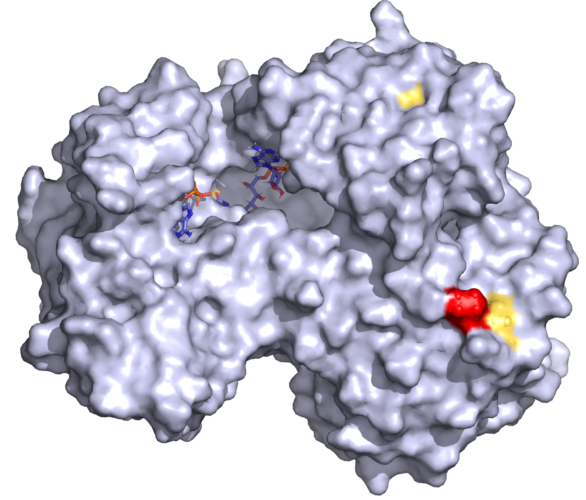
Cytochrome P450 oxidoreductase (POR) is the redox partner of steroid and drug-metabolizing cytochromes P450 located in the endoplasmic reticulum. Mutations in POR cause a broad range of metabolic disorders. The POR variant rs17853284 (P228L) identified by genome sequencing has been linked to lower testosterone levels and reduced P450 activities. We expressed POR wild type and the P228L variant in bacteria, purified the proteins, and performed protein stability and catalytic functional studies. Variant P228L affected the stability of the protein as evidenced by lower unfolding temperatures and higher sensitivity to urea denaturation. A significant reduction of small molecule metabolism was observed with POR P228L while activities of CYP3A4 were reduced by 25%, and activities of CYP3A5, and CYP2C9 were reduced by more than 40% compared to WT POR. The 17,20 lyase activity of CYP17A1 responsible for production of main androgen precursor dehydroepiandrosterone, was reduced to 27% of WT in presence of P228L variant of POR. Based on in silico and in vitro studies we predict that the change of proline to leucine may change the rigidity of the protein, causing conformational changes in POR, leading to altered electron transfer to redox partners. A single amino acid change can affect protein stability and cause a severe reduction in POR activity. Molecular characterization of individual POR mutations is crucial for a better understanding of the impact on different redox partners of POR.