Submitted:
22 April 2025
Posted:
22 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Gas Exchange and Chlorophyll Fluorescence Measurements
2.3. Photosynthesis Models and Calculations
2.3.1. Rectangular Hyperbola (RH) Model
2.3.2. Non-Rectangular Hyperbola (NRH) Model
2.3.3. Photosynthetic Mechanistic Model (Ye Model)
2.4. Statistical Analysis
3. Results
3.1. Photosynthetic and Electron Transport Responses
3.2. Quantum Yield and Photophysical Traits of Light-Harvesting Pigment Molecules
3.3. Photoprotection and Metabolic Efficiency Dynamics
4. Discussion
4.1. Applicability of Ye Mechanistic Model in Aquatic Plants Photosynthesis
4.2. Evolutionary Adaptations of E. crassipes
| Plants | Pnmax | Isat | Jmax | Ie-sat | Reference |
| E. crassipes | 23.1–30.8 | ― | ― | ― | [6] |
| E. crassipes | 34.5 ± 0.72 | 2358 ± 69 | ― | ― | [19] |
| E. crassipes | 24.70 ± 1.01 | 2200.0 ± 81.7 | 186.1 ± 10.0 | 1750.0 ± 170.8 | This study |
| Nymphoides peltate | 12.66 | 219.98 | ― | ― | [38] |
| Nelumbo nucifera | 7.1–9.2 | ― | ― | ― | [39] |
| Phragmites australis | 9.0~19.5 | 924.1–2186.3 | ― | ― | [40] |
| Oryza sativa L. | 17.51–27.89 | ≈ 2000 | ― | ― | [41] |
| Oryza sativa L. (Kitaake) | 19.56 ± 0.62 | 1641 ± 32.0 | ― | ― | [19] |
| Tamarix ramosissima | 17.2–24.4 | 957–1360 | ― | ― | [42] |
| Solanum lycopersicum L. | 6.34–17.82 | ― | ― | ― | [43] |
| Malus pumila Mill. | 15.25–20.29 | 1413.8–1874.9 | ― | ― | [44] |
| Glycine max L. (Merr.) | 19.73 | 1800 | 143.51 ± 5.21 | 1601.6 ± 0.64 | [45] |
| Zea mays L. (Nongda 108) | 30.36 ± 0.42 | 2550 ± 37.0 | ― | ― | [19] |
| Sorghum bicolor L. (KFJT-4) | 37.49 ± 0.90 | 1866.7 ± 33.3 | 170.15 ± 4.45 | 1640.0 ± 74.83 | [16,18] |
| Sorghum bicolor L. (KFJT-1) | ― | ― | 133.84 ± 5.52 | 1600.0 ± 63.24 | [18] |
4.3. Synergistic Efficiency Metrics Supports Invasiveness of E. crassipes
5. Conclusions
Author Contributions
Funding
References
- Fan, D.; Schwinghamer, T.; Liu, S.; Xia, O.; Ge, C.; Chen, Q.; Smith, D. L. Characterization of endophytic bacteriome diversity and associated beneficial bacteria inhabiting a macrophyte Eichhornia crassipes. Front. Plant Sci. 2023, 14, 1176648. [Google Scholar] [CrossRef]
- López-Pozo, M.; Adams, W. W.; Polutchko, S. K.; Demmig-Adams, B. Terrestrial and floating aquatic plants differ in acclimation to light environment. Plants 2023, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Pilon, J.; Santamaría, L. Clonal variation in the thermal response of the submerged aquatic macrophyte Potamogeton pectinatus. J. Ecol. 2002, 90, 141–152. [Google Scholar] [CrossRef]
- Pasos-Panqueva, J.; Baker, A.; Camargo-Valero, M. A. Unravelling the impact of light, temperature and nutrient dynamics on duckweed growth: A meta-analysis study. J. Environ. Manage. 2024, 366, 121721. [Google Scholar] [CrossRef] [PubMed]
- Meneguelli-Souza, A. C.; Vitória, A. P.; Vieira, T. O.; Degli-Esposti, M. S. O.; Souza, C. M. M. Ecophysiological responses of Eichhornia crassipes (Mart.) Solms to As5+ under different stress conditions. Photosynthetica.
- Pereira, F. J.; Castro, E. M.; Oliveira, C.; Pires, M. F.; Pereira, M. P.; Ramos, S. J.; Faquin, V. Lead tolerance of water hyacinth (Eichhornia crassipes Mart. - Pontederiaceae) as defined by anatomical and physiological traits. An. Acad. Bras. Cienc. 1433. [Google Scholar]
- Downing-Kunz, M.; Stacey, M. Flow-induced forces on free-floating macrophytes. Hydrobiologia 2011, 671, 121–135. [Google Scholar] [CrossRef]
- Kühlbrandt, W.; Wang, D. N.; Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 1994, 367, 614–621. [Google Scholar] [CrossRef]
- Ripley, B. S.; Muller, E.; Behenna, M.; Whittington-Jones, G. M.; Hill, M. P. Biomass and photosynthetic productivity of water hyacinth (Eichhornia crassipes) as affected by nutrient supply and mirid (Eccritotarus catarinensis) biocontrol. Biol. Control 2006, 39, 392–400. [Google Scholar] [CrossRef]
- Maranho, L. T.; Gomes, M. P. Morphophysiological adaptations of aquatic macrophytes in wetland-based sewage treatment systems: strategies for resilience and efficiency under environmental stress. Plants 2024, 13, 2870. [Google Scholar] [CrossRef]
- Long, S. P.; Taylor, S. H.; Burgess, S. J.; Carmo-Silva, E.; Lawson, T.; De Souza, A. P.; Leonelli, L.; Wang, Y. Into the shadows and back into sunlight: Photosynthesis in fluctuating light. Ann. Rev. Plant Biol. 2022, 73, 617–648. [Google Scholar] [CrossRef]
- Ruan, M.; Li, H.; Zhang, Y.; Zhao, R.; Zhang, J.; Wang, Y.; Gao, J.; Wang, Z.; Wang, Y.; Sun, D.; Ding, W.; Weng, Y. Cryo-EM structures of LHCII in photo-active and photo-protecting states reveal allosteric regulation of light harvesting and excess energy dissipation. Nat. Plants 2023, 9, 1547–1557. [Google Scholar] [CrossRef]
- Baker, N. R. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Sun, Z.; Struik, P. C.; Gu, J. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J. Exp. Bot. 2011, 62, 3489–3499. [Google Scholar] [CrossRef] [PubMed]
- Yin, X. Y.; Struik, P. C. Theoretical reconsiderations when estimating the mesophyll conductance to CO2 diffusion in leaves of C3 plants by analysis of combined gas exchange and chlorophyll fluorescence measurements. Plant Cell Environ. 2009, 32, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Ma, X.-F.; Ye, Z.-P.; Yang, L.-S.; Shi, J.-B.; Wang, X.; Zhou, B.-B.; Wang, F.-B.; Deng, Z.-F. Simulating short-term light responses of photosynthesis and water use efficiency in sweet sorghum under varying temperature and CO2 conditions. Front. Plant Sci. 2024, 15, 1291630. [Google Scholar] [CrossRef]
- Ye, Z. P.; Kang, H. J.; An, T.; Duan, H. L.; Wang, F. B.; Yang, X. L.; Zhou, S. X. Modeling light response of electron transport rate and its allocation for ribulose biphosphate carboxylation and oxygenation. Front. Plant Sci. 2020, 11, 581851. [Google Scholar] [CrossRef]
- Yang, X.-L.; An, T.; Ye, Z.-W.-Y.; Kang, H.-J.; Robakowski, P.; Ye, Z.-P.; Wang, F.-B.; Zhou, S.-X. Modeling light response of effective quantum efficiency of photosystem II for C3 and C4 crops. Front. Plant Sci. 2025, 16, 1478346. [Google Scholar] [CrossRef]
- Xia, L.; Jianchu, Z.; Shaohua, Y.; Chenggang, R.; Man, W.; Wei, C.; Jing, S.; Puping, Z. Dynamics of photosynthesis in Eichhornia crassipes dolms of Jiangsu of China and their influencing factors. African Journal of Biotechnology 2010, 9, 7302–7311. [Google Scholar]
- Baly, E. C. C. The kinetics of photosynthesis. Proceedings of the Royal Society of London 1935, 149, 596–596. [Google Scholar] [CrossRef]
- Farquhar, G. D.; Wong, S. C. An empirical model of stomatal conductance. Aust. J. Plant Physiol. 1984, 11, 191–210. [Google Scholar] [CrossRef]
- Thornley, J. H. M. Mathematical Models in Plant Physiology: A quantitative approach to problems in plant and crop physiology (Experimental botany) London, UK: Academic Press, 1976.
- Farquhar, G. D.; von Caemmerer, S.; Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Ye, Z. P.; Suggett, J. D.; Robakowski, P.; Kang, H. J. A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z. P. Nonlinear optical absorption of photosynthetic pigment molecules in leaves. Photosynth. Res. 2012, 112, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, E.; Rubio, E.; García-Morote, F. A.; Andrés-Abellán, M.; Miettinen, H.; López-Serrano, F. R. Net ecosystem production in a Spanish black pine forest after a low burn-severity fire: Significance of different modelling approaches for estimating gross primary production. Agr. Forest Meteorol. 2017, 246, 178–193. [Google Scholar] [CrossRef]
- Atsushi, S.; Tatsuya, K.; Shigeto, T.; Masashi, Y. Effect of temperature on photosynthesis characteristics in the passion fruits ‘Summer Queen’ and ‘Ruby Star’. Horticult. J. 2017, 86, 194–199. [Google Scholar]
- Liu, S.; Liu, W.; Shi, X.; Li, S.; Hu, T.; Song, L.; Wu, C. Dry-hot stress significantly reduced the nitrogenase activity of epiphytic cyanolichen. Sci. Total Environ.
- Ye, Z. P.; Zhang, H. L.; Huang, Z. A.; Yang, X. L.; Kang, H. J. Model construction of light use efficiency and water use efficiency based on a photosynthetic mechanistic model of light response. J. Plant Physiol. 2017, 53, 1116–1122. [Google Scholar]
- Ye, Z. P.; Ling, Y.; Yu, Q.; Duan, H. L.; Kang, H. J.; Huang, G. M.; Duan, S. H.; Chen, X. M.; Liu, Y. G.; Zhou, S. X. Quantifying light response of leaf-scale water-use efficiency and its interrelationships with photosynthesis and stomatal conductance in C3 and C4 species. Front. Plant Sci. 2020, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yin, H.; Xiong, P.; Wan, C.; Liu, Q. Short-term responses of Picea asperata seedlings of different ages grown in two contrasting forest ecosystems to experimental warming. Environ. Exp. Bot. 2012, 77, 1–11. [Google Scholar] [CrossRef]
- Yang, X. L.; Dong, W.; Liu, L. H.; Bi, Y. H.; Xu, W. Y.; Wang, X. Uncovering the differential growth of Microcystis aeruginosa cultivated under nitrate and ammonium from a pathophysiological perspective. ACS ES&T Water.
- Yang, X. L.; Liu, L. H.; Yin, Z. K.; Wang, X. Y.; Wang, S. B.; Ye, Z. P. Quantifying photosynthetic performance of phytoplankton based on photosynthesis–irradiance response models. Environ. Sci. Eur. 2020, 32, 24. [Google Scholar] [CrossRef]
- Smyth, T. J.; Pemberton, K. L.; Aiken, J.; Geider, R. J. A methodology to determine primary production and phytoplankton photosynthetic parameters from Fast Repetition Rate Fluorometry. J. Plankton Res. 2004, 26, 1337–1350. [Google Scholar] [CrossRef]
- Silsbe, G. M.; Kromkamp, J. C. Modeling the irradiance dependency of the quantum efficiency of photosynthesis. Limnol. Oceanogr. - Meth.
- Buckley, T. N.; Diaz-Espejo, A. Reporting estimates of maximum potential electron transport rate. New Phytol. 2015, 205, 14–17. [Google Scholar] [CrossRef]
- Krall, J. P.; Edwards, G. E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiologia Plantarum 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Yu, H.; Niu, Y.; Hu, Y.; Du, D. Photosynthetic response of the floating-leaved macrophyte Nymphoides peltata to a temporary terrestrial habitat and its implications for ecological recovery of Lakeside zones. Knowl. Manag. Aquat. Ec. 2014, 412, 08. [Google Scholar] [CrossRef]
- Zhao, S.; Ruan, F.; Shen, W.; Deng, K.; Jiang, T.; Wu, P.; Feng, K.; Li, L. The effect of nitrogen fertilizer on rhizome quality and starch physicochemical properties in Nelumbo nucifera. In Agronomy 2022, 12, 794. [Google Scholar] [CrossRef]
- An, S.; Liu, X.; Wen, B.; Li, X.; Qi, P.; Zhang, K. Comparison of the photosynthetic capacity of Phragmites australis in five habitats in saline‒alkaline wetlands. Plants 2020, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. Z.; Yu, Y. M.; Peng, S. Z.; Yang, S. H.; Liao, L. X. A modified nonrectangular hyperbola equation for photosynthetic light-response curves of leaves with different nitrogen status. Photosynthetica 2014, 52, 117–123. [Google Scholar] [CrossRef]
- Tiemuerbieke, B.; Ma, J.-Y.; Sun, W. Differential eco-physiological performance to declining groundwater depth in Central Asian C3 and C4 shrubs in the Gurbantunggut Desert. Front. Plant Sci. 2024, 14. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Zheng, M.; Mu, W.; Wang, Q.; Zhang, J.; Bai, Y.; Sun, Y.; Lu, Z.; Wei, X. Case study on the effects of sodium carboxymethyl cellulose and biostimulants on physiological and photosynthetic characteristics, yield, and quality of apples. Agronomy 2024, 14, 1403. [Google Scholar] [CrossRef]
- Ye, Z. P.; Stirbet, A.; An, T.; Robakowski, P.; Kang, H. J.; Yang, X. L.; Wang, F. B. Investigation on absorption cross-section of photosynthetic pigment molecules based on a mechanistic model of the photosynthetic electron flow-light response in C3, C4 species and cyanobacteria grown under various conditions. Front. Plant Sci. 2023, 14, 1234462. [Google Scholar] [CrossRef]
- Ye, Z.-P.; An, T.; Govindjee, G.; Robakowski, P.; Stirbet, A.; Yang, X.-L.; Hao, X.-Y.; Kang, H.-J.; Wang, F.-B. Addressing the long-standing limitations of double exponential and non-rectangular hyperbolic models in quantifying light-response of electron transport rates in different photosynthetic organisms under various conditions. Front. Plant Sci. 2024, 15, 581851. [Google Scholar] [CrossRef]
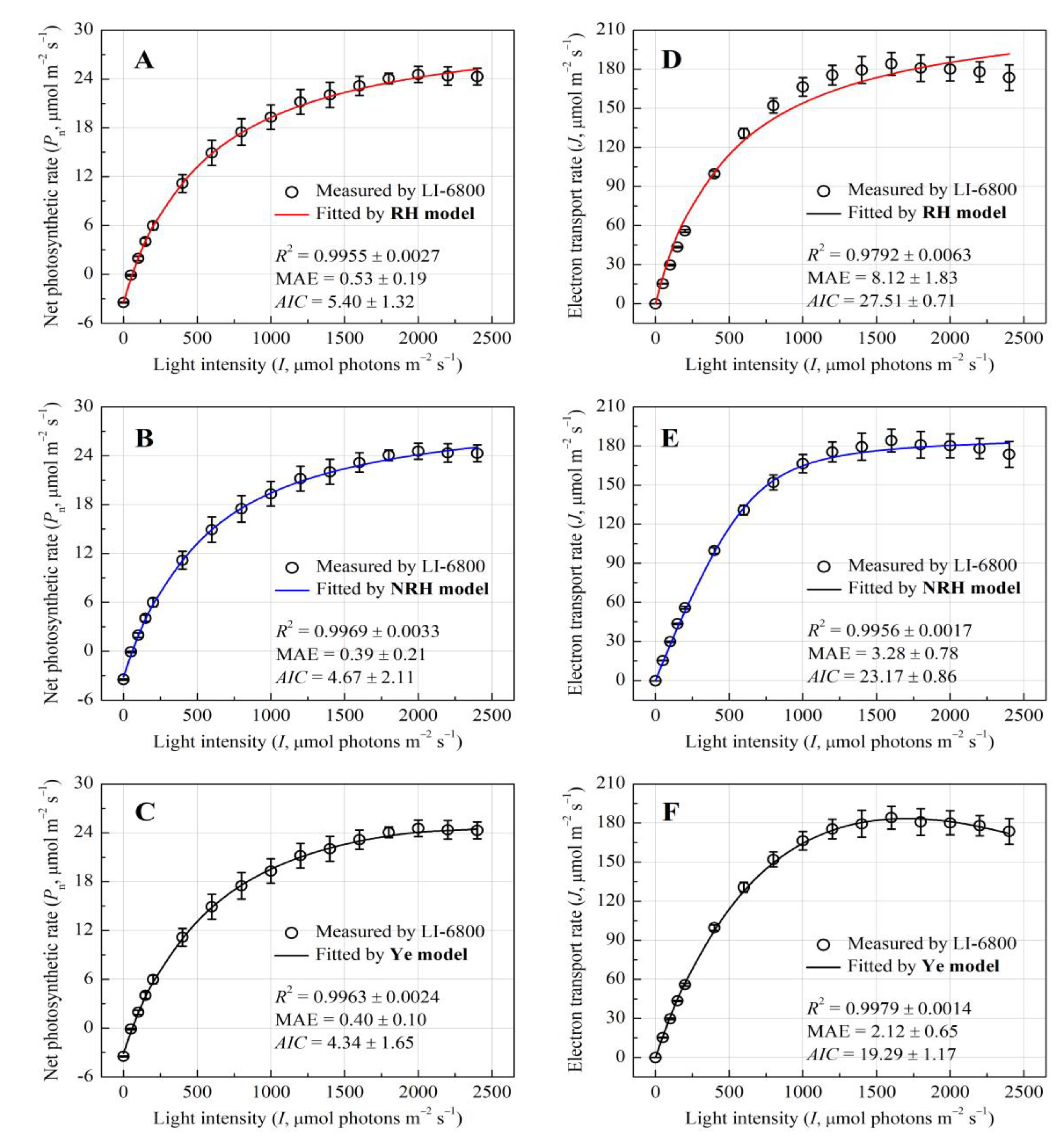
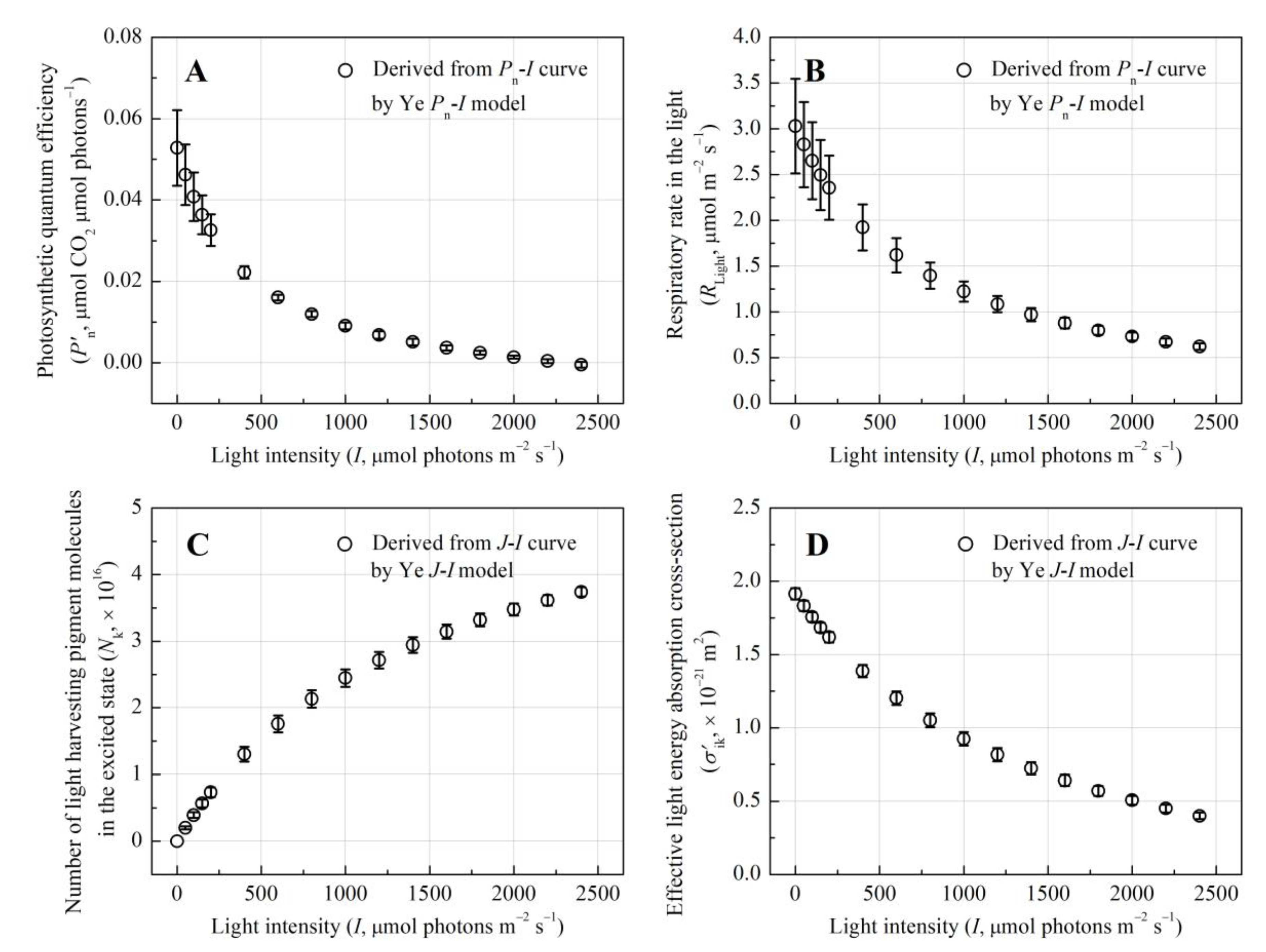
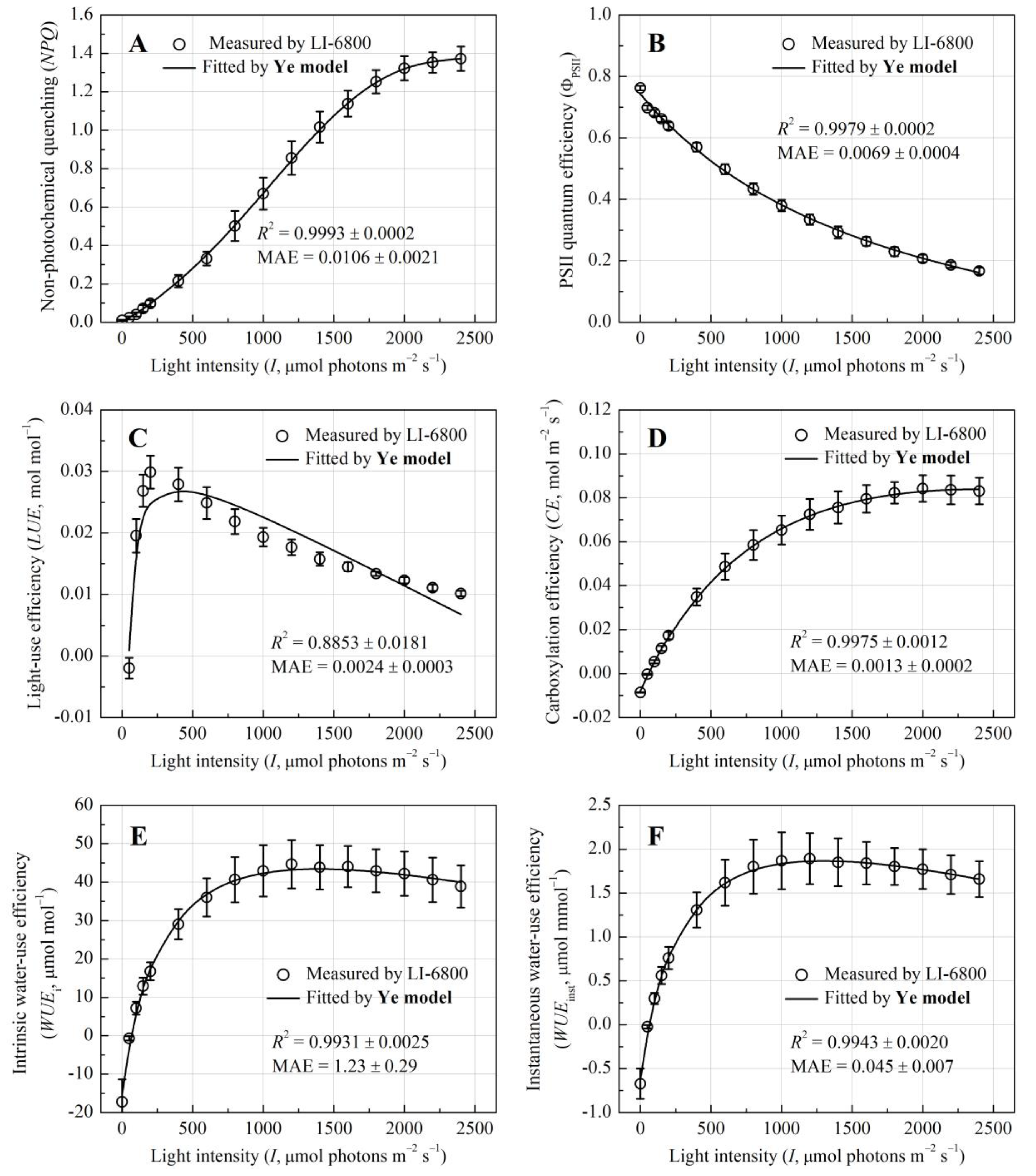
| Traits | Fitted value | Measured value | ||
| RH Model | NRH Model | Ye Model | ||
| αp (μmol mol−1) | 0.0659 ± 0.0111a | 0.0522 ± 0.0058a | 0.0528 ± 0.0107a | ― |
| Pnmax (μmol m−2 s−1) | 35.93 ± 1.33a | 33.70 ± 1.65a | 24.64 ± 1.08b | 24.70 ± 1.01b |
| Isat (μmol m−2 s−1) | ― | ― | 2520.41 ± 243.03a | 2200.00 ± 81.65a |
| Ic (μmol m−2 s−1) | 58.78 ± 3.59a | 60.02 ± 3.33a | 54.17 ± 2.45a | 53.06 ± 2.11a |
| Rd (μmol m−2 s−1) | 3.41 ± 0.46a | 2.91 ± 0.24b | 3.46 ± 0.16a | 3.46 ± 0.06a |
| αe (μmol mol−1) | 0.4658 ± 0.0109a | 0.2779 ± 0.0052c | 0.3424 ± 0.0076b | ― |
| Jmax (μmol m−2 s−1) | 232.08 ± 16.78a | 188.85 ± 11.66b | 184.10 ± 10.84b | 186.07 ± 10.04b |
| Ie-sat (μmol m−2 s−1) | ― | ― | 1699.64 ± 40.39a | 1750.00 ± 170.78a |
| na | 6.45 ± 0.34b | 5.62 ± 0.31b | 7.46 ± 0.12a | 7.52 ± 0.12a |
| σik (10−21 m2) | ― | ― | 1.91 ± 0.04 | ― |
| τmin (ms) | ― | ― | 11.53 ± 1.27 | ― |
| N0 (1016 m2) | ― | ― | 9.46 ± 0.08 | ― |
| Chl content (mg m−2) | ― | ― | ― | 707.34 ± 5.86 |
| Traits | Fitted value | Measured value |
| NPQmax | 1.366 ± 0.058a | 1.375 ± 0.062a |
| INPQ-sat (μmol m−2 s−1) | 2278.76 ± 41.25a | 2350.00 ± 50.00a |
| ΦPSIImax | 0.743 ± 0.007b | 0.762 ± 0.007a |
| LUEmax (mol mol−1) | 0.027 ± 0.002a | 0.030 ± 0.003a |
| ILUE-sat (μmol m−2 s−1) | 384.65 ± 17.60a | 250.00 ± 50.00b |
| CEmax (mol m−2 s−1) | 0.084 ± 0.006a | 0.085 ± 0.006a |
| ICE-sat (μmol m−2 s−1) | 2242.99 ± 82.55a | 2000.00 ± 81.65b |
| WUEi-max (μmol mol−1) | 44.17 ± 6.09a | 45.91 ± 6.28a |
| Ii-sat (μmol m−2 s−1) | 1621.82 ± 267.39a | 1500.00 ± 173.21a |
| WUEinst-max (μmol mmol−1) | 1.88 ± 0.28a | 1.96 ± 0.29a |
| Iinst-sat (μmol m−2 s−1) | 1391.42 ± 139.03a | 1300.00 ± 173.21a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





