Submitted:
16 April 2025
Posted:
17 April 2025
You are already at the latest version
Abstract
Keywords:
Introduction
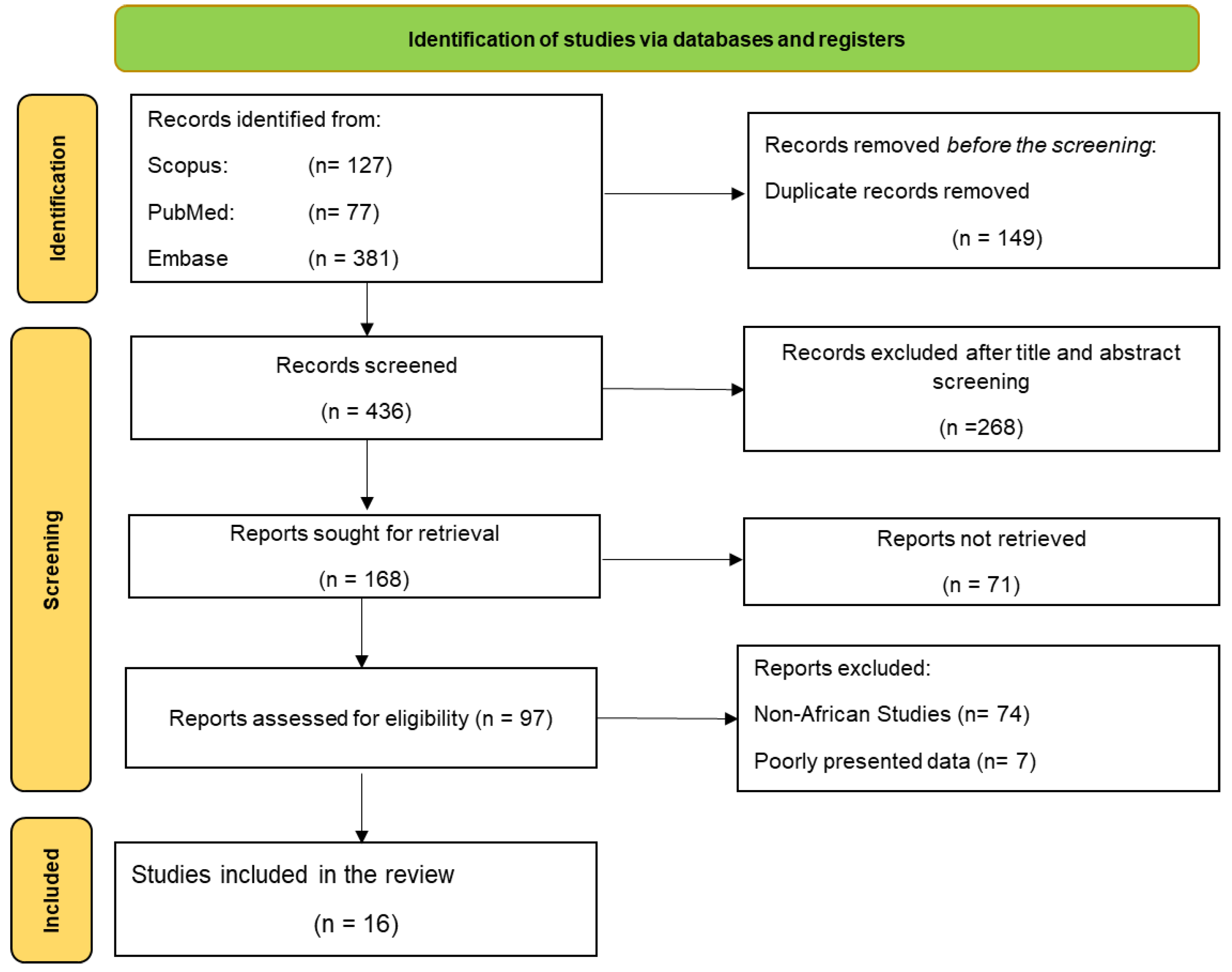
Methods
Search Strategy
Study Selection Criteria
Data Extraction
Data Analysis
Results
Study Selection and Characteristics
Plant Species and Parts Used
Extraction Methods and Solvents
Antimicrobial Activity
Minimum Inhibitory Concentration (MIC)
Bioactive Compounds
Discussion
Extraction Methods and Solvents
Bioactive Compounds and Mechanisms
Implications for Drug Discovery and Antimicrobial Resistance
Limitations and Future Directions
Conclusion
Funding
Data availability
Conflict of interests
References
- Abdella, G., Mirutse, G., Gobena, A., & Adane, W. (2013). In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complementary and Alternative Medicine, 13(291), 1–6. http://www.biomedcentral.com/1472-6882/13/291.
- Adeniyi, B. A., Onwubuche, B. C., Anyiam, F. M., Ekundayo, O., & Mahady, G. B. (2009). Anti-Helicobacter pylori activities of Eucalyptus grandis: Effects on susceptibility, urease activity and cell surface hydrophobicity. Pharmaceutical Biology, 47(1), 13–17. [CrossRef]
- Adeniyi, C. B. A., Lawal, T. O., & Mahady, G. B. (2009). In vitro susceptibility of Helicobacter pylori to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana. Pharmaceutical Biology, 47(1), 99–102. [CrossRef]
- Ahmed, S. K., Hussein, S., Qurbani, K., Ibrahim, R. H., Fareeq, A., Mahmood, K. A., & Mohamed, M. G. (2024). Antimicrobial resistance: Impacts, challenges, and future prospects. Journal of Medicine, Surgery, and Public Health. [CrossRef]
- Aleksic Sabo, V., & Knezevic, P. (2019). Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. In Industrial Crops and Products. [CrossRef]
- Ameur, E., Sarra, M., Yosra, D., Mariem, K., Nabil, A., Lynen, F., & Larbi, K. M. (2021). Chemical composition of essential oils of eight Tunisian Eucalyptus species and their antibacterial activity against strains responsible for otitis. BMC Complementary Medicine and Therapies. [CrossRef]
- Bouharb, H., Badaoui, K. El, Zair, T., Shisseh, H., Chakir, S., & Alaoui, T. (2014). Antibacterial evaluation and phytochemical screening of Eucalyptus gomphocephala DC against Pseudomonas aeruginosa. Asian Journal of Pharmaceutical and Clinical Research, 7(5), 264–267.
- Boukhalfoun, L., Kirouani, A., Behidj, N., & Gana, S. (2020). Assessment of Some Biological Activities of Eucalyptus blakelyi Maiden Using the Essential Oil, Methanolic and Aqueous Extracts. Journal of Essential Oil-Bearing Plants, 23(2), 266–275. [CrossRef]
- Boulekbache-Makhlouf, L., Slimani, S., & Madani, K. (2013). Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Industrial Crops and Products, 41(1), 85–89. [CrossRef]
- Bouras, M., Abbaci, N. B., & Bennadja, S. (2016). In vitro antibacterial proprieties of aqueous extract and essential oil of Eucalyptus globulus against multi-resistant Klebseilla pneumoniae isolated from hospitalized patients. Der Pharma Chemica, 8(16), 48–51.
- Barbieri, R., Coppo, E., Marchese, A., Daglia, M., Sobarzo-Sánchez, E., Nabavi, S. F., & Nabavi, S. M. (2017). Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. In Microbiological Research. [CrossRef]
- Chandorkar, N., Tambe, S., Amin, P., & Madankar, C. (2021). A systematic and comprehensive review on current understanding of the pharmacological actions, molecular mechanisms, and clinical implications of the genus Eucalyptus. In Phytomedicine Plus. [CrossRef]
- De Siqueira Mota, V., Turrini, R. N. T., & De Brito Poveda, V. (2015). Antimicrobial activity of Eucalyptus globulus oil, xylitol and papain: A pilot study. Revista Da Escola de Enfermagem. [CrossRef]
- Elaissi, A., Rouis, Z., Salem, N. A. B., Mabrouk, S., ben Salem, Y., Salah, K. B. H., Aouni, M., Farhat, F., Chemli, R., Harzallah-Skhiri, F., & Khouja, M. L. (2012). Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complementary and Alternative Medicine. [CrossRef]
- Elangovan, S., & Mudgil, P. (2023). Antibacterial Properties of Eucalyptus globulus Essential Oil against MRSA: A Systematic Review. In Antibiotics. [CrossRef]
- Elbhnsawi, N. A., Elwakil, B. H., Hassanin, A. H., Shehata, N., Elshewemi, S. S., Hagar, M., & Olama, Z. A. (2023). Nano-Chitosan/Eucalyptus Oil/Cellulose Acetate Nanofibers: Manufacturing, Antibacterial and Wound Healing Activities. Membranes. [CrossRef]
- Egwaikhide, P. A., Okeniyi, S. O., & Gimba, C. E. (2009). Screening for anti-microbial activity and phytochemical constituents of some Nigerian medicinal plants. Journal of Medicinal Plants Research, 3(12), 1088–1091.
- Elkolli, H., Elkolli, M., Ataya, F. S., Salem-Bekhit, M. M., Zahrani, S. Al, Abdelmageed, M. W. M., Ernst, B., & Benguerba, Y. (2023). In Vitro and In Silico Activities of E. radiata and E. cinerea as an Enhancer of Antibacterial, Antioxidant, and Anti-Inflammatory Agents. Molecules, 28(20). [CrossRef]
- Evans, C. E., Banso, A., & Samuel, O. A. (2002). Efficacy of some nupe medicinal plants against Salmonella typhi: An in vitro study. Journal of Ethnopharmacology, 80(1), 21–24. [CrossRef]
- Fokunang, C. N., Ndikum, V., Tabi, O. Y., Jiofack, R. B., Ngameni, B., Guedje, N. M., Tembe-Fokunang, E. A., Tomkins, P., Barkwan, S., Kechia, F., Asongalem, E., Ngoupayou, J., Torimiro, N. J., Gonsu, K. H., Sielinou, V., Ngadjui, B., Angwafor, I., Nkongmeneck, A., Abena, O. M., … Kamsu-Kom. (2011). Traditional medicine: Past, present and future research and development prospects and integration in the national health system of Cameroon. African Journal of Traditional, Complementary and Alternative Medicines. [CrossRef]
- Ghareeb, M. A., Habib, M. R., Mossalem, H. S., & Abdel-Aziz, M. S. (2018). Phytochemical analysis of Eucalyptus camaldulensis leaves extracts and testing its antimicrobial and schistosomicidal activities. Bulletin of the National Research Centre. [CrossRef]
- Gilles, M., Zhao, J., An, M., & Agboola, S. (2010). Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chemistry. [CrossRef]
- Hajibonabi, A., Yekani, M., Sharifi, S., Nahad, J. S., Dizaj, S. M., & Memar, M. Y. (2023). Antimicrobial activity of nanoformulations of carvacrol and thymol: New trend and applications. In OpenNano. [CrossRef]
- Ikinyom, N., Lamwaka, A. V., Malagala, A. T., Ndyomugyenyi, E. K. (2024). Antimicrobial activity of selected nutraceutical plants used in Northern. African Journal of Clinical and Experimental Microbiology, 25(1), 103–111. [CrossRef]
- Kouki, H., Polito, F., De Martino, L., Mabrouk, Y., Hamrouni, L., Amri, I., Fratianni, F., De Feo, V., & Nazzaro, F. (2022). Chemistry and Bioactivities of Six Tunisian Eucalyptus Species. Pharmaceuticals, 15(10). [CrossRef]
- Khedhri, S., Polito, F., Caputo, L., Manna, F., Khammassi, M., Hamrouni, L., Amri, I., Nazzaro, F., De Feo, V., & Fratianni, F. (2022). Chemical Composition, Phytotoxic and Antibiofilm Activity of Seven Eucalyptus Species from Tunisia. Molecules, 27(23). [CrossRef]
- Kouki, H., Polito, F., De Martino, L., Mabrouk, Y., Hamrouni, L., Amri, I., Fratianni, F., De Feo, V., & Nazzaro, F. (2022). Chemistry and Bioactivities of Six Tunisian Eucalyptus Species. Pharmaceuticals, 15(10), 1–17. [CrossRef]
- Kwansa-Bentum, B., Okine, B. A., Dayie, A. D., Tetteh-Quarcoo, P. B., Kotey, F. C. N., Donkor, E. S., & Dayie, N. T. K. D. (2023). In Vitro effects of petroleum ether, dichloromethane, methanolic and aqueous leaf extracts of Eucalyptus grandis on selected multidrug-resistant bacteria. PLoS ONE, 18(3 March), 1–10. [CrossRef]
- Lawal, T. O., Adeniyi, B. A., Adegoke, A. O., Franzblau, S. G., & Mahady, G. B. (2012). In vitro susceptibility of Mycobacterium tuberculosis to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana and isolated compounds. Pharmaceutical Biology, 50(1), 92–98. [CrossRef]
- Lawal, T. O., Adeniyi, B. A., Moody, J. O., & Mahady, G. B. (2012). Combination studies of eucalyptus torelliana F. Muell. leaf extracts and clarithromycin on helicobacter pylori. Phytotherapy Research, 26(9), 1393–1398. [CrossRef]
- Matouskova, P., Marova, I., Bokrova, J., & Benesova, P. (2016). Effect of encapsulation on antimicrobial activity of herbal extracts with lysozyme. Food Technology and Biotechnology. [CrossRef]
- Muhamad, N., Muhmed, S. A., Yusoff, M. M., & Gimbun, J. (2014). Influence of solvent polarity and conditions on extraction of antioxidant, flavonoids and phenolic content from Averrhoa bilimbi. Journal of Food Science and Engineering. [CrossRef]
- Muteeb, G., Rehman, M. T., Shahwan, M., & Aatif, M. (2023). Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. In Pharmaceuticals. [CrossRef]
- Nasir Shah, S., Khan, I., Tul Muntaha, S., Hayat, A., Ur Rehman, M., Ali Shah, T., Siddique, F., Salamatullah, A. M., Mekonnen, A. B., & Bourhia, M. (2023). Bioactive, antioxidant and antimicrobial properties of chemically fingerprinted essential oils extracted from Eucalyptus globulus: in-vitro and in-silico investigations. Frontiers in Chemistry. [CrossRef]
- Nayim, P., Mbaveng, A. T., & Kuete, V. (2023). Anti-Helicobacter pylori activities of African medicinal plants. Advances in Botanical Research. [CrossRef]
- Nortjie, E., Basitere, M., Moyo, D., & Nyamukamba, P. (2024). Assessing the Efficiency of Antimicrobial Plant Extracts from Artemisia afra and Eucalyptus globulus as Coatings for Textiles. Plants, 13(4). [CrossRef]
- Nwabor, O. F., Singh, S., Syukri, D. M., & Voravuthikunchai, S. P. (2021). Bioactive fractions of Eucalyptus camaldulensis inhibit important foodborne pathogens, reduce listeriolysin O-induced haemolysis, and ameliorate hydrogen peroxide-induced oxidative stress on human embryonic colon cells. Food Chemistry. [CrossRef]
- Okba, M. M., El-Shiekh, R. A., Abu-Elghait, M., Sobeh, M., & Ashour, R. M. S. (2021). Hplc-pda-esi-ms/ms profiling and anti-biofilm potential of eucalyptus sideroxylon flowers. Antibiotics, 10(7), 1–17. [CrossRef]
- Ouattara, L. P., Maiga, I., Bazie, B. V., Zerbo, M., Bationo, K. R., Zongo, C., Savadogo, A., & Nebie, C. H. R. (2022). Phytochemical screening and antimicrobial activity of extracts of five aromatic and medicinal plants from Burkina Faso. International Journal of Biological and Chemical Sciences, 16(5), 2228–2237. [CrossRef]
- OUMAIMA NAILI, HADJER TAOUS, R. M. (2022). Phytochemical Screening and Effect of Three Plants Collected from the Region of Khenchela, Algeria against Multi-drug Resistant Pathogenic Bacteria. Indian Journal of Novel Drug Delivery, 14(1), 29–35.
- Parham, S., Kharazi, A. Z., Bakhsheshi-Rad, H. R., Nur, H., Ismail, A. F., Sharif, S., Ramakrishna, S., & Berto, F. (2020). Antioxidant, antimicrobial and antiviral properties of herbal materials. In Antioxidants. [CrossRef]
- Parreira, P., Soares, B. I. G., Freire, C. S. R., Silvestre, A. J. D., Reis, C. A., Martins, M. C. L., & Duarte, M. F. (2017). Eucalyptus spp. outer bark extracts inhibit Helicobacter pylori growth: in vitro studies. Industrial Crops and Products. [CrossRef]
- Pereira, V., Dias, C., Vasconcelos, M. C., Rosa, E., & Saavedra, M. J. (2014). Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Industrial Crops and Products. [CrossRef]
- Plaskova, A., & Mlcek, J. (2023). New insights of the application of water or ethanol-water plant extract rich in active compounds in food. In Frontiers in Nutrition. [CrossRef]
- Prestinaci, F., Pezzotti, P., & Pantosti, A. (2015). Antimicrobial resistance: A global multifaceted phenomenon. In Pathogens and Global Health. [CrossRef]
- Rehman, R., Hayat, U., Idrees Jilani, M., & Nadeem, F. (2015). A Review on Eucalyptus globulus: A New Perspective in Therapeutics. In IJCBS.
- Shaaban, H. A. (2020). Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. In Essential Oils - Bioactive Compounds, New Perspectives and Applications. [CrossRef]
- Safavi, M., Shams-Ardakani, M., & Foroumadi, A. (2015). Medicinal plants in the treatment of Helicobacter pylori infections. In Pharmaceutical Biology. [CrossRef]
- Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., & Alqumber, M. A. A. (2023). Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. In Healthcare (Switzerland). [CrossRef]
- Salvatori, E. S., Morgan, L. V., Ferrarini, S., Zilli, G. A. L., Rosina, A., Almeida, M. O. P., Hackbart, H. C. S., Rezende, R. S., Albeny-Simões, D., Oliveira, J. V., Gasparetto, A., Müller, L. G., & Dal Magro, J. (2023). Anti-Inflammatory and Antimicrobial Effects of Eucalyptus spp. Essential Oils: A Potential Valuable Use for an Industry Byproduct. Evidence-Based Complementary and Alternative Medicine. [CrossRef]
- Sebei, K., Sakouhi, F., Herchi, W., Khouja, M. L., & Boukhchina, S. (2015). Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biological Research, 48, 7. [CrossRef]
- Shekar, C., Nagarajappa, R., Singh, R., & Thakur, R. (2015). Antimicrobial efficacy of Acacia nilotica, Murraya koenigii L. Sprengel, Eucalyptus hybrid, and Psidium guajava on primary plaque colonizers: An in vitro comparison between hot and cold extraction process. Journal of Indian Society of Periodontology. [CrossRef]
- Siddique, S., Parveen, Z., Firdaus-E-Bareen, Mazhar, S., & Chaudhary, M. N. (2018). Antibacterial and antioxidant activities of essential oils from leaves of seven Eucalyptus species grown in Pakistan. Journal of Animal and Plant Sciences.
- Takahashi, T., Kokubo, R., & Sakaino, M. (2004). Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Letters in Applied Microbiology. [CrossRef]
- Tyagi, A. K., & Malik, A. (2011). Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chemistry. [CrossRef]
- Tankeo, S. B., Lacmata, S. T., Noumedem, J. A. K., Dzoyem, J. P., Kuiate, J. R., & Kuete, V. (2014). Antibacterial and antibiotic-potentiation activities of some Cameroonian food plants against multi-drug resistant gram-negative bacteria. Chinese Journal of Integrative Medicine, 20(7), 546–554. [CrossRef]
- WHO. (2018). World Health Organization Antimicrobial Resistance. Department of Agriculture and Water Resources.
- Yuan, H., Ma, Q., Ye, L., & Piao, G. (2016). The traditional medicine and modern medicine from natural products. Molecules. [CrossRef]
| Author | Year | Country | Plant Specie | Part of Plant used |
Organisms tested | Solvent used | Type of extraction | Extract MIC (mg/ml) | Positive Control | Control Conc. (µg/ml) |
Bioactive Compounds |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ikinyom et al | 2024 | Uganda | E. globulus Labill | Leaves |
S. pneumoniae K. pneumoniae |
Acetone | Maceration | 1.25 2.5 |
Ciprofloxacin | 0.04 0.04 |
Tannins, saponins, terpenoids, glycosides, alkaloids, phenolic compounds, cardiac glycosides, terpenes, reducing sugars, carbohydrates, flavonoids. |
| Evans et al | 2002 | Nigeria | E. cassia | Leaves Stem inflorenscence |
S. typhi | Distilled water Ethanol |
Decoction | 1 2 |
Not reported | Not reported |
Saponins, Alkaloid Tannins |
| Kouki et al | 2022 | Tunisia |
E. melliodora, E. paniculata, E. transcontinentalis E. bosistoana E. salmonopholia |
Leaves |
S. aureus L.monocytogenes, P. aeruginosa E. coli, A. baumannii |
N-hexane | Hydrodistillation | 0.026 -0.032 | Tetracycline | 23-27 µL/mL | Eucalyptol, α-Pinene, trans-Pinocarveol, p-cymene, neoverbanol, Monoterpenoids, Sesquiterpenoids. |
| Adeniyi et al | 2009 | Nigeria |
E. camaldulensis E. torelliana |
Leaves Stem bark |
H. pylori | Chloroform Methanol |
Soxhlet extraction |
0.0125 – 0.4 | Clarithromycin | 0.5 | Tannins saponins, cardenolides |
| Bouharb et al | 2014 | Morocco | E. gomphocephala | Leaves | P. aeruginosa | Distilled water | Maceration | Aq = 6.25-12.5 Hex = 9.37- 50 |
Gentamicin | 15 | tannins, flavonoids, saponins triterpenes |
| Adeniyi et al | 2009 | Nigeria | E. grandis | Stem bark | H. pylori | N-Hexane Methanol |
Soxhlet extraction |
0.00039 -0.00156 | Bismuth citrate | 25 | Tannins, essential oils and saponins |
| Gemechu et al | 2013 | Ethiopia | E. camaldulensis | Leaves |
M. tuberculosis M. bovis |
Methanol | Maceration | 0.00625-0.1 | Rifampicin | 32 | Tannins and saponins |
| Okba et al | 2021 | Egypt | E. sideroxylon | Flowers |
B. subtilis S. aureus E. coli P. aeruginosa C. albicans |
Methanol | Maceration | 0.5-1.2 1.2 1.2 3.0 |
Gentamicin | 10 | Phloroglucinols, Flavonoids Tannins. |
| Ouattara et al | 2022 | Burkina-Faso | E. camaldulensis | Whole plant |
E. coli K. pneumoniae S. aureus S. epidermidis S. saprophyticus C. albicans Aspergillus sp |
Methanol | Maceration | 0.156 - 5 | Ciprofloxacin | 5 | Tannins Flavonoids Saponins |
| Naili et al | 2022 | Algeria | E. globulus | Leaves |
E. coli K. pneumoniae P. aeruginosa E. aerogenes S. aureus B. subtilis |
Ethanol & Distilled water |
Maceration |
6.25 |
Tetracycline Vancomycin, Oxacillin. | 30 30 5 |
Flavonoids Tannins, free quinones Terpenoids |
| Lawal et al | 2012 | Nigeria |
E. camaldulensis E. torelliana |
Leaves Stem bark |
M. tuberculosis | N-hexane, Chloroform Methanol | Maceration | 0.0495 0.04699 |
Rifampicin | 4–0.0156 | Tannins Triterpenes saponins, Anthraquinones Glycosides |
| Boulekbache-Makhlouf et al | 2013 | Algeria | E. globulus | Fruits |
S. aureus B. subtilis K. pneumoniae |
Acetone & distilled water | Maceration | 0.03 0.08 |
Gallic and tannic acids | none | Phénols Tannins Flavonoids |
| Khedhri et al | 2022 | Tunisia |
E. griffithsii E. hemiphloia E. lesouefii E. longicornis E. pyriformis, E. viminalis E. wandoo |
Leaves |
baumannii P. aeruginosa E. coli S. aureus L. monocytogenes |
N-hexane | Hydrodistillation | 25-38 µL/mL | tetracycline | 23- 24 µL/mL | The oxygenated monoterpenes, hydrocarbon monoterpenes, Eucalyptol, Eucalyptol, α-pinene, o-cymene, trans-pinocarveol, neo-verbenol, α-terpineol, cumin aldehyde, terpinene-4-ol, dihydrocarveol, pinocarvone, p-menth-1-en-7-al, carvacrol, p-cymene, iso-menthol, β-pinene, m-cymen-8-ol, sabina ketone, spathulenol, sabina ketone, pinocarvone, cryptone, acetate, pinocarvone, sesquiterpenes, β-eudesmol, rosifoliol, α-eudesmol. |
| Tankeo et al | 2014 | Cameroon | E. robusta | Leaves |
P. stuartii P. aeruginosa K. pneumoniae E. coli E. aerogenes E. cloacae |
Methanol | Soxhlet extraction |
0.064 |
Chloramphenicol | 2.5 | Alkaloids Anthocyanins, anthraquinones, Flavonoids Phenols saponins sterols tannins triterpenes |
| Lawal et al | 2012 | Nigeria | E. torelliana | Leaves Stem bark |
H. pylori | N-Hexane Chloroform Methanol |
Soxhlet extraction | 0.050 to 0.1 | Clarithromycin | 0.25–0.0625 | tannins, triterpenoid saponins and cardiac glycosides |
| Bouras et al | 2016 | Algeria | E. golobulus | Leaves | K. pneumoniae | Distilled water | decoction | 0.3 – 0.4 | dimethylsulfoxid (DMSO) | 10 | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




