14, 647259. [Google Scholar] [CrossRef]
- Sha, Q.; Escobar Galvis, M.L.; Madaj, Z.B.; Keaton, S.A.; Smart, L.; Edgerly, Y.M.; Anis, E.; Leach, R.; Osborne, L.M.; Achtyes, E.; et al. Dysregulated placental expression of kynurenine pathway enzymes is associated with inflammation and depression in pregnancy. Brain Behav Immun 2024, 119, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun 2020, 83, 239–247. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. Journal of Neural Transmission 2024, 1–21. [Google Scholar]
- Tanaka, M.; Vécsei, L. A decade of dedication: pioneering perspectives on neurological diseases and mental illnesses. 2024, 12, 1083. [Google Scholar] [CrossRef]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain, behavior, and immunity 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S. Inflammation-associated synaptic alterations as shared threads in depression and multiple sclerosis. Frontiers in Cellular Neuroscience 2020, 14, 169. [Google Scholar] [CrossRef]
- Gustafsson, H.C.; Sullivan, E.L.; Nousen, E.K.; Sullivan, C.A.; Huang, E.; Rincon, M.; Nigg, J.T.; Loftis, J.M. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain, behavior, and immunity 2018, 73, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, G.; Cepas, M.B.; Broeders, T.A.A.; Koubiyr, I.; Schoonheim, M.M. Network Analysis in Multiple Sclerosis and Related Disorders. Neuroimaging Clin N Am 2024, 34, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Z.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; Liu, S.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun 2024, 15, 3003. [Google Scholar] [CrossRef]
- Molina Galindo, L.S.; Gonzalez-Escamilla, G.; Fleischer, V.; Grotegerd, D.; Meinert, S.; Ciolac, D.; Person, M.; Stein, F.; Brosch, K.; Nenadić, I.; et al. Concurrent inflammation-related brain reorganization in multiple sclerosis and depression. Brain Behav Immun 2024, 119, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int J Mol Med 2024, 53. [Google Scholar] [CrossRef]
- Cui, J.-J.; Huang, Z.-Y.; Xie, Y.-H.; Wu, J.-B.; Xu, G.-H.; Li, C.-F.; Zhang, M.-M.; Yi, L.-T. Gut microbiota mediated inflammation, neuroendocrine and neurotrophic functions involved in the antidepressant-like effects of diosgenin in chronic restraint stress. Journal of Affective Disorders 2023, 321, 242–252. [Google Scholar] [CrossRef]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Molecular neurobiology 2018, 55, 6282–6306. [Google Scholar] [CrossRef]
- Adzic, M.; Brkic, Z.; Mitic, M.; Francija, E.; Jovicic, M.J.; Radulovic, J.; Maric, N.P. Therapeutic strategies for treatment of inflammation-related depression. Current neuropharmacology 2018, 16, 176–209. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting dots between mitochondrial dysfunction and depression. Biomolecules 2023, 13, 695. [Google Scholar] [CrossRef]
- Casaril, A.M.; Dantzer, R.; Bas-Orth, C. Neuronal mitochondrial dysfunction and bioenergetic failure in inflammation-associated depression. Frontiers in neuroscience 2021, 15, 725547. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug discovery today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Current pharmaceutical design 2014, 20, 3812–3847. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting Dots between Mitochondrial Dysfunction and Depression. Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The kynurenine and serotonin pathway, neopterin and biopterin in depressed children and adolescents: an impact of omega-3 fatty acids, and association with markers related to depressive disorder. A randomized, blinded, prospective study. Front Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knock-out Mice: A Novel Model for Experience-Based Depression and Post-Traumatic Stress Disorder. 2024. [Google Scholar]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog Neuropsychopharmacol Biol Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Larrea, A.; Sánchez-Sánchez, L.; Diez-Martin, E.; Elexpe, A.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Mitochondrial Metabolism in Major Depressive Disorder: From Early Diagnosis to Emerging Treatment Options. J Clin Med 2024, 13. [Google Scholar] [CrossRef]
- Chen, H.; Lu, M.; Lyu, Q.; Shi, L.; Zhou, C.; Li, M.; Feng, S.; Liang, X.; Zhou, X.; Ren, L. Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomed Pharmacother 2024, 175, 116656. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The impact of C-3 side chain modifications on Kynurenic Acid: a behavioral analysis of its analogs in the Motor Domain. International journal of molecular sciences 2024, 25, 3394. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. 2024, 12, 574. [Google Scholar]
- Cao, Y.; Chen, H.; Tan, Y.; Yu, X.D.; Xiao, C.; Li, Y.; Reilly, J.; He, Z.; Shu, X. Protection of p-Coumaric acid against chronic stress-induced neurobehavioral deficits in mice via activating the PKA-CREB-BDNF pathway. Physiol Behav 2024, 273, 114415. [Google Scholar] [CrossRef]
- Song, Y.; Cao, H.; Zuo, C.; Gu, Z.; Huang, Y.; Miao, J.; Fu, Y.; Guo, Y.; Jiang, Y.; Wang, F. Mitochondrial dysfunction: A fatal blow in depression. Biomed Pharmacother 2023, 167, 115652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy Contributes to the Pathogenesis of Inflammatory Diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, M.; Jeong, Y.Y.; Margolis, D.J.; Cai, Q. The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy 2021, 17, 4182–4201. [Google Scholar] [CrossRef]
- Visentin, A.P.V.; Colombo, R.; Scotton, E.; Fracasso, D.S.; da Rosa, A.R.; Branco, C.S.; Salvador, M. Targeting Inflammatory-Mitochondrial Response in Major Depression: Current Evidence and Further Challenges. Oxid Med Cell Longev 2020, 2020, 2972968. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; Dantzer, R.; Bas-Orth, C. Neuronal Mitochondrial Dysfunction and Bioenergetic Failure in Inflammation-Associated Depression. Front Neurosci 2021, 15, 725547. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr Neuropharmacol 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol (Lausanne) 2023, 14, 1134025. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Moreno, J.J.; Bodega, P.; de Cos-Gandoy, A.; de Miguel, M.; Santos-Beneit, G.; Fernández-Alvira, J.M.; Fernández-Jiménez, R.; Martínez-Gómez, J.; et al. Metabolic syndrome, adiposity, diet, and emotional eating are associated with oxidative stress in adolescents. Front Nutr 2023, 10, 1216445. [Google Scholar] [CrossRef]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep 2021, 35, 109163. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, L.; Cen, M.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Oxidative balance scores and depressive symptoms: Mediating effects of oxidative stress and inflammatory factors. J Affect Disord 2023, 334, 205–212. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Takeda, L.N.; Omine, A.; Laurindo, L.F.; Araújo, A.C.; Machado, N.M.; Dias, J.A.; Kavalakatt, J.; Banerjee, S.; de Alvares Goulart, R.; Atanasov, A.G.; et al. Brazil nut (Bertholletia excelsa Bonpl.) in health and disease: A narrative review. Food Chem 2025, 477, 143425. [Google Scholar] [CrossRef] [PubMed]
- Corraliza-Gómez, M.; Gallardo, A.B.; Díaz-Marrero, A.R.; de la Rosa, J.M.; D’Croz, L.; Darias, J.; Arranz, E.; Cózar-Castellano, I.; Ganfornina, M.D.; Cueto, M. Modulation of Glial Responses by Furanocembranolides: Leptolide Diminishes Microglial Inflammation in Vitro and Ameliorates Gliosis In Vivo in a Mouse Model of Obesity and Insulin Resistance. Mar Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Y.; Qin, Y.; Zhou, Y.; Tang, R.; Wang, Q.; Li, X.; Wang, H.; Weston-Green, K.; Huang, X.F.; et al. Alterations to the microbiota-colon-brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J Nutr Biochem 2019, 65, 54–65. [Google Scholar] [CrossRef]
- Tobe, E.H. Mitochondrial dysfunction, oxidative stress, and major depressive disorder. Neuropsychiatr Dis Treat 2013, 9, 567–573. [Google Scholar] [CrossRef]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2016, 65, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front Neurosci 2018, 12, 386. [Google Scholar] [CrossRef]
- Li, W.; Zhu, L.; Chen, Y.; Zhuo, Y.; Wan, S.; Guo, R. Association between mitochondrial DNA levels and depression: a systematic review and meta-analysis. BMC Psychiatry 2023, 23, 866. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Sheng, H. Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems. CNS Neurosci Ther 2024, 30, e14576. [Google Scholar] [CrossRef] [PubMed]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Rezin, G.T.; Amboni, G.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 2009, 34, 1021–1029. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J Clin Invest 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res Rev 2023, 88, 101955. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 2019, 593, 1566–1579. [Google Scholar] [CrossRef]
- Scaini, G.; Mason, B.L.; Diaz, A.P.; Jha, M.K.; Soares, J.C.; Trivedi, M.H.; Quevedo, J. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: Does inflammation play a role? Molecular psychiatry 2022, 27, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Lin, G.; Su, L.; Xue, L.; Duan, K.; Chen, L.; Ni, J. The association between methylmalonic acid, a biomarker of mitochondrial dysfunction, and cause-specific mortality in Alzheimer’s disease and Parkinson’s disease. Heliyon 2024, 10, e29357. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Wan, X.; Liu, X.; Wang, Z.; Liang, C.; Zhang, Z.; Wang, Y.; Yan, M.; Wu, P.; et al. Mitochondria-derived methylmalonic acid aggravates ischemia-reperfusion injury by activating reactive oxygen species-dependent ferroptosis. Cell Commun Signal 2024, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xiao, Y.; Liu, D. Associations of methylmalonic acid and depressive symptoms with mortality: a population-based study. Transl Psychiatry 2024, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.N.; Watanabe, F.; Koseki, K.; He, R.E.; Lee, H.L.; Chiu, T.H.T. Effect of roasted purple laver (nori) on vitamin B(12) nutritional status of vegetarians: a dose-response trial. European journal of nutrition 2024, 63, 3269–3279. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Zhao, Y.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci Rep 2017, 7, 6932. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24. [Google Scholar] [CrossRef]
- de Moraes, M.S.; Guerreiro, G.; Sitta, A.; de Moura Coelho, D.; Manfredini, V.; Wajner, M.; Vargas, C.R. Oxidative damage in mitochondrial fatty acids oxidation disorders patients and the in vitro effect of l-carnitine on DNA damage induced by the accumulated metabolites. Arch Biochem Biophys 2020, 679, 108206. [Google Scholar] [CrossRef]
- Butkowski, E.G.; Al-Aubaidy, H.A.; Jelinek, H.F. Interaction of homocysteine, glutathione and 8-hydroxy-2’-deoxyguanosine in metabolic syndrome progression. Clin Biochem 2017, 50, 116–120. [Google Scholar] [CrossRef]
- Zhao, X.; Abulikemu, A.; Lv, S.; Qi, Y.; Duan, J.; Zhang, J.; Chen, R.; Guo, C.; Li, Y.; Sun, Z. Oxidative stress- and mitochondrial dysfunction-mediated cytotoxicity by silica nanoparticle in lung epithelial cells from metabolomic perspective. Chemosphere 2021, 275, 129969. [Google Scholar] [CrossRef]
- Park, A.; Oh, M.; Lee, S.J.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Han, B.S.; Kim, W.K. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Riou, A.; Broeglin, A.; Grimm, A. Mitochondrial transplantation in brain disorders: Achievements, methods, and challenges. Neurosci Biobehav Rev 2025, 169, 105971. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct Target Ther 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Hamad, R.S.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. Role of brain renin-angiotensin system in depression: A new perspective. CNS Neurosci Ther 2024, 30, e14525. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr Med Chem 2015, 22, 2690–2711. [Google Scholar] [CrossRef]
- Koh, J.H.; Kim, J.Y. Role of PGC-1α in the Mitochondrial NAD(+) Pool in Metabolic Diseases. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol Sci 2019, 40, 50–70. [Google Scholar] [CrossRef]
- Tao, J.; Chen, H.; Wang, Y.J.; Qiu, J.X.; Meng, Q.Q.; Zou, R.J.; Li, L.; Huang, J.G.; Zhao, Z.K.; Huang, Y.L.; et al. Ketogenic Diet Suppressed T-Regulatory Cells and Promoted Cardiac Fibrosis via Reducing Mitochondria-Associated Membranes and Inhibiting Mitochondrial Function. Oxid Med Cell Longev 2021, 2021, 5512322. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Tian, Y.; Shi, J. Sirtuin 3 and mitochondrial permeability transition pore (mPTP): A systematic review. Mitochondrion 2022, 64, 103–111. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Yang, X.X.; Pang, L.; Liu, J.; Ng, K.T.P.; Yeung, O.W.H.; Lam, Y.F.; Zhang, W.Y.; Lo, C.M.; et al. Inhibition of Carnitine Palmitoyltransferase 1A Aggravates Fatty Liver Graft Injury via Promoting Mitochondrial Permeability Transition. Transplantation 2021, 105, 550–560. [Google Scholar] [CrossRef]
- Kalani, K.; Yan, S.F.; Yan, S.S. Mitochondrial permeability transition pore: a potential drug target for neurodegeneration. Drug Discov Today 2018, 23, 1983–1989. [Google Scholar] [CrossRef]
- Khiroya, K.; Sekyere, E.; McEwen, B.; Bayes, J. Nutritional considerations in major depressive disorder: current evidence and functional testing for clinical practice. Nutr Res Rev 2023, 1–12. [Google Scholar] [CrossRef]
- Bernier, V.; Debarge, M.H.; Hein, M.; Ammendola, S.; Mungo, A.; Loas, G. Major Depressive Disorder, Inflammation, and Nutrition: A Tricky Pattern? Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front Nutr 2022, 9, 867150. [Google Scholar] [CrossRef]
- Muha, J.; Schumacher, A.; Campisi, S.C.; Korczak, D.J. Depression and emotional eating in children and adolescents: A systematic review and meta-analysis. Appetite 2024, 200, 107511. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G.N.; Sanlier, N. The relationship between nutrition and depression in the life process: A mini-review. Exp Gerontol 2023, 172, 112072. [Google Scholar] [CrossRef] [PubMed]
- Chrzastek, Z.; Guligowska, A.; Sobczuk, P.; Kostka, T. Dietary factors, risk of developing depression, and severity of its symptoms in older adults-A narrative review of current knowledge. Nutrition 2023, 106, 111892. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’Neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mardi, P.; Hejrani, B.; Mahdavi, F.S.; Ghoreshi, B.; Gohari, K.; Heidari-Beni, M.; Qorbani, M. Association between junk food consumption and mental health problems in adults: a systematic review and meta-analysis. BMC Psychiatry 2024, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Mahdavi, F.S.; Ejtahed, H.S.; Kazemian, E.; Chaharrahi, A.; Mohammadian Khonsari, N.; Mahdavi-Gorabi, A.; Qorbani, M. Junk food consumption and psychological distress in children and adolescents: a systematic review and meta-analysis. Nutr Neurosci 2023, 26, 807–827. [Google Scholar] [CrossRef]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes Through Their Stomach: Dietary Factors in Major Depressive Disorder. Front Neurosci 2020, 14, 582853. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin Neurosci 2023, 77, 420–433. [Google Scholar] [CrossRef]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J Affect Disord 2023, 327, 270–278. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. [Google Scholar] [CrossRef]
- Gorbachev, D.; Markina, E.; Chigareva, O.; Gradinar, A.; Borisova, N.; Syunyakov, T. Dietary Patterns as Modifiable Risk Factors for Depression: a Narrative Review. Psychiatr Danub 2023, 35, 423–431. [Google Scholar] [PubMed]
- Zhang, Q.; Yi, J.; Wu, Y. Oxidative stress and inflammation mediate the association between elevated oxidative balance scores and improved sleep quality: evidence from NHANES. Front Nutr 2024, 11, 1469779. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Driller, M.; Winwood, P.; Clissold, T.; Johnston, B.; Gill, N. The Effectiveness of a Combined Healthy Eating, Physical Activity, and Sleep Hygiene Lifestyle Intervention on Health and Fitness of Overweight Airline Pilots: A Controlled Trial. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Bolognese, M.A.; Franco, C.B.; Ferrari, A.; Bennemann, R.M.; Lopes, S.M.A.; Bertolini, S.; Júnior, N.N.; Branco, B.H.M. Group Nutrition Counseling or Individualized Prescription for Women With Obesity? A Clinical Trial. Front Public Health 2020, 8, 127. [Google Scholar] [CrossRef]
- Mehdi, S.; Wani, S.U.D.; Krishna, K.L.; Kinattingal, N.; Roohi, T.F. A review on linking stress, depression, and insulin resistance via low-grade chronic inflammation. Biochem Biophys Rep 2023, 36, 101571. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, J.W.; Turco, C.V.; Ramdeo, K.; Rehsi, R.S.; Foglia, S.D.; Nelson, A.J. The Combined Influences of Exercise, Diet and Sleep on Neuroplasticity. Front Psychol 2022, 13, 831819. [Google Scholar] [CrossRef]
- Magzal, F.; Turroni, S.; Fabbrini, M.; Barone, M.; Vitman Schorr, A.; Ofran, A.; Tamir, S. A personalized diet intervention improves depression symptoms and changes microbiota and metabolite profiles among community-dwelling older adults. Front Nutr 2023, 10, 1234549. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, W.; Zhu, X.; Hu, Z.; Li, S.; Zheng, B.; Xu, H.; Long, W.; Xiong, X. Association between dietary intake and symptoms of depression and anxiety in pregnant women: Evidence from a community-based observational study. Food Sci Nutr 2023, 11, 7555–7564. [Google Scholar] [CrossRef]
- Serrano Ripoll, M.J.; Oliván-Blázquez, B.; Vicens-Pons, E.; Roca, M.; Gili, M.; Leiva, A.; García-Campayo, J.; Demarzo, M.P.; García-Toro, M. Lifestyle change recommendations in major depression: Do they work? J Affect Disord 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.K.; Ho, F.Y.; Yeung, W.F.; Chung, K.F.; Ng, C.H.; Oliver, G.; Sarris, J. Effects of a group-based lifestyle medicine for depression: A pilot randomized controlled trial. PLoS One 2021, 16, e0258059. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; O’Neil, A.; Coulson, C.E.; Schweitzer, I.; Berk, M. Lifestyle medicine for depression. BMC Psychiatry 2014, 14, 107. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom Med 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Dale, E.; Bang-Andersen, B.; Sánchez, C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol 2015, 95, 81–97. [Google Scholar] [CrossRef]
- Fagiolini, A.; Florea, I.; Loft, H.; Christensen, M.C. Effectiveness of Vortioxetine on Emotional Blunting in Patients with Major Depressive Disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord 2021, 283, 472–479. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Wang, J.; Zhou, D.; Liu, A.; Sun, Y.; Yuan, Y.; Li, J.; Guo, W. The pharmacological mechanism of chaihu-jia-longgu-muli-tang for treating depression: integrated meta-analysis and network pharmacology analysis. Front Pharmacol 2023, 14, 1257617. [Google Scholar] [CrossRef] [PubMed]
- Zhichao, H.; Ching, L.W.; Huijuan, L.; Liang, Y.; Zhiyu, W.; Weiyang, H.; Zhaoxiang, B.; Linda, Z.L.D. A network meta-analysis on the effectiveness and safety of acupuncture in treating patients with major depressive disorder. Sci Rep 2021, 11, 10384. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Owens, D.K.; Etxeandia-Ikobaltzeta, I.; Tufte, J.E.; Cross, J.T.; Wilt, T.J.; Physicians, C.G.C.f.t.A.C.o. Nonpharmacologic and Pharmacologic Treatments of Adults in the Acute Phase of Major Depressive Disorder: A Living Clinical Guideline From the American College of Physicians (Version 1, Update Alert 2). Ann Intern Med 2024. [Google Scholar] [CrossRef]
- Direito, R.; Barbalho, S.M.; Sepodes, B.; Figueira, M.E. Plant-Derived Bioactive Compounds: Exploring Neuroprotective, Metabolic, and Hepatoprotective Effects for Health Promotion and Disease Prevention. Pharmaceutics 2024, 16. [Google Scholar] [CrossRef]
- Marx, W.; Manger, S.H.; Blencowe, M.; Murray, G.; Ho, F.Y.; Lawn, S.; Blumenthal, J.A.; Schuch, F.; Stubbs, B.; Ruusunen, A.; et al. Clinical guidelines for the use of lifestyle-based mental health care in major depressive disorder: World Federation of Societies for Biological Psychiatry (WFSBP) and Australasian Society of Lifestyle Medicine (ASLM) taskforce. World J Biol Psychiatry 2023, 24, 333–386. [Google Scholar] [CrossRef]
- Smith, R.D.; Dang, W.; Shen, S.; Hung, S.C.; Lam, I.H.; Kwok, J.Y.Y.; Choi, E.P.H.; Fong, D.Y.T.; Ali, S.; Wilson, C.A.; et al. Comparative effectiveness of interventions for the prevention and treatment of perinatal depression: A systematic review and network meta-analysis. Asian J Psychiatr 2025, 103, 104316. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr Dis Treat 2020, 16, 221–234. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Filteau, M.J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 2014, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Raedt, R.; Vanderhasselt, M.A.; Baeken, C. Neurostimulation as an intervention for treatment resistant depression: From research on mechanisms towards targeted neurocognitive strategies. Clin Psychol Rev 2015, 41, 61–69. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Pariante, C.M.; Cattaneo, A.; Sforzini, L. Understanding treatment-resistant depression using “omics” techniques: A systematic review. J Affect Disord 2022, 318, 423–455. [Google Scholar] [CrossRef]
- Bayes, A.; Parker, G. How to choose an antidepressant medication. Acta Psychiatr Scand 2019, 139, 280–291. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 2007, 151, 737–748. [Google Scholar] [CrossRef]
- Arakawa, R.; Stenkrona, P.; Takano, A.; Svensson, J.; Andersson, M.; Nag, S.; Asami, Y.; Hirano, Y.; Halldin, C.; Lundberg, J. Venlafaxine ER Blocks the Norepinephrine Transporter in the Brain of Patients with Major Depressive Disorder: a PET Study Using [18F]FMeNER-D2. Int J Neuropsychopharmacol 2019, 22, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Settle, E.C., Jr. Antidepressant drugs: disturbing and potentially dangerous adverse effects. J Clin Psychiatry 1998, 59 Suppl 16, 25–30, discussion 40-22. [Google Scholar]
- Teply, R.M.; Packard, K.A.; White, N.D.; Hilleman, D.E.; DiNicolantonio, J.J. Treatment of Depression in Patients with Concomitant Cardiac Disease. Prog Cardiovasc Dis 2016, 58, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Han, C.; Bahk, W.M.; Lee, S.J.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Addressing the Side Effects of Contemporary Antidepressant Drugs: A Comprehensive Review. Chonnam Med J 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of action of antidepressant medications. J Clin Psychiatry 1999, 60 Suppl 4, 4–11, discussion 12-13. [Google Scholar]
- Zhou, S.; Li, P.; Lv, X.; Lai, X.; Liu, Z.; Zhou, J.; Liu, F.; Tao, Y.; Zhang, M.; Yu, X.; et al. Adverse effects of 21 antidepressants on sleep during acute-phase treatment in major depressive disorder: a systemic review and dose-effect network meta-analysis. Sleep 2023, 46. [Google Scholar] [CrossRef]
- Muir, K.W.; Lees, K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995, 26, 503–513. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother Psychosom 2016, 85, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: a systematic review and network meta-analysis. Mol Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr Psychiatry Rep 2017, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Beach, S.R.; Kostis, W.J.; Celano, C.M.; Januzzi, J.L.; Ruskin, J.N.; Noseworthy, P.A.; Huffman, J.C. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J Clin Psychiatry 2014, 75, e441–449. [Google Scholar] [CrossRef]
- Gheysens, T.; Van Den Eede, F.; De Picker, L. The risk of antidepressant-induced hyponatremia: A meta-analysis of antidepressant classes and compounds. Eur Psychiatry 2024, 67, e20. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W. Reminiscence therapy-based care program alleviates anxiety and depression, as well as improves the quality of life in recurrent gastric cancer patients. Front Psychol 2023, 14, 1133470. [Google Scholar] [CrossRef]
- Yeung, W.F.; Chung, K.F.; Ng, K.Y.; Yu, Y.M.; Ziea, E.T.; Ng, B.F. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J Psychiatr Res 2014, 57, 165–175. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phytother Res 2018, 32, 1147–1162. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Carvalho, G.M.; de Oliveira Zanuso, B.; Figueira, M.E.; Direito, R.; de Alvares Goulart, R.; Buglio, D.S.; Barbalho, S.M. Curcumin-Based Nanomedicines in the Treatment of Inflammatory and Immunomodulated Diseases: An Evidence-Based Comprehensive Review. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.; Direito, R.; Goulart, R.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Liu, L.Y.; Feng, B.; Chen, J.; Tan, Q.R.; Chen, Z.X.; Chen, W.S.; Wang, P.R.; Zhang, Z.J. Herbal medicine for hospitalized patients with severe depressive episode: a retrospective controlled study. J Affect Disord 2015, 170, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Yang, Z.; Huang, X.J.; Li, J.; Bao, W.Y.; Hurilebagen; Wulanqiqige; Wuyunsiriguleng; Cui, J.W.; Ma, L.Q.; et al. Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway. J Ethnopharmacol 2022, 293, 115310. [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Singh, P.K.; Chopra, R.; Kumari, A.; Mittal, A. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon 2023, 9, e14932. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Q. A Review of Antidepressant Effects and Mechanisms of Three Common Herbal Medicines: Panax ginseng, Bupleurum chinense, and Gastrodia elata. CNS Neurol Disord Drug Targets 2023, 22, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, T. Targeting autophagy and neuroinflammation pathways with plant-derived natural compounds as potential antidepressant agents. Phytother Res 2022, 36, 3470–3489. [Google Scholar] [CrossRef]
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals That Act on Synaptic Plasticity as Potential Prophylaxis against Stress-Induced Depressive Disorder. Biomol Ther (Seoul) 2023, 31, 148–160. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Xi, Y.; Zhou, H.; Tang, Z.; Xiong, L.; Qin, D. Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals (Basel) 2024, 17. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Li, Z.; Li, T.; Zhang, R.; Zhao, Y.; Cheng, T.; Zong, Z.; Ma, Y.; Zhang, D.; et al. Flavonoid 4,4’-dimethoxychalcone selectively eliminates senescent cells via activating ferritinophagy. Redox Biol 2024, 69, 103017. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother Res 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int J Mol Sci 2025, 26. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J Med Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Costa, R.O.; Martins, L.F.; Tahiri, E.; Duarte, C.B. Brain-derived neurotrophic factor-induced regulation of RNA metabolism in neuronal development and synaptic plasticity. Wiley Interdiscip Rev RNA 2022, 13, e1713. [Google Scholar] [CrossRef] [PubMed]
- Gadad, B.S.; Vargas-Medrano, J.; Ramos, E.I.; Najera, K.; Fagan, M.; Forero, A.; Thompson, P.M. Altered levels of interleukins and neurotrophic growth factors in mood disorders and suicidality: an analysis from periphery to central nervous system. Transl Psychiatry 2021, 11, 341. [Google Scholar] [CrossRef]
- Afzal, A.; Batool, Z.; Sadir, S.; Liaquat, L.; Shahzad, S.; Tabassum, S.; Ahmad, S.; Kamil, N.; Perveen, T.; Haider, S. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem Res 2021, 46, 3273–3285. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur J Pharmacol 2014, 745, 59–68. [Google Scholar] [CrossRef]
- Hassan, S.S.U.; Samanta, S.; Dash, R.; Karpiński, T.M.; Habibi, E.; Sadiq, A.; Ahmadi, A.; Bunagu, S. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: Focus on the role of oxidative stress. Front Pharmacol 2022, 13, 1015835. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J Nutr Biochem 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Idayu, N.F.; Hidayat, M.T.; Moklas, M.A.; Sharida, F.; Raudzah, A.R.; Shamima, A.R.; Apryani, E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine 2011, 18, 402–407. [Google Scholar] [CrossRef]
- Ahmad, I.; Prabowo, W.C.; Arifuddin, M.; Fadraersada, J.; Indriyanti, N.; Herman, H.; Purwoko, R.Y.; Nainu, F.; Rahmadi, A.; Paramita, S.; et al. Species as Pharmacological Agents: From Abuse to Promising Pharmaceutical Products. Life (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Chen, L.; Fei, S.; Olatunji, O.J. LC/ESI/TOF-MS Characterization, Anxiolytic and Antidepressant-like Effects of. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr Neuropharmacol 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and Neuroprotective Effects of Hericium erinaceus. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Zanuso, B.; de Oliveira Dos Santos, A.R.; Miola, V.F.B.; Guissoni Campos, L.M.; Spilla, C.S.G.; Barbalho, S.M. Panax ginseng and aging related disorders: A systematic review. Exp Gerontol 2022, 161, 111731. [Google Scholar] [CrossRef]
- Hou, Y.; Peng, S.; Li, X.; Yao, J.; Xu, J.; Fang, J. Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. ACS chemical neuroscience 2018, 9, 3108–3116. [Google Scholar] [CrossRef]
- Wang, D.; Cao, L.; Zhou, X.; Wang, G.; Ma, Y.; Hao, X.; Fan, H. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. Journal of hazardous materials 2022, 437, 129381. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, N. Berberine: Pathways to protect neurons. Phytotherapy Research 2018, 32, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, Z.; Zhu, L.; Zhou, J.; Zhuo, L.; Zhang, J.; Zhang, X.; Liu, W.; Han, L.; Liao, W. Rhynchophylline alleviates neuroinflammation and regulates metabolic disorders in a mouse model of Parkinson’s disease. Food & Function 2023, 14, 3208–3219. [Google Scholar]
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr Pharm Des 2019, 25, 738–745. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic Effects of Phytochemicals and Medicinal Herbs on Depression. Biomed Res Int 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Manosso, L.M.; Arent, C.O.; Borba, L.A.; Abelaira, H.M.; Réus, G.Z. Natural Phytochemicals for the Treatment of Major Depressive Disorder: A Mini-Review of Pre-and Clinical Studies. CNS & Neurological Disorders-Drug Targets-CNS & Neurological Disorders) 2023, 22, 237–254. [Google Scholar]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X. Therapeutic potential of plant iridoids in depression: a review. Pharmaceutical Biology 2022, 60, 2167–2181. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, B.; Wu, P.-F.; Tian, J.; Shi, L.-L.; Gu, J.; Hu, Z.-L.; Fu, H.; Wang, F.; Chen, J.-G. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biological and Pharmaceutical Bulletin 2011, 34, 253–259. [Google Scholar] [CrossRef]
- Gupta, J.; Gupta, R.; Varshney, K.K. Emerging mechanisms and potential antidepressant action of medicinal plants. Int J Pharm Sci Res 2020, 11, 1–3. [Google Scholar]
- Picheta, N.; Piekarz, J.; Daniłowska, K.; Mazur, K.; Piecewicz-Szczęsna, H.; Smoleń, A. Phytochemicals in the treatment of patients with depression: a systemic review. Frontiers in Psychiatry 2024, 15, 1509109. [Google Scholar] [CrossRef]
- Soltani, M.; Hosseinzadeh-Attar, M.J.; Rezaei, M.; Alipoor, E.; Vasheghani-Farahani, A.; Yaseri, M.; Rezayat, S.M. Effect of nano-curcumin supplementation on cardiometabolic risk factors, physical and psychological quality of life, and depression in patients with coronary slow flow phenomenon: a randomized double-blind clinical trial. Trials 2024, 25, 515. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Vafa, M.; Ebrahimkhani, A.; Găman, M.A.; Sezavar Seyedi Jandaghi, S.H. Effects of quercetin supplementation on endothelial dysfunction biomarkers and depression in post-myocardial infarction patients: A double-blind, placebo-controlled, randomized clinical trial. Clin Nutr ESPEN 2023, 56, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: a randomized, double-blind, placebo-controlled study. Arch Gynecol Obstet 2016, 293, 609–615. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.H.; Park, M.; Lee, H.J. Effects of Flavonoid-Rich Orange Juice Intervention on Major Depressive Disorder in Young Adults: A Randomized Controlled Trial. Nutrients 2022, 15. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Honmou, O.; Sasaki, M.; Haseda, A.; Kagami-Katsuyama, H.; Shoji, T.; Namioka, A.; Namioka, T.; Magota, H.; Oka, S.; et al. The Impact of Purple-Flesh Potato. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Barfoot, K.L.; Forster, R.; Lamport, D.J. Mental Health in New Mothers: A Randomised Controlled Study into the Effects of Dietary Flavonoids on Mood and Perceived Quality of Life. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clinical nutrition (Edinburgh, Scotland) 2021, 40, 5556–5567. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Ostan, R.; Regueme, S.C.; Pinto, A.; Pryen, F.; Charrouf, Z.; d’Alessio, P.; Baudron, C.R.; Guerville, F.; Durrieu, J.; et al. Quality of Life: Psychological Symptoms-Effects of a 2-Month Healthy Diet and Nutraceutical Intervention; A Randomized, Open-Label Intervention Trial (RISTOMED). Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Smetanka, A.; Stara, V.; Farsky, I.; Tonhajzerova, I.; Ondrejka, I. Pycnogenol supplementation as an adjunct treatment for antidepressant-induced sexual dysfunction. Physiol Int 2019, 106, 59–69. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox Res 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Khalid, S.; Barfoot, K.L.; May, G.; Lamport, D.J.; Reynolds, S.A.; Williams, C.M. Effects of Acute Blueberry Flavonoids on Mood in Children and Young Adults. Nutrients 2017, 9. [Google Scholar] [CrossRef]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin J Integr Med 2015, 21, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: a randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Beckett, S.; Rigby, A.S.; Mellor, D.D.; Atkin, S.L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutrition journal 2010, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Malau, I.A.; Chang, J.P.-C.; Lin, Y.-W.; Chang, C.-C.; Chiu, W.-C.; Su, K.-P. Omega-3 Fatty Acids and Neuroinflammation in Depression: Targeting Damage-Associated Molecular Patterns and Neural Biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Wang, J.; Behl, T.; Rana, T.; Sehgal, A.; Wal, P.; Saxena, B.; Yadav, S.; Mohan, S.; Anwer, M.K.; Chigurupati, S. Exploring the pathophysiological influence of heme oxygenase-1 on neuroinflammation and depression: A study of phytotherapeutic-based modulation. Phytomedicine 2024, 155466. [Google Scholar] [CrossRef]
- Haase, J.; Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression—a central role for the serotonin transporter? Pharmacology & therapeutics 2015, 147, 1–11. [Google Scholar]
- Halaris, A. Inflammation and depression but where does the inflammation come from? Current opinion in psychiatry 2019, 32, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, J.; Chung, S.K.; Xu, B. New insights into anti-depression effects of bioactive phytochemicals. Pharmacological Research 2024, 107566. [Google Scholar] [CrossRef]
- Yoon, S.; Iqbal, H.; Kim, S.M.; Jin, M. Phytochemicals that act on synaptic plasticity as potential prophylaxis against stress-induced depressive disorder. Biomolecules & Therapeutics 2023, 31, 148. [Google Scholar]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Natural polyphenols and terpenoids for depression treatment: Current status. Studies in natural products chemistry 2018, 55, 181–221. [Google Scholar]
- Shi, X.; Fu, Y.; Zhang, S.; Ding, H.; Chen, J. Baicalin attenuates subarachnoid hemorrhagic brain injury by modulating blood-brain barrier disruption, inflammation, and oxidative damage in mice. Oxidative Medicine and Cellular Longevity 2017, 2017, 1401790. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundamental & Clinical Pharmacology 2023, 37, 1050–1064. [Google Scholar]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [CrossRef]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals 2014, 7, 913–942. [Google Scholar] [CrossRef]
- Mu, D.; Ma, Q. A review of antidepressant effects and mechanisms of three common herbal medicines: Panax ginseng, Bupleurum chinense, and Gastrodia elata. CNS & Neurological Disorders-Drug Targets-CNS & Neurological Disorders) 2023, 22, 1164–1175. [Google Scholar]
- Bozzuto, G.; Calcabrini, A.; Colone, M.; Condello, M.; Dupuis, M.L.; Pellegrini, E.; Stringaro, A. Phytocompounds and nanoformulations for anticancer therapy: A review. Molecules 2024, 29, 3784. [Google Scholar] [CrossRef]
- Aburel, O.M.; Pavel, I.Z.; Dănilă, M.D.; Lelcu, T.; Roi, A.; Lighezan, R.; Muntean, D.M.; Rusu, L.C. Pleiotropic effects of eugenol: The good, the bad, and the unknown. Oxidative medicine and cellular longevity 2021, 2021, 3165159. [Google Scholar] [CrossRef]
- Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting multiple pathways by naturally occurring phytochemicals. Biomedicines 2020, 8, 284. [Google Scholar] [CrossRef]
- Lippert, A.; Renner, B. Herb–drug interaction in inflammatory diseases: Review of phytomedicine and herbal supplements. Journal of clinical medicine 2022, 11, 1567. [Google Scholar] [CrossRef]
- Tayeb, B.A.; Kusuma, I.Y.; Osman, A.A.; Minorics, R. Herbal compounds as promising therapeutic agents in precision medicine strategies for cancer: A systematic review. Journal of Integrative Medicine 2024. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed research international 2017, 2017, 6596241. [Google Scholar] [CrossRef] [PubMed]
- Dobrek, L.; Głowacka, K. Depression and its phytopharmacotherapy—a narrative review. International Journal of Molecular Sciences 2023, 24, 4772. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rashid, M.; Chaturvedi, S.; Agarwal, A.; Chauhan, D.; Gayen, J.R.; Wahajuddin, M. Preclinical pharmacokinetics, absolute bioavailability and dose proportionality evaluation of bioactive phytochemical Withanone in rats. Bioorganic Chemistry 2025, 155, 108128. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. International journal of nanomedicine 2021, 6983–7022. [Google Scholar] [CrossRef]
- Gouws, C.; Hamman, J.H. What are the dangers of drug interactions with herbal medicines? Expert opinion on drug metabolism & toxicology 2020, 16, 165–167. [Google Scholar]
- Holleran, G.; Scaldaferri, F.; Gasbarrini, A.; Currò, D. Herbal medicinal products for inflammatory bowel disease: A focus on those assessed in double-blind randomised controlled trials. Phytother Res 2020, 34, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; McMahon, F.J. Pharmacogenetics of antidepressants, mood stabilizers, and antipsychotics in diverse human populations. Discov Med 2013, 16, 113–122. [Google Scholar]
- Zhao, R.; Wang, J.; Chung, S.K.; Xu, B. New insights into anti-depression effects of bioactive phytochemicals. Pharmacol Res 2025, 212, 107566. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr Neurosci 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Magni, G.; Riboldi, B.; Petroni, K.; Ceruti, S. Flavonoids bridging the gut and the brain: Intestinal metabolic fate, and direct or indirect effects of natural supporters against neuroinflammation and neurodegeneration. Biochem Pharmacol 2022, 205, 115257. [Google Scholar] [CrossRef]
- Coen, M.; Want, E.J.; Clayton, T.A.; Rhode, C.M.; Hong, Y.S.; Keun, H.C.; Cantor, G.H.; Metz, A.L.; Robertson, D.G.; Reily, M.D.; et al. Mechanistic aspects and novel biomarkers of responder and non-responder phenotypes in galactosamine-induced hepatitis. J Proteome Res 2009, 8, 5175–5187. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat Rev Genet 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Almeida, S.S.; Zizzi, F.B.; Cattaneo, A.; Comandini, A.; Di Dato, G.; Lubrano, E.; Pellicano, C.; Spallone, V.; Tongiani, S.; Torta, R. Management and Treatment of Patients With Major Depressive Disorder and Chronic Diseases: A Multidisciplinary Approach. Front Psychol 2020, 11, 542444. [Google Scholar] [CrossRef]
- Pepe, M.; Bartolucci, G.; Marcelli, I.; Pesaresi, F.; Brugnami, A.; Caso, R.; Fischetti, A.; Grisoni, F.; Mazza, M.; Camardese, G.; et al. The Patient’s Perspective on the Effects of Intranasal Esketamine in Treatment-Resistant Depression. Brain Sci 2023, 13. [Google Scholar] [CrossRef]
- Stolfi, F.; Abreu, H.; Sinella, R.; Nembrini, S.; Centonze, S.; Landra, V.; Brasso, C.; Cappellano, G.; Rocca, P.; Chiocchetti, A. Omics approaches open new horizons in major depressive disorder: from biomarkers to precision medicine. Front Psychiatry 2024, 15, 1422939. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, T.; Liu, J.; Gao, C.; Zhang, X. Physical exercise and mitochondrial function: New therapeutic interventions for psychiatric and neurodegenerative disorders. Front Neurol 2022, 13, 929781. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Holtzheimer, P.E.; Gao, S.; Kirwin, D.S.; Price, R.B. Leveraging Neuroplasticity to Enhance Adaptive Learning: The Potential for Synergistic Somatic-Behavioral Treatment Combinations to Improve Clinical Outcomes in Depression. Biol Psychiatry 2019, 85, 454–465. [Google Scholar] [CrossRef]
- Ashdown-Franks, G.; Firth, J.; Carney, R.; Carvalho, A.F.; Hallgren, M.; Koyanagi, A.; Rosenbaum, S.; Schuch, F.B.; Smith, L.; Solmi, M.; et al. Exercise as Medicine for Mental and Substance Use Disorders: A Meta-review of the Benefits for Neuropsychiatric and Cognitive Outcomes. Sports Med 2020, 50, 151–170. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch Gerontol Geriatr 2023, 106, 104855. [Google Scholar] [CrossRef]
- Gamage, E.; Orr, R.; Travica, N.; Lane, M.M.; Dissanayaka, T.; Kim, J.H.; Grosso, G.; Godos, J.; Marx, W. Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neurosci Biobehav Rev 2023, 151, 105225. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Garg, M.; Prajapati, P.; Singh, P.K.; Chopra, R.; Kumari, A.; Mittal, A. Adaptogenic property of Asparagus racemosus: Future trends and prospects. Heliyon 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L.; Giménez-Llort, L. Emerging translational research in neurological and psychiatric diseases: from in vitro to in vivo models. 2023, 24, 15739. [Google Scholar]
- Kessler, R.C.; van Loo, H.M.; Wardenaar, K.J.; Bossarte, R.M.; Brenner, L.; Ebert, D.; De Jonge, P.; Nierenberg, A.; Rosellini, A.; Sampson, N. Using patient self-reports to study heterogeneity of treatment effects in major depressive disorder. Epidemiology and psychiatric sciences 2017, 26, 22–36. [Google Scholar] [CrossRef]
- Malgaroli, M.; Calderon, A.; Bonanno, G.A. Networks of major depressive disorder: A systematic review. Clinical Psychology Review 2021, 85, 102000. [Google Scholar] [CrossRef]
- Mew, E.J.; Monsour, A.; Saeed, L.; Santos, L.; Patel, S.; Courtney, D.B.; Watson, P.N.; Szatmari, P.; Offringa, M.; Monga, S. Systematic scoping review identifies heterogeneity in outcomes measured in adolescent depression clinical trials. Journal of Clinical Epidemiology 2020, 126, 71–79. [Google Scholar] [CrossRef] [PubMed]
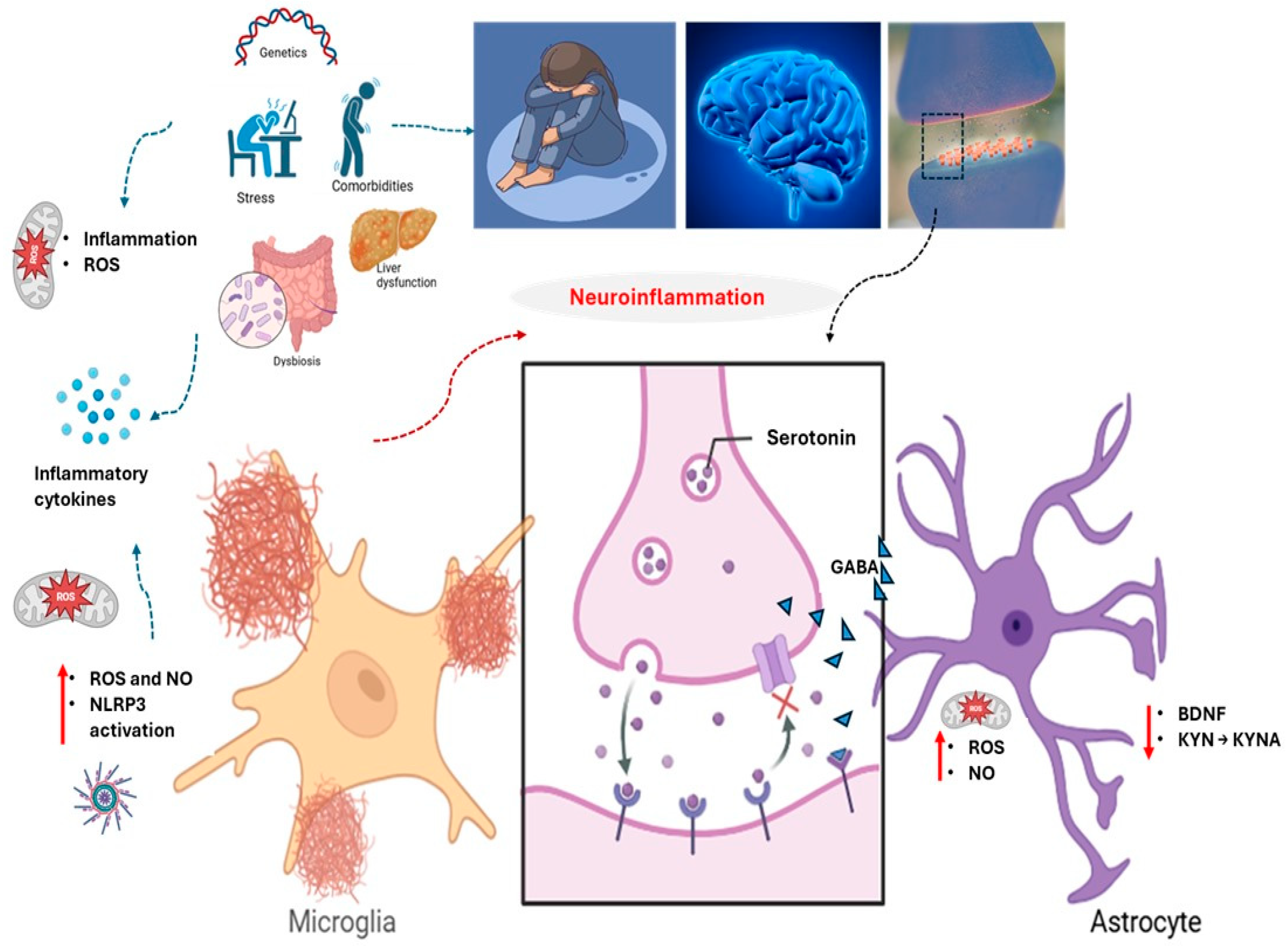
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





