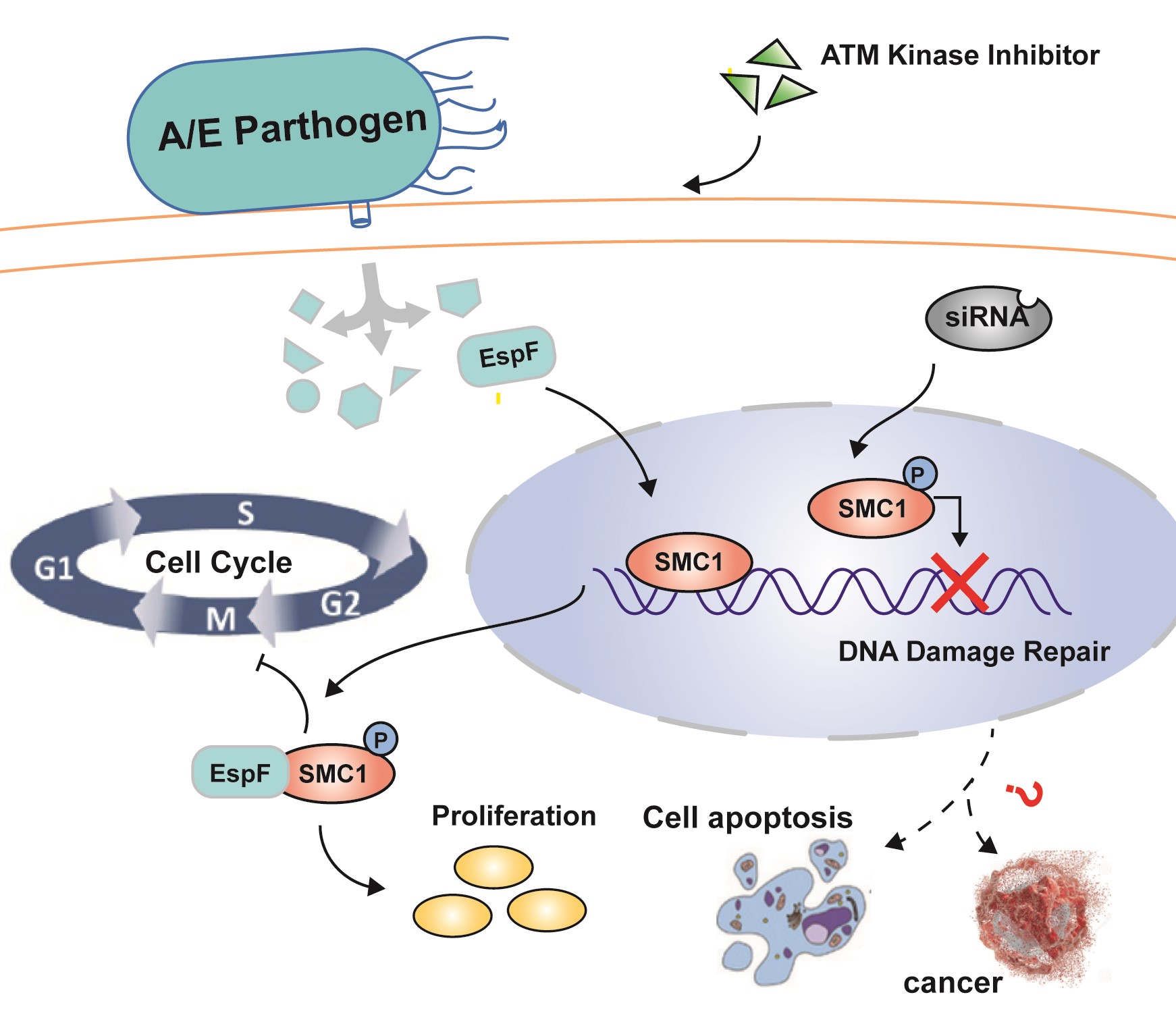
The enterohemorrhagic Escherichia coli (EHEC) O157: H7 EspF is known to be a multifunctional effector that triggers several damage processes in the host cells. However, in the process of EHEC O157: H7 infection, the interactions between EspF, its N- or C-terminus, and host proteins are still unclear. In this work, we use co-immunoprecipitation combined with mass spectrometry (CoIP-MS) to screen the interactions between EspF/EspF-N/EspF-C terminus and host proteins. A total of 311 host proteins are analyzed. The N-terminus of EspF is found to interact with 192 proteins, whereas 205 proteins interact with the C-terminus. These proteins are mainly involved in RNA splicing, endoplasmic reticulum stress, and a variety of metabolic signaling pathways. Surprisingly, MS results reveal that EspF can also phosphorylate H2AX, suggesting that EspF may directly mediate DNA damage. Here, by western blot and immunofluorescence (IF), we verified that EspF can cause phosphorylation of H2AX and mediates cell multi-nuclearation and cell hypertrophy. Furthermore, we verify here for the first time that SMC1 interacts with EspF -C-terminus, and provide evidence that EspF increases p-SMC1 levels. p-SMC1 is known to influence S-phase cell cycle arrest and usually increases during periods of DNA damage. Our work revealed a novel interaction between EspF and the host protein SMC1 and lays a foundation for further research on EspF-mediated host DNA damage, apoptosis, and even colorectal carcinogenesis.