Submitted:
14 April 2025
Posted:
15 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Photosynthesis and Respiration
3. Results
3.1. Photosynthesis and Respiration
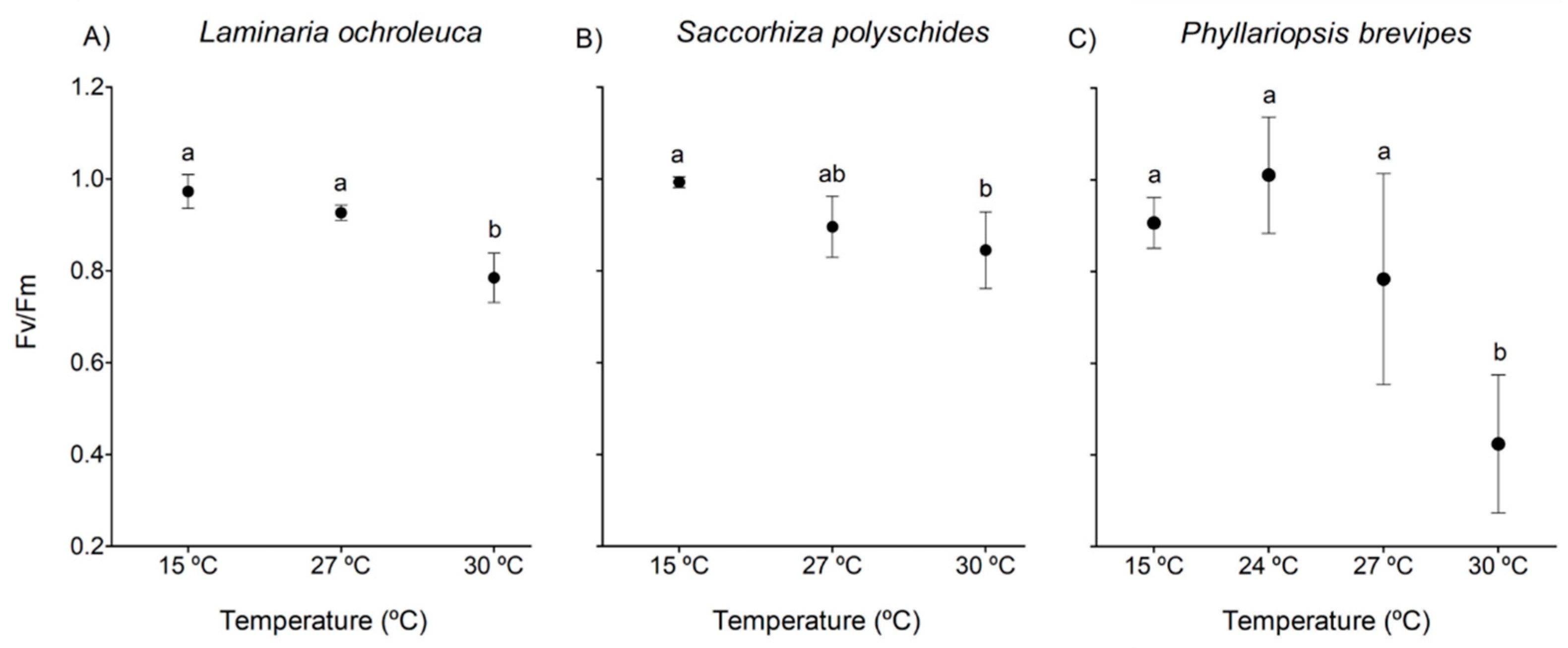
3.2. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Dry Mass |
| Fv/Fm | Maximum quantum yield of photosystem II |
| LMax | Photon fluence rate at which maximum production was attained |
| NPP | Net Primary Production |
| RMax | Maximum respiration rate |
References
- Wernberg, T.; Krumhansl, K.A.; Filbee-Dexter, K.; Pedersen, M. Status and Trends for the World’s Kelp Forests. 2019.
- Jayathilake, D.R.M.; Costello, M.J. A modelled global distribution of the kelp biome. Biological Conservation 2020, 252, 108815. [Google Scholar] [CrossRef]
- Dayton, P. Ecology of Kelp Communities. Annual Review of Ecology, Evolution, and Systematics 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, I.; Vogt, J.; Pehlke, C.; Hanelt, D. Prevailing sea surface temperatures inhibit summer reproduction of the kelp aminaria digitata at Helgoland (North Sea). Journal of Phycology 2013, 49, 1061–1073. [Google Scholar] [CrossRef]
- Markager, S.; Sand-Jensen, K. Light requirements and depth zonation of marine macroalgae. Marine Ecology Progress Series 1992, 88, 83–92. [Google Scholar] [CrossRef]
- Dieter, H.; Melchersmann, B.; Wiencke, C.; Nultsch, W. Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Marine Ecology-progress Series - MAR ECOL-PROGR SER 1997, 149, 255–266. [Google Scholar] [CrossRef]
- Franco, J.N.; Tuya, F.; Bertocci, I.; Rodríguez, L.; Martínez, B.; Sousa-Pinto, I.; Arenas, F. The ‘golden kelp’ Laminaria ochroleuca under global change: Integrating multiple eco-physiological responses with species distribution models. Journal of Ecology 2018, 106, 47–58. [Google Scholar] [CrossRef]
- Franco, J.N.; Wernberg, T.; Bertocci, I.; Duarte, P.; Jacinto, D.; Vasco-Rodrigues, N.; Tuya, F. Herbivory drives kelp recruits into ’hiding’ in a warm ocean climate. Marine Ecology Progress Series 2015, 536, 1–9. [Google Scholar] [CrossRef]
- Schoenrock, K.; O’Callaghan, T.; O’Callaghan, R.; Krueger-Hadfield, S. First record of Laminaria ochroleuca Bachelot de la Pylaie in Ireland in Béal an Mhuirthead, county Mayo. Marine Biodiversity Records 2019, 12. [Google Scholar] [CrossRef]
- Lüning, K.; Yarish, C.; Kirkman, H. Seaweeds: Their Environment, Biogeography, and Ecophysiology. 1990.
- Birkett, D.; Maggs, C.; Dring, M.; Boaden, P. An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs. Scott. Assoc. Mar. Sci. (SAMS) 1998, 5. [Google Scholar]
- Henry, E. The life history of Phyllariopsis brevipes (= Phyllaria reniformis) (Phyllariaceae, Laminariales, Phaeophyceae), a kelp with dioecious but sexually monomorphic gametophytes. Phycologia 1987, 26, 17–22. [Google Scholar] [CrossRef]
- Dieck, I. Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta) - Ecological and biogeographical implications. Marine Ecology Progress Series 1993, 100, 253–253. [Google Scholar] [CrossRef]
- Pereira, T.R.; Engelen, A.H.; Pearson, G.A.; Serrão, E.A.; Destombe, C.; Valero, M. Temperature effects on the microscopic haploid stage development of Laminaria ochroleuca and Sacchoriza polyschides, kelps with contrasting life histories. Cahiers De Biologie Marine 2011, 52, 395–403. [Google Scholar]
- García-Sánchez, M.; Delgado Huertas, A.; Fernández, J.; Flores-Moya, A. Photosynthetic use of inorganic carbon in deep-water kelps from the Strait of Gibraltar. Photosynthesis Research 2016, 2016, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Tempera, F.; Milla-Figueras, D.; Sinde-Mano, A.L.; Atchoi, E.; Afonso, P. Range Extension of Mesophotic Kelps (Ochrophyta: Laminariales and Tilopteridales) in the Central North Atlantic: Opportunities for Marine Forest Research and Conservation. Journal of Phycology 2021, 57, 1140–1150. [Google Scholar] [CrossRef]
- Pearson, G.; Lago-Leston, A.; Mota, C. Frayed at the edges: Selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. Journal of Ecology 2009, 97, 450–462. [Google Scholar] [CrossRef]
- Dieck, I.t. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 1992, 31, 147–163. [Google Scholar] [CrossRef]
- Biškup, S.; Bertocci, I.; Arenas, F.; Tuya, F. Functional responses of juvenile kelps, Laminaria ochroleuca and Saccorhiza polyschides, to increasing temperatures. Aquatic Botany 2014, 113, 117–122. [Google Scholar] [CrossRef]
- Pereira, T.; Engelen, A.; Pearson, G.; Valero, M.; Serrao, E. Response of kelps from different latitudes to consecutive heat shock. Journal of Experimental Marine Biology and Ecology 2015, 463, 57–62. [Google Scholar] [CrossRef]
- King, N.; Leathers, T.; Smith, K.; Smale, D. The influence of pre-exposure to marine heatwaves on the critical thermal maxima (CTmax) of marine foundation species. Functional Ecology 2024. [Google Scholar] [CrossRef]
- Leathers, T.; King, N.G.; Foggo, A.; Smale, D.A. Marine heatwave duration and intensity interact to reduce physiological tipping points of kelp species with contrasting thermal affinities. Ann Bot 2024, 133, 51–60. [Google Scholar] [CrossRef]
- Strasser, F.-E.; Barreto, L.M.; Kaidi, S.; Sabour, B.; Serrão, E.A.; Pearson, G.A.; Martins, N. Population level variation in reproductive development and output in the golden kelp Laminaria ochroleuca under marine heat wave scenarios. In Proceedings of the Frontiers in Marine Science; 2022. [Google Scholar]
- Izquierdo, J.; Pérez-ruzafa, I.; Gallardo, T. Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgoland Marine Research 2001, 55, 285–292. [Google Scholar] [CrossRef]
- Smale, D.; Wernberg, T.; Yunnie, A.L.E.; Vance, T. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Marine Ecology 2015, 36, 1033–1044. [Google Scholar] [CrossRef]
- Smale, D.A.; Moore, P.J. Variability in kelp forest structure along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology 2017, 486, 255–264. [Google Scholar] [CrossRef]
- Norton, T.A. Experiments on the Factors Influencing the Geographical Distributions of Saccorhiza polyschides and Saccorhiza dermatodea. The New Phytologist 1977, 78, 625–635. [Google Scholar] [CrossRef]
- Fernández, C. The retreat of large brown seaweeds on the north coast of Spain: The case of Saccorhiza polyschides. Eur. J. Phycol. 2011, 46, 352–360. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Global Change Biology 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Pereira, T.R.; Engelen, A.H.; Pearson, G.A.; Valero, M.; Serrão, E.A. Population dynamics of temperate kelp forests near their low-latitude limit. Aquatic Botany 2017, 139, 8–18. [Google Scholar] [CrossRef]
- Flores-Moya, A.; A., F.J.; Niell, F.X. Reproductive phenology, growth and primary production of Phyllariopsis purpurascens (Phyllariaceae, Phaeophyta) from the Straits of Gibraltar. European Journal of Phycology 1993, 28, 223–230. [Google Scholar] [CrossRef]
- Lemos, R.; Pires, H. The upwelling regime off the West Portuguese Coast, 1941–2000. International Journal of Climatology 2004, 24, 511–524. [Google Scholar] [CrossRef]
- Gómez-Gesteira, M.; de Castro, M.; Alvarez, I.; Lorenzo, M.N.; Gesteira, J.L.; Crespo, A.J. Spatio-temporal upwelling trends along the Canary Upwelling System (1967-2006). Ann N Y Acad Sci 2008, 1146, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat Commun 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
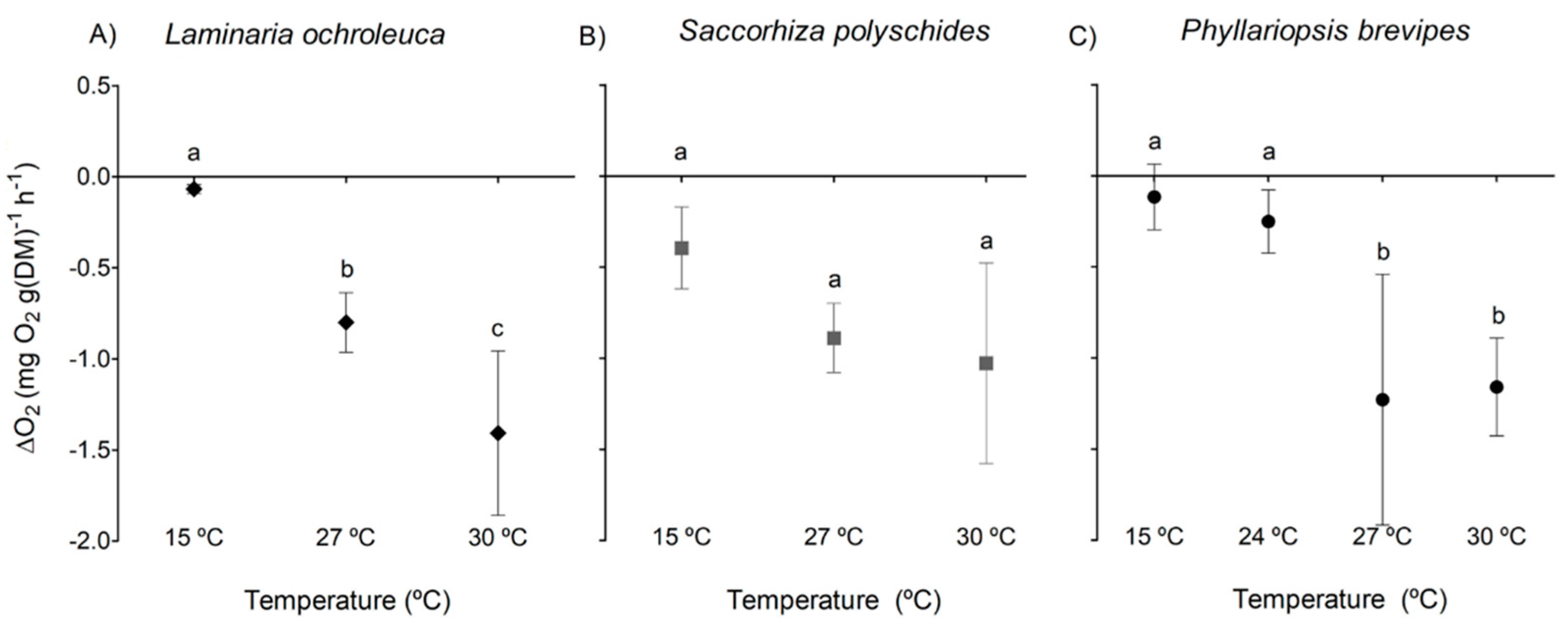
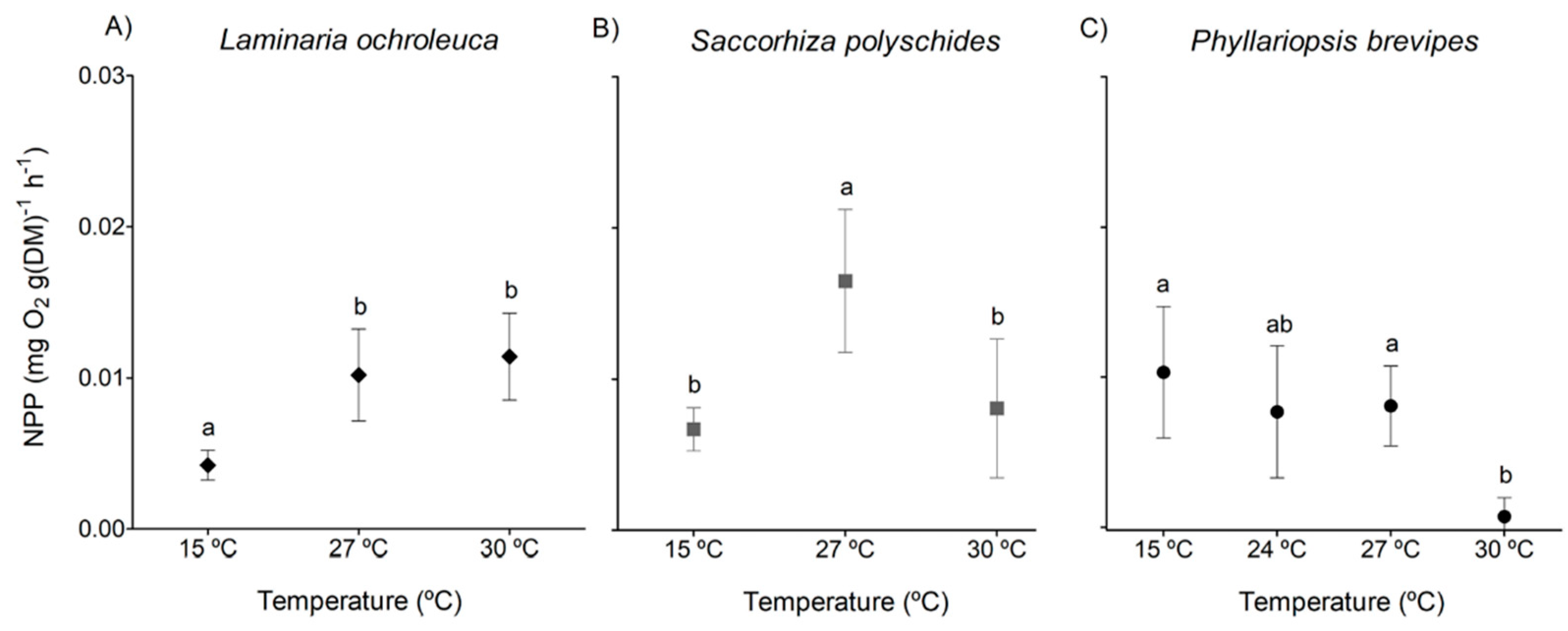
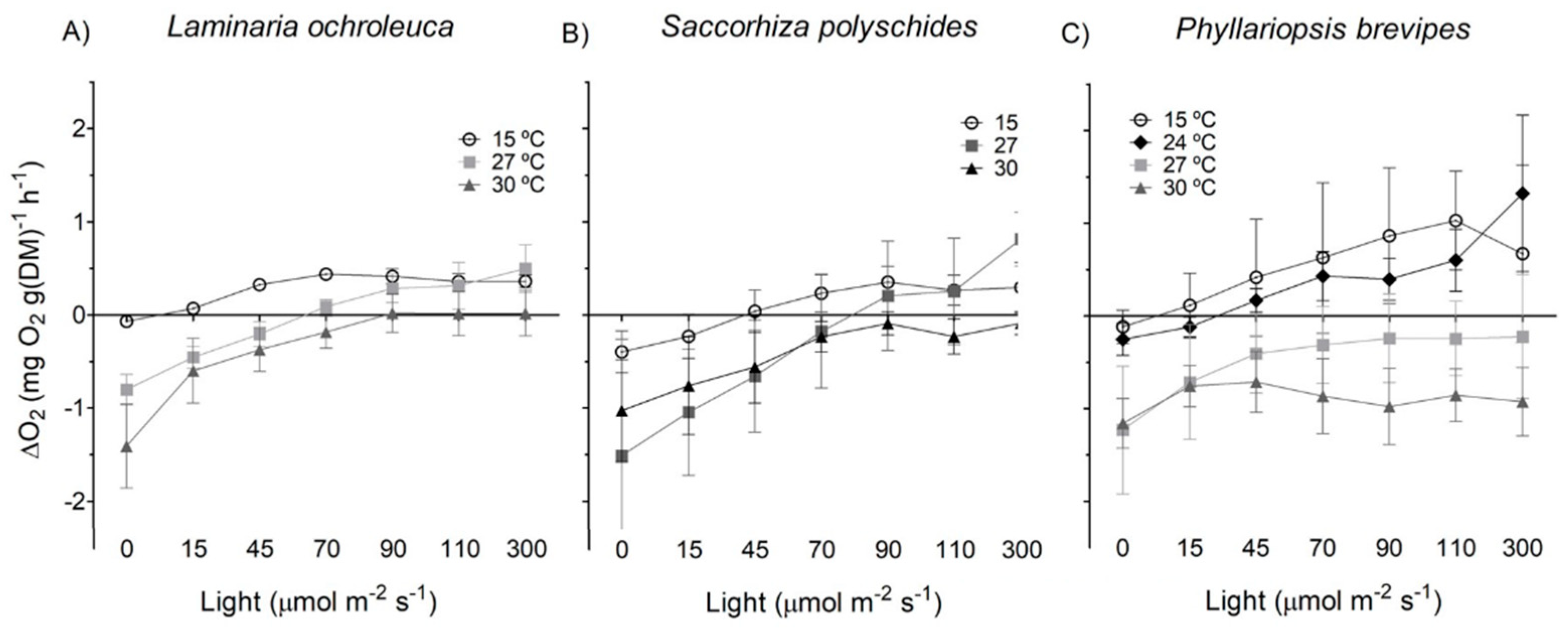
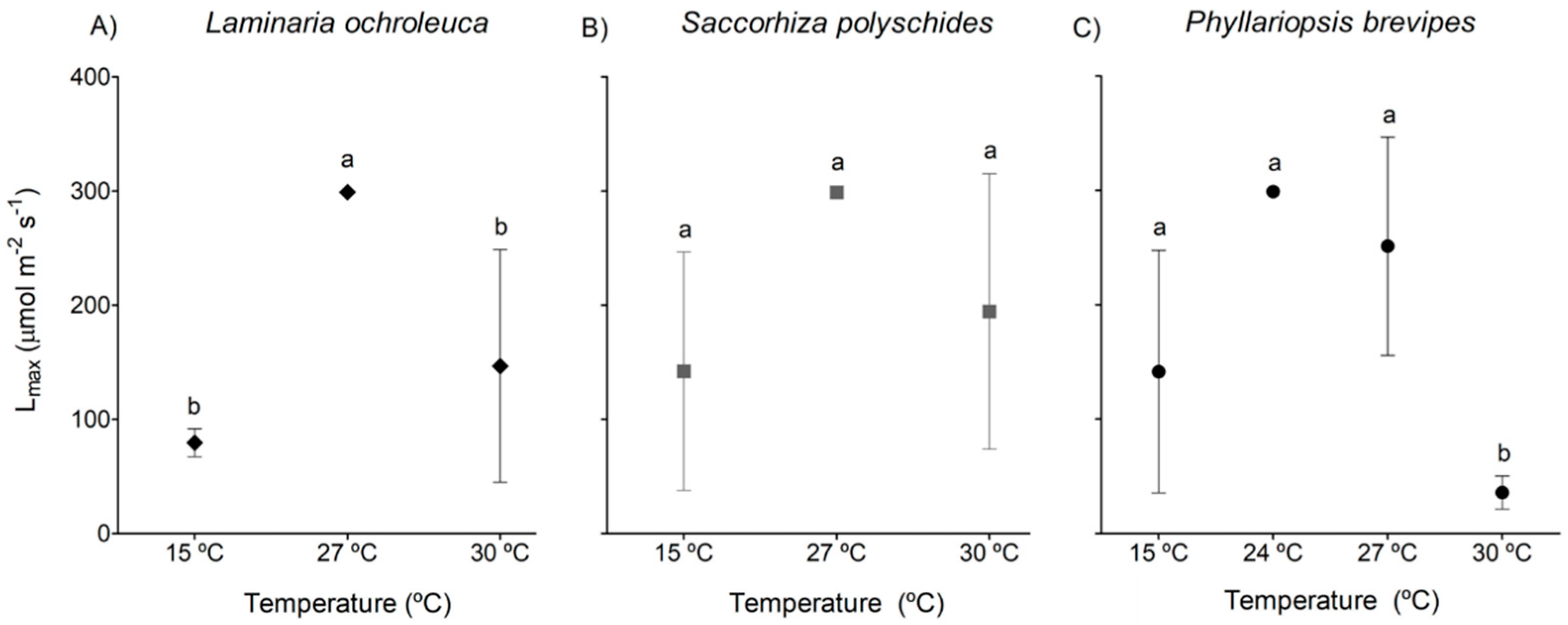
| Species | p-value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.0001 | 23.419 | 2 |
| Saccorhiza polyschides | 0.197 | 1.956 | 2 |
| Phyllariopsis brevipes | 0.002 | 9.051 | 3 |
| Species | p-value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.006 | 9.622 | 2 |
| Saccorhiza polyschides | 0.012 | 7.427 | 2 |
| Phyllariopsis brevipes | 0.011 | 5.861 | 3 |
| Species | p-value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.002 | 14.425 | 2 |
| Saccorhiza polyschides | 0.1 | 3.000 | 2 |
| Phyllariopsis brevipes | 0.001 | 10.743 | 3 |
| Species | p-value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | <0.001 | 25.621 | 2 |
| Saccorhiza polyschides | 0.023 | 5.922 | 2 |
| Phyllariopsis brevipes | 0.001 | 11.010 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





