1. Introduction
Serotonin (5-HT) and its receptors represent one of the most ancient and widely distributed signaling systems among Animal kingdom. It is a ligand for seven transmembrane receptors [
1,
2,
3]. The majority of studies is involved in search for agonists/antagonists and receptors molecules and their biochemical and pharmacological characteristics. Serotonylation is a posttranslational protein modification [
4]. At first it was shown on vertebate model. A recent study demonstrated serotonylation on invertebrate model [
5].
Annelid
D. gyrociliatus has short-term life cycle and they are quite easy to maintain in stock. There data on nervous system morphology and development [
6,
7,
8,
9,
10,
11]. There are prominent identifiable and countable 5-HT-like immunoreactive neurons, especially in males that contain only 5 neurons [
6].
D. gyrociliatus demonstrates relatively simple organization. Thus juveniles only 500 microns in size, while adult females 1.5-2 mm. They utilize gliding ciliary locomotion. They have circular ciliary bands on the body region for swimming and filter feeding apparatus in the anterior region. CNS contains 5-HT in the most part of nervous elements in juveniles and adult individuals There are also data on 5-HT impact on ciliary locomotion in juveniles and adults [
12].Thus it was shown that serotonin and its' biochemical precursor 5-hydroxy tryptophan (HTP) increase the speed ciliary locomotion in juveniles and have no effect on ciliary locomotion in adults. The authors hypothesize a possible change in receptor system. Nothing is known about 5-HT impact on lifespan longevity.
In this study I show the difference in lifespan longevity with long-term increased, decreased levels of serotonin and decreased level of serotonylation in treated worms and in F1 worms from treated mothers.
2. Results
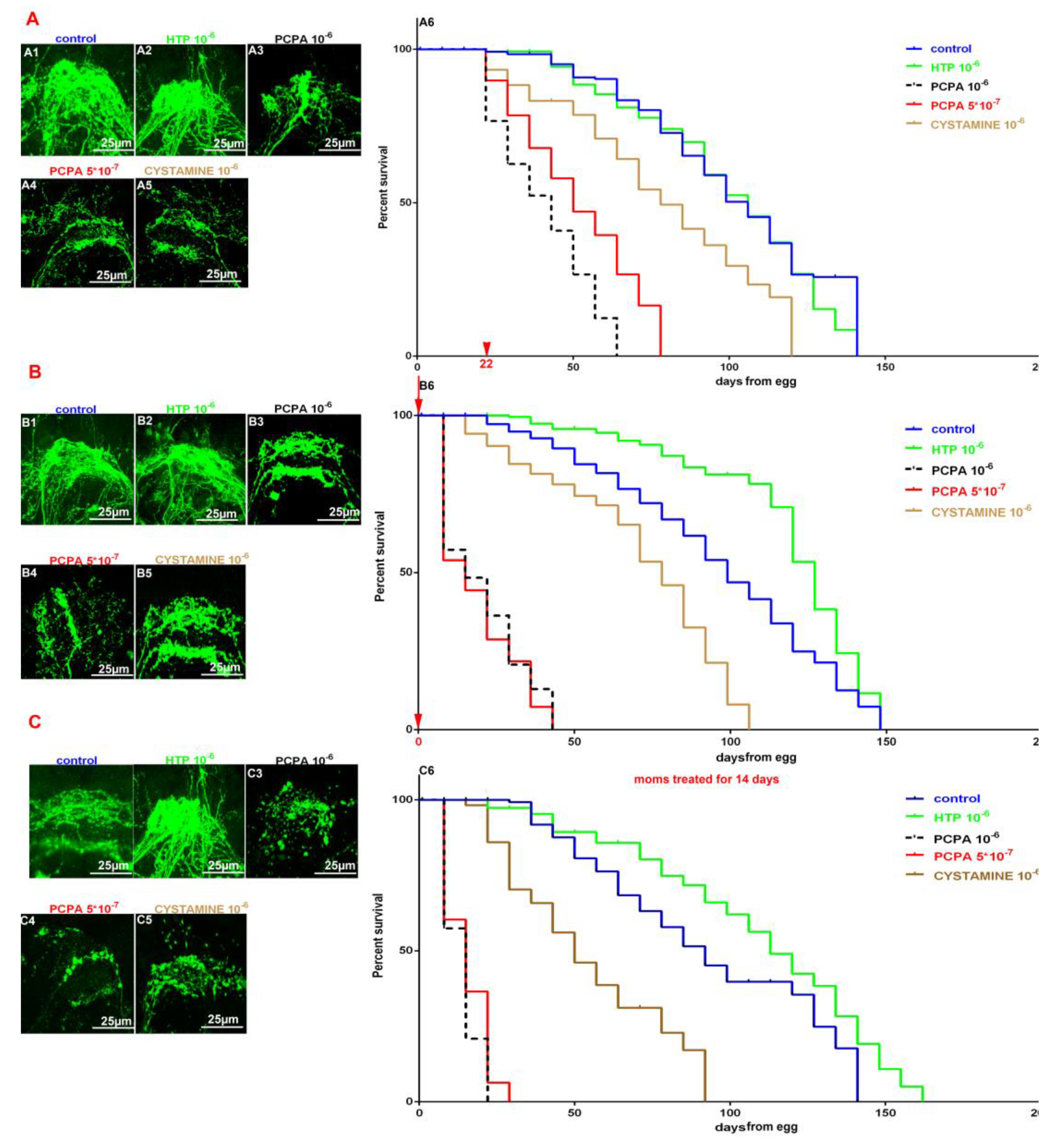
In this section I compare the fluorescence intensity of brain neuropiles in control and treated animals and the respective survival curves.
2.1. Treatment since 22day Age
The results show the increased level of fluorescence during HTP treatment (
Figure 2A2) and decreased level of fluorescence during PCPA (
Figure 2A3, A4) and cystamine treatment (
Figure 2A5). PCPA 10
-6M (black line,
Figure 2A6), 5*10
-7M (red line,
Figure 2A6) and cystamine 10
-6M (brown line,
Figure 2A6) treatment of adult specimens reduces lifespan (
Figure 2A6) with mean life longevity 43, 50 and 78 days respectively. HTP 10
-6M treatment (green line,
Figure 2A6)does not change the lifespan with mean life longevity 106 days, the same as control group (blue line,
Figure 2A6).
2.2. Treatment since Laid Egg
The higher rate of fluorescence during HTP treatment (
Figure 2B2) and lower rate of fluorescence during PCPA (
Figure 2B3, B4) and cystamine treatment (
Figure 2B5). PCPA 10
-6M (black line,
Figure 2B6), 5*10
-7M (red line,
Figure 2B6) and cystamine 10
-6M (brown line,
Figure 2B6) treatment of adult specimens shortens lifespan (
Figure 2B6) with mean life longevity 15, 15 and 78 days respectively. HTP 10
-6M treatment (green line,
Figure 2B6) increases the lifespan with mean life longevity 127 days, while control group mean lifespan was 99 days (blue line,
Figure 2B6).
2.3. Mother Treatment
An increased fluorescence in F
1 from mothers treated with HTP (
Figure 2C2) and lower rate of fluorescence in F
1 from mothers treated with PCPA (
Figure 2C3, C4) and cystamine (
Figure 2C5) comparing to control group (
Figure 2C1). Offspings from mothers treated with PCPA 10
-6M (black line,
Figure 2C6), 5*10
-7M (red line,
Figure 2C6) and cystamine 10
-6M (brown line,
Figure 2B6) show reduced lifespan (
Figure 2B6) with mean life longevity 15, 15 and 50 days respectively. Descendants from mothers treated with HTP 10
-6M (green line,
Figure 2C6) demonstrated prolonged lifespan with mean life longevity 113 days, while control group mean life span was 92 days (blue line,
Figure 2C6).
3. Discussion
The present study provides data on 5-HT impact on lifespan longevity. The previous lifespan investigations used Drosophila melanogaster and C. elegance as model organims and demonstrated quite similar results. Thus, our results demonstrated that serotonin impacts lifespan longevity and moreover the level of serotonin in mother organism affects offspring lifespan longevity.
4. Materials and Methods
4.1. Annelid Stock
The Dimorphilus gyrociliatus stock has been supported since 2007. The animals were reared in plastic tanks with artificial seawater (33‰ salinity) at 21º C and fed with homogenized frozen nettle leaves (Urtica sp.) once every 7 days during water and tank changes. In this work we synchronized the age of animals. To obtain enough individuals of the same age we monitored tanks with adults daily. We collected all freshly laid egg cocoons using Pasteur pippette. Using this technique we able to collect up to 60 cocoons (each contains 1-3 female eggs) in one day.
4.2. Survivalship Trial
Life-time assays were performed in 24-well plates in artificial sea water, each well was 2 ml total volume and contained 6-10 worms. Age-synchronized worms were seeded in the plates at the cleavage stage and then were monitored weekly. The portion of individuals alive was scored using binocular on the basis of gliding or swimming and pharyngeal bulbs movement. In the experiments we used HTP (cas #4350-09-8, Sigma Aldrich), PCPA (cas #14173-39-8, Sigma Aldrich) and Cystamine (cas#14173-39-8, Sigma Aldrich) at final concentrations 10-6M, 10-6M and 5*10-7M and 10-6M, respectively.
During first experimental series drug HTP 10-6 M, PCPA (10-6 and 5*10-7M) and cystamine (10-6 M) was added to 22-days age individuals. In each experimental trial a group of treated and control worms were fixed for immunochemistry to visualise 5-HT-containing nervous structures.
4.3. Immunohistochemistry
The individuals were fixed with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS, 0.01mM, pH=7.4) at 4°C overnight. Then, the specimens were subjected to immunostaining according to the protocol used in [
6]. Each staining was performed with at least 50-60 adult female specimens. After fixation, the specimens were washed three times in PBS and then incubated for half an hour at room temperature in 10% normal goat serum and 1% bovine serum albumin in PBS. Subsequently, the samples were incubated at 10 °C for 3 days in a solution containing primary antibodies. Aanti-serotonin (5-HT) antibodies (Immunostar, Hudson, WI, USA; 428002; rabbit; polyclonal; Product ID: 20080) diluted 1:2000 in PBS with 0.1% Triton X-100 (PBS-TX) were used to label 5-HT-like immunoreactive elements. The primary antibodies were washed three times with PBS-TX solution and labeled with secondary goat anti-rabbit antibodies with Alexa-555 (1:1000, Molecular Probes, USA; A-11008; goat; polyclonal) in PBS containing 0.1% Triton X-100 overnight at 10 C. The secondary antibodies were then rinsed with PBS. The samples were then mounted on slides in 90% glycerol.
4.4. Image Acquisition
A Zeiss LSM-880 confocal scanning microscope (Karl Zeiss, Jena, Germany) was used to analyze the specimens. Optical stacks were acquired with an x40 objective and processed at 0.7 μm intervals and 30 stacks in total using ZEN (Karl Zeiss, Germany) and Image J (NIH, USA) to obtain two-dimensional images. The stacks were projected onto an image and then imported into Adobe Photoshop CC.
4.5. Statistical Analysis
For the statistical analysis Prism 7 (GraphPad) package was used. For survival analysis Log rank (Mantel–Haenszel) test was used. We observed the death of all the worms.
5. Conclusions
This study results demonstrate that 5-HT impacts on D.gyrociliatus lifespan longevity. The increased level of serotonin extends lifespan longevity up to 22%. The decreased 5-HT level shortens lifespan longevity drastically. The decreased level of serotonylation reduces lifespan longevity as well. Moreover, 5-HT concentration and serotonylation in mother organism affects the F1 offspring lifespan longevity.
Funding
The reported study was funded by the Russian Foundation for Basic Research, grant № 19-34-60040. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
I am grateful to E.E. Voronezhskaya for methodological recommendations. The research was done using the equipment of the Core Centrum of the Institute of Developmental Biology RAS. I thank the anonymous native speakers from the Flarus agency for the professional language proofreading. The research was conducted under IDB RAS RP # 0008-2021-0020 using Core Centrum facility equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin Receptors in Depression and Anxiety: Insights from Animal Studies. Life Sciences 2018, 210, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Tierney, A.J. Invertebrate Serotonin Receptors: A Molecular Perspective on Classification and Pharmacology. Journal of Experimental Biology 2018, 221, jeb184838. [Google Scholar] [CrossRef]
- Bader, M. Serotonylation: Serotonin Signaling and Epigenetics. Front. Mol. Neurosci. 2019, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Ivashkin, E.; Melnikova, V.; Kurtova, A.; Brun, N.R.; Obukhova, A.; Khabarova, M.Yu.; Yakusheff, A.; Adameyko, I.; Gribble, K.E.; Voronezhskaya, E.E. Transglutaminase Activity Determines Nuclear Localization of Serotonin Immunoreactivity in the Early Embryos of Invertebrates and Vertebrates. ACS Chem. Neurosci. 2019, 10, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Fofanova, E.; Mayorova, T.D.; Voronezhskaya, E.E. Dinophiliformia Early Neurogenesis Suggests the Evolution of Conservative Neural Structures across the Annelida Phylogenetic Tree. PeerJ 2021, 9, e12386. [Google Scholar] [CrossRef] [PubMed]
- Fofanova, E.; Voronezhskaya, E. The Structure of Archiannelid Dinophilus Gyrociliatus Ventral Nerve Cords. Acta Biologica Hungarica 2012, 63, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Fofanova, E.G.; Nezlin, L.P.; Voronezhskaya, E.E. Ciliary and Nervous Structures in Juvenile Females of the Annelid Dinophilus Gyrociliatus (O. Schmidt, 1848) (Annelida: Polychaeta). Russ J Mar Biol 2014, 40, 43–52. [Google Scholar] [CrossRef]
- Kerbl, A.; Fofanova, E.G.; Mayorova, T.D.; Voronezhskaya, E.E.; Worsaae, K. Comparison of Neuromuscular Development in Two Dinophilid Species (Annelida) Suggests Progenetic Origin of Dinophilus Gyrociliatus. Front Zool 2016, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Windoffer, R.; Westheide, W. The Nervous System of the Male Dinophilus Gyrociliatus (Polychaeta, Dinophilidae): II. Electron Microscopical Reconstruction of Nervous Anatomy and Effector Cells. J Comp Neurol 1988, 272, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Windoffer, R.; Westheide, W. The Nervous system of the male Dinophilus gyrociliatus (Annelida: Polychaeta). I. Number, types and distribution pattern of sensory cells. Acta Zoologica 1988, 69, 55–64. [Google Scholar] [CrossRef]
- Fofanova, E.G.; Mayorova, T.D.; Voronezhskaya, E.E. Paradoxical effect of serotonin on ciliary locomotion of the adult archiannelid worms Dinophilus gyrociliatus and D. taeniatus (Annelida: Polychaeta). Invertzool 2017, 14, 114–120. [Google Scholar] [CrossRef]
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).