1. Introduction
Chemotherapy is still considered as the major treatment option for advanced stage cancer. Transition metals
e.g. platinum, gold, ruthenium, copper and palladium are playing significant roles in developing chemotherapeutic agents since 1960s after serendipitous discovery of cisplatin. Even though earlier investigations demonstrated that palladium complexes were more toxic and less active than cisplatin, recently introduction of bulky ligands into palladium complexes demonstrated promising anticancer potential [1-3]. The quinoline scaffold has been used to develop various bio-active compounds which have been investigated against cancer, malaria, microbial infection, fungal infection, tuberculosis and leishmaniasis [
4]. Recently, review articles have been published on quinolone based tumour active compounds [
5,
6]. However, quinoline complexed palladium compounds have not been reported yet as anticancer drug candidates. In the present study synthesis of a palladium complex [Bis(quinoline)palladium (II) chloride] (coded as NH1) has been described followed by its characterization. Anticancer activity of the designed complex has been studied against seven different cancer cell lines.
Combination chemotherapy has gained popularity over monotherapy among clinicians in overcoming drug resistance. Phytochemicals possess great potential of chemopreventive properties and numerous research data suggest that phytochemicals can modulate various signaling pathways associated with tumour development and progression. We have been working on synergism from combination of metal-based drugs used in the clinic and designed compounds with phytochemicals against different cancer models [
7,
8]. Curcumin is the chief active constituent of the dried rhizomes of turmeric (
Curcuma longa) which has already demonstrated tumour suppressor activity in numerous preclinical and clinical studies [
9]. Recently, curcumin has been reported to potentiate the antitumour activity of cisplatin through enhancing cell death and cycle arrest. Moreover, curcumin increases sensitivity of cisplatin and prevent drug resistance by suppressing STAT3 and NF-ĸB signaling pathways [
10]. 6-shogaol is the dehydrated form of 6-gingerol obtained from ginger (
Zingiber officinale) which has been reported to have anti-proliferative, anti-metastatic and pro-apoptotic activities. But in combination with other chemotherapeutics 6-shogaol has not much studied.
In the present study, newly designed complex NH1 has been administered in binary sequenced combination with two phytochemicals curcumin and 6-shogaol against ovarian and colorectal cancer models. In addition, proteomic study has been conducted to identify the proteins believed to be responsible for antitumour action of the designed complex. Finally, preliminary toxicity profile of NH1 has been investigated in mice model.
2. Results
2.1. Synthesis
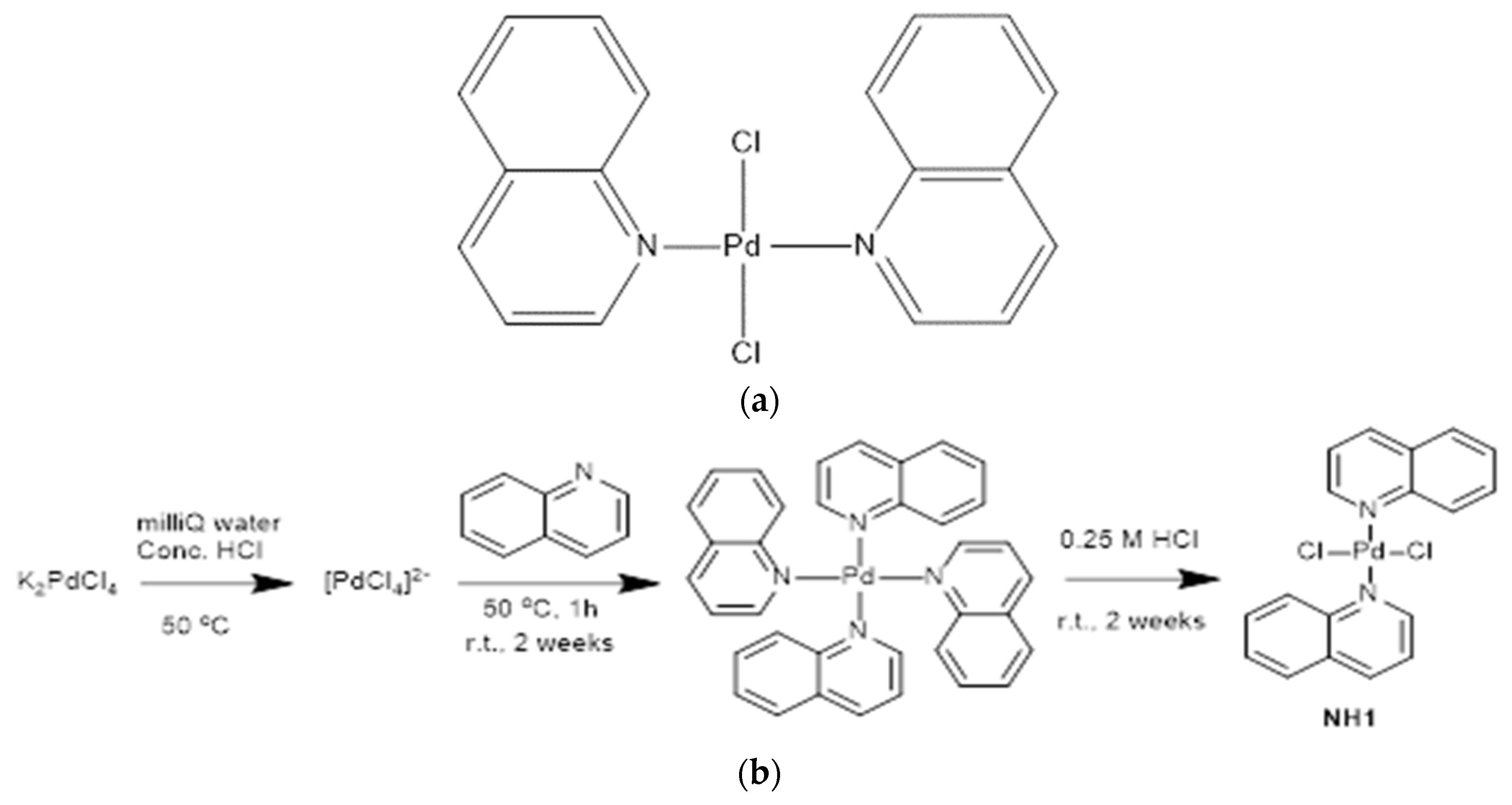
Quinoline chelated palladium compound encoded as NH1 was synthesized by reacting potassium tetrachloropalladate with the ligand quinoline. The chemical structure of the designed compound along with its synthesis scheme have been given in
Figure 1 (a) and
Figure 1 (b) respectively.
2.2. Antitumour activity of the complex
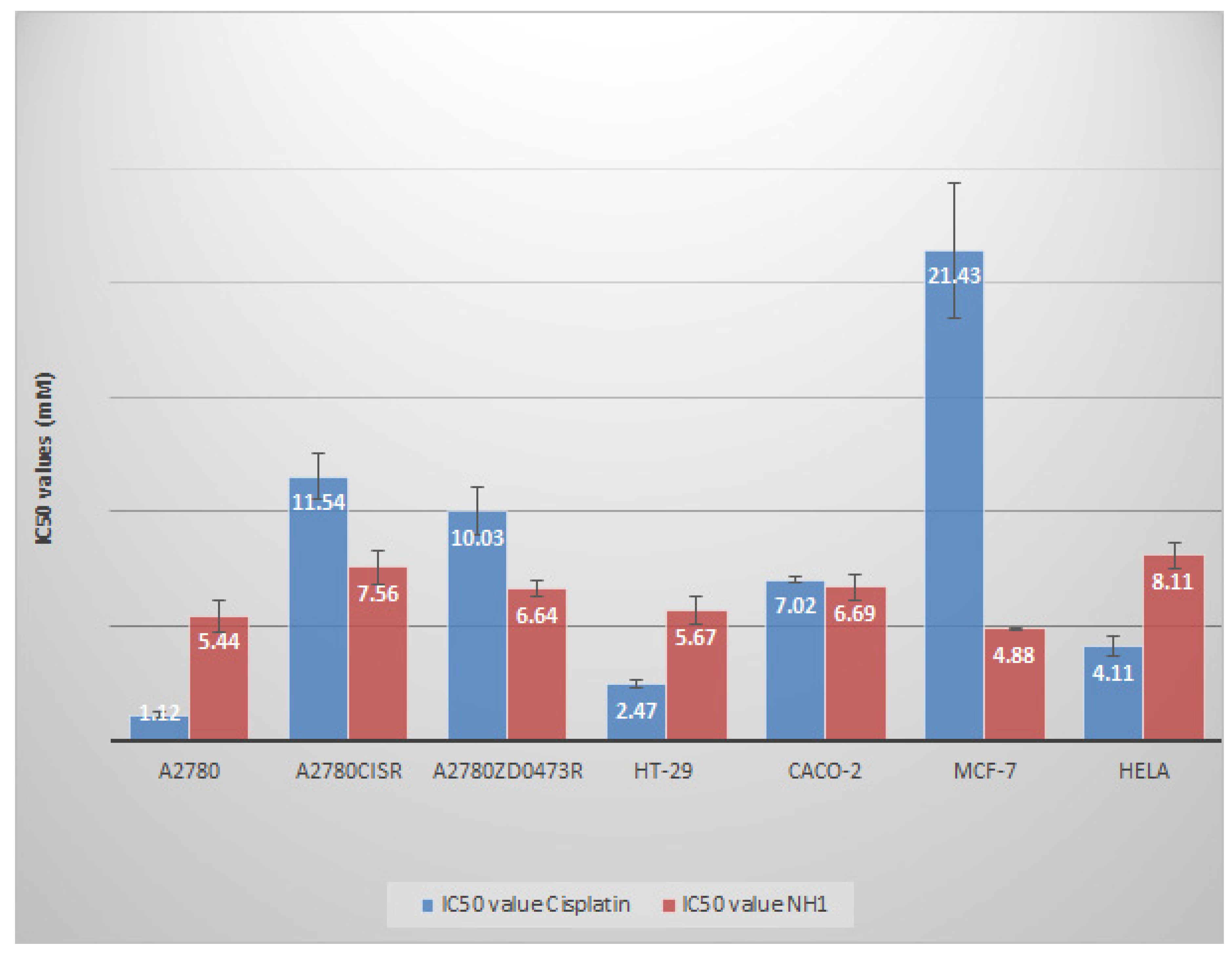
It is evident from
Figure 2 that NH1 possesses significant antitumour activity against the tested cancer cell lines that include ovarian, colorectal, breast and cervical cancers. Although NH1 is less active than cisplatin against parent ovarian A2780 cell line, it has significantly greater activity than cisplatin against resistant cell lines A2780
cisR and A2780
ZDO473R. NH1 demonstrates 4.4 times lower IC
50 value than cisplatin against MCF-7 breast cancer cell line but exhibits greater IC
50 value against Hela cervical cancer cell line. In colorectal cancer models, NH1 exhibits comparable activity with cisplatin against CACO-2 cell line but has lower activity against HT-29 cell line. Dose response curves have been displayed in supplementary information (figures S1 to S5).
Quinoline and its derivatives have been investigated for antitumour potential since 1950s, and now considered as privileged scaffold for development of anticancer drugs. In the last decade, several articles have been demonstrated encouraging antitumour activity of quinoline derivatives against a panel of cancer cell lines. In most of the cases ligation of quinolines with metal ions such as copper, platinum, chromium and cobalt were found to display greater cytotoxicity against cancer cells compared to cisplatin, irinotecan, topotecan taken as standards [11-13].
2.3. Combination study
Binary sequenced combinations of NH1 with curcumin and 6-shogaol have been investigated in A2780 ovarian cancer cell line with the results shown in
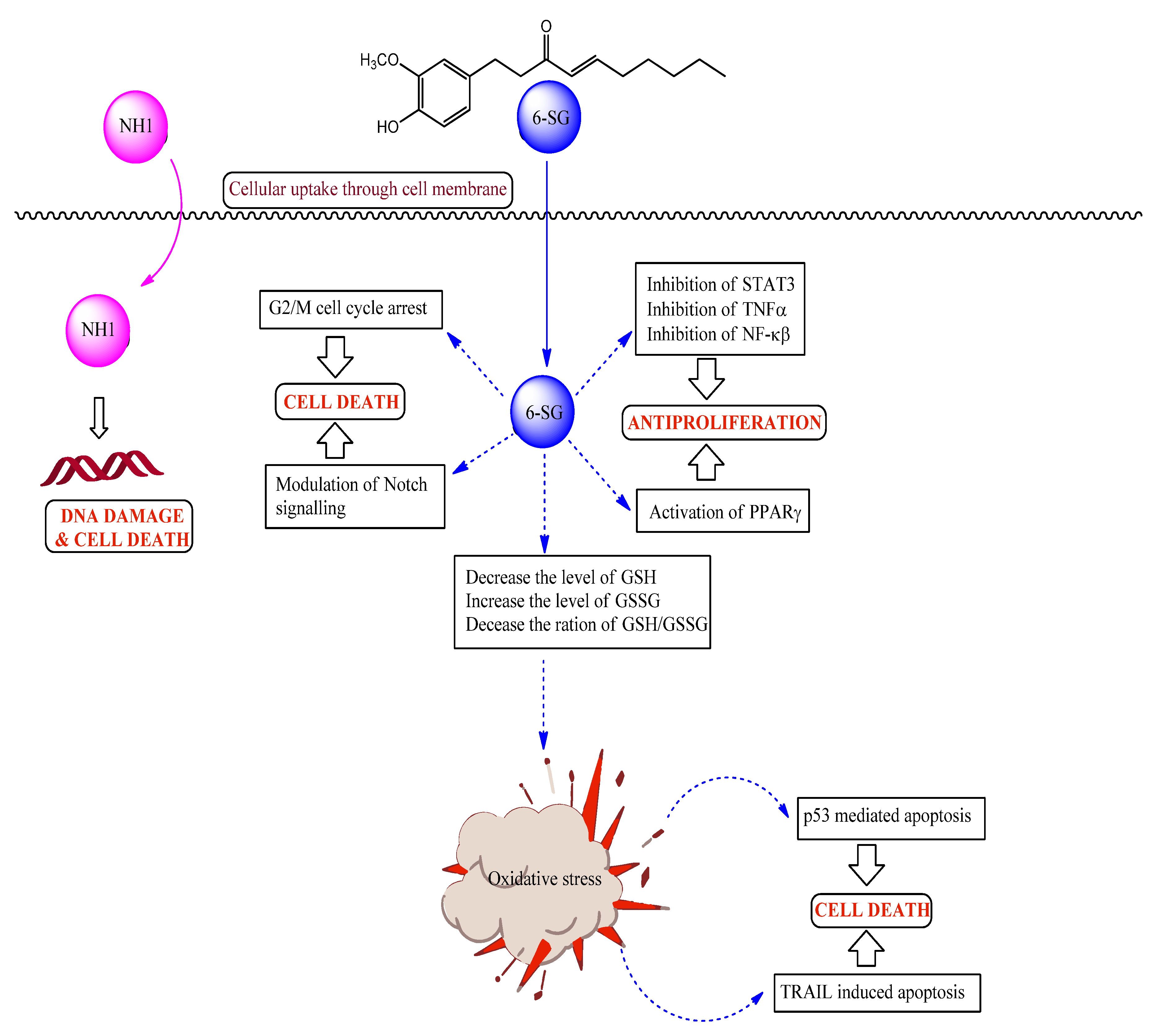
Table 1. The combined drug action from combination of NH1 and curcumin was mostly additive to antagonistic. In contrast, combination of NH1 with 6-shogaol demonstrated synergism depending on dose and sequence. When NH1 was combined with 6-shogaol, very strong synergism (combination index 0.11) was found at lower concentrations (ED
50 level) for all sequences of administration against the tested cell line. 0/4 sequence of administration produced greatest synergism followed by 4/0 while bolus displayed the least. Both 0/4 and 4/0 sequences of administration displayed strong synergism at ED
75 level as well while bolus administration caused additiveness at the same concentration in A2780 cell line. Anticancer mechanism of 6-shogaol has been correlated with oxidative stress mediated p53 apoptotic pathway [
14], inhibition of cell proliferation via activation of peroxisomal proliferator activated receptor γ [
15], TRAIL-induced apoptosis [
16], G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk [
17].
Figure 3 depicts the suggested pathways for synergism observed from combination of NH1 with 6-shogaol.
2.4. Proteomics
The study has revealed underlying mechanism on antitumour activity of NH1 alone in HT-29 colorectal cancer cell line as well as obtained synergism from combination of NH1 with 6-shogaol at 0/4 sequence of addition in A2780 ovarian cancer cell line. Eight proteins have been recognized which experienced significant changes in expression following treatment of NH1 alone in HT-29 colorectal cancer cell line. In contrast, nine proteins underwent significant changes in expression following combined treatment of NH1 with 6-shogaol at 0/4 sequence of addition. Change in folds of the respective proteins after treatment is given in
Table 2 (NH1 alone in HT-29 cell line) and
Table 3 (synergism from NH1 and 6-shogaol in A2780 cell line).
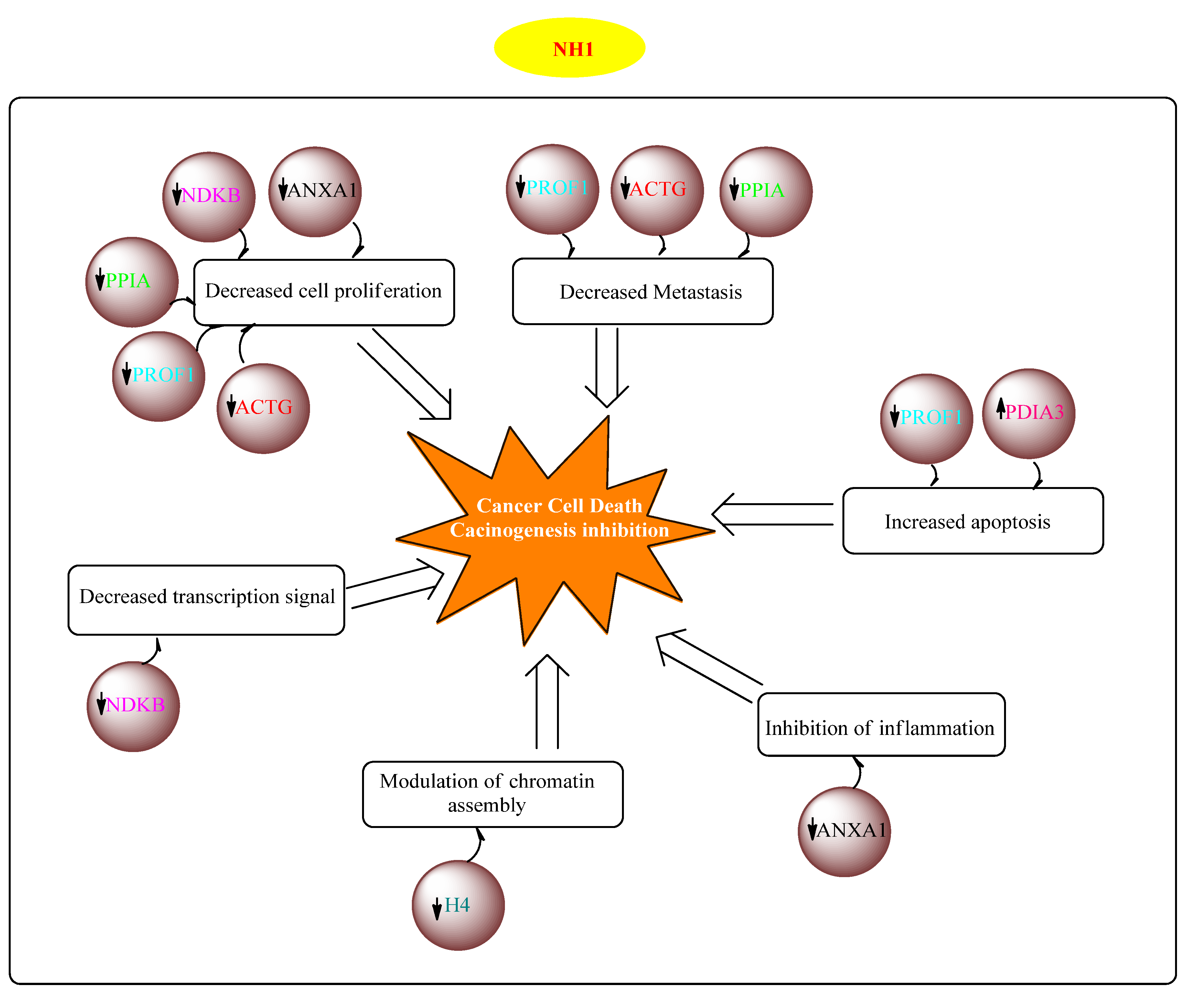
Among the eight proteins which were considered to be associated with the anticancer action of NH1 in colorectal cancer, seven of them were downregulated which are as: actin, cytoplasmic 2 (ACTG), profilin-1 (PROF1), peptidyl-propyl cis-trans isomerase A (PPIA), nucleoside diphosphate kinase B (NDKB), annexin A1 (ANXA1), histone H4 (H4) and ALDH class 2 (ALDH2). In addition, upregulation of protein disulfide-isomerase A3 (PDIA3) was correlated with the antitumour activity of NH1 in the colorectal cancer model.
Figure 4 provides proposed the mechanism of antitumour activity of NH1 based on proteomics.
Whereas, downregulation of eight proteins namely: 60 kDa heat shock protein, (CH60), 78 kDa glucose-regulated protein (HSP7C), Calreticulin (CALR), Polyubiquitin-B (UBB), Histone H3.3 (H3), Annexin A (ANXA1), Nascent polypeptide-associated complex subunit alpha (NACA) and Peptidyl-prolyl cis-trans isomerase A (PPIA) were associated with the synergistic anticancer activity from the combination of NH1 with 6-shogaol. Moreover, upregulation of Actin cytoplasmic 1 (ACTB) was thought to be involved with the synergism from combination of NH1 with 6-shogaol.
2.5. In vivo anticancer activity study
Ehrlich ascites carcinoma is one of the transplantable tumors in laboratory animals, which has been used in the present study to evaluate the anticancer activity of NH1. It has been observed from the study that, NH1 decrease the tumour volume significantly by 36.1 percent at a dose of 0.75 mg/kg compared to the control group. Increased tumour volume in the control group may be attributed to either angiogenesis, which is observed in the tumour-bearing peritoneal wall, or reduced lymphatic recovery system, which is associated with the impediment of the lymphatic by cancer cells, or owing to both of them. Similarly, hyperpermeability of micro-blood vessels in the peritoneal cavity may result in an inflamed abdomen [
18]. It can also be seen from
Table 4 that, NH1 decreases tumour cell count by 29 percent compared to control group which again indicates the treatment response. All the values are presented as mean±S.E.M (n=6). One-way ANOVA followed by Dunnett’s multiple comparisons was performed to analyze this dataset when compared against control. For Dunnett test, *P<0.05, **P<0.01, ***<0.001.
Anemia is a commonly encountered problem in cancer and is connected with fatigue and reduced quality of life. From table 4 below, it is evident that hemoglobin level is increased by 4% compared to control group followed by the treatment of NH1 at a dose of 0.75 mg/kg. Moreover, treatment of NH1 results increase in RBC count by 16 percent compared to control group. Thus, it can be said that investigated anticancer agent might counteracts the suppressive effect of EAC toxin on the bone marrow erythropoiesis which happens due to the deficiency of thiamine in the liver and blood of mice bearing EAC [
19].
White blood cells are a part of our immune system that protects the body from infection. Total white blood cell (WBC) count is often raised throughout infections, which is one of the nonspecific markers of inflammation and can be related to some particular kinds of cancers, such as leukemia. The normal number of WBCs in the blood is 4,500 to 11,000 WBCs per microliter. During cancer chemotherapy, WBC either returns to normal or may be lower than normal and may expose the patient to infection [
20]. In the present study, administration of NH1 at a dose of 0.75 mg/kg caused reduction of WBC by 48% compared to control group which is statistically highly significant at P value 0.005.
2.6. In vivo toxicological study
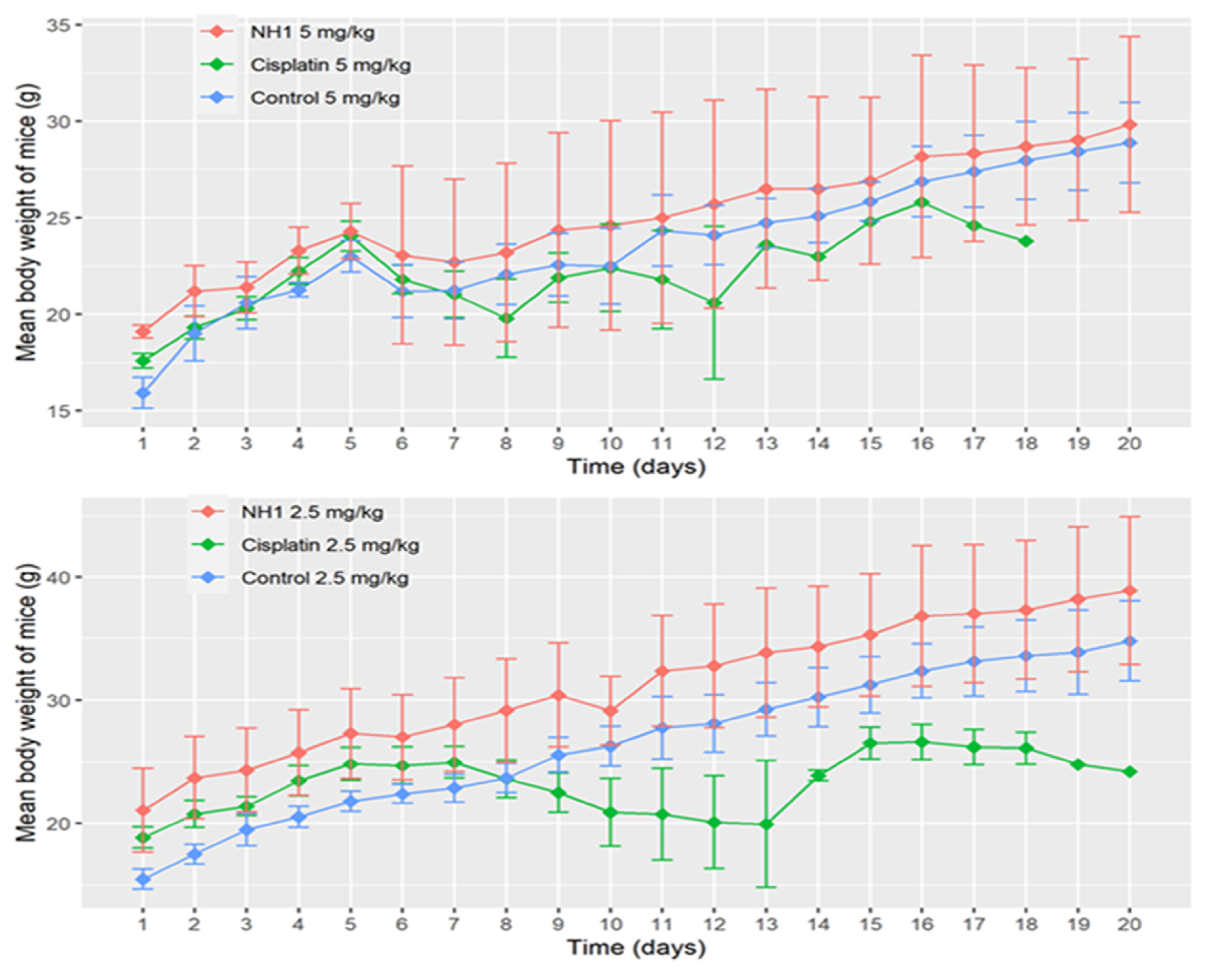
NH1 did not show any toxicity at any of the administered doses, either 2.5 mg/kg or 5 mg/kg. All mice were alive in control group and NH1 treated group at the lower dose. In Contrast, only one mouse was alive in cisplatin treated group till the end of the experiment at the same dose. Five mice of cisplatin treated group died within 15 days of experiment at the lower dose (2.5 mg/kg). Similarly, at the higher dose of cisplatin (5 mg/kg) none of the mice was alive till the end of the experiment and most of them died within ten days of the experiment. In contrast, none of the mice died in the control group orNH1 treated group at 5 mg/kg dose. Change in mean body weight of mice in different treatment groups is shown in
Figure 5, indicating that body weight has decreased drastically in cisplatin treated group at both lower and higher doses. Repeated measures two-way analysis of variance showed that decrease in body weight was highly significant (p- values shown in
Table 5) in cisplatin treated groups at both doses compared to corresponding control groups. However, change in body weight is not statistically significant in NH1 treated groups at any of the treatment doses compared to corresponding control groups.
Biochemical investigations for serum SGOT, SGPT and creatinine level have been presented in
Table 6. The results also supported that NH1 is not toxic towards liver or kidney, however cisplatin demonstrated significant toxicity towards both organs.
3. Materials and Methods
3.1. Chemistry (Reagents and Chemicals)
Potassium tetrachloropalladate (K2[PdCl4]); quinoline (Sigma Chemical Company, St. Louis, MO, USA); HCl (Ajax chemicals, Auburn, NSW, Australia); ethanol (Merck Pty. Ltd., Kilsyth, Australia).
3.2. Synthesis of [Bis(quinoline)palladium (II) chloride] coded as NH1
0.5 millimol of K2PdCl4 (0.163 g) was dissolved in 7.5 mL of milliQ water followed by addition of 0.25 mL of concentrated HCl. Five millimoles of quinoline (0.60 mL) were added dropwise over 1 h to the resulting solution at 50°C. The reaction mixture was stirred for 2 weeks at room temperature followed by addition of 4 mL of 0.25 M hydrochloric acid and continued stirring for further 2 weeks at room temperature. The mixture was centrifuged at 5500 rpm for 10 min to provide precipitate of NH1 that was purified by dissolving in 0.05 M HCl, followed by filtration and collection after washing successively with ice-cold water and ethanol.
3.3. Elemental and Spectral Characterization
Elemental microanalysis for C, H, and N was done using the micro analyser (Model PE2400 CHNS/O (PerkinElmer, Shelton, CT, USA)) available at Macquarie University. Palladium content was determined by graphite furnace atomic absorption spectroscopy. IR spectrum was recorded using a PerkinElmer FT-IR spectrometer. To obtain high resolution mass spectra, solution of NH1 made in methanol was injected via syringe infusion at 120 µL/hr into a Bruker Apex Qe 7T Fourier Transform Ion Cyclotron Resonance mass spectrometer (Bellerica, MA, USA) equipped with an electrospray ionisation source. The 1H NMR spectrum of NH1 (dissolved in deuterated DMSO) was recorded on a Bruker DPX400 spectrometer for which a 5 mm high-precision Wilmad NMR tube at 300 K (±1 K) was used.
NH1: Yield: 0.205 g (94.47%). Anal. Calcd. for [Pd(C
9H
7N)
2Cl
2] (435.65 g/mol): C= 49.63 %, H= 3.24%, N= 6.43%, Pd= 24.43%. Found: C=50.01%, H= 3.24%, N=6.45%, Pd=24.71%. Selected IR data (KBR, cm
-1): ῦ=1588, 1461, 1392, 1316, 1210, 1130, 869, 753, 644, 514.
1H NMR (400 MHz, DMSO): δ =8.91 (d, due to C
2H); 8.69 (t, due to C
3H); 8.33 (d, due to C
4H); 8.14 (d, due to C
5H); 8.02 (m, due to C
6H); 7.85 (t, due to C
7H); 7.79 (t, due to C
8H); 2.49 (s, due to DMSO). HRMS (ESI) m/z (%): Calculated for [{Pd(C
9H
7N)
2Cl
2}
2 - Cl]
+ 832.9444; found 832.9439(100), Calculated for [Pd(C
9H
7N)
2Cl
2 - Cl]
+ 398.9875; found 398.9878 (30). IR,
1H-NMR and Mass spectra of NH1 have been displayed in supplementary information (
Figure S6-S8).
3.4. Biological activity
In vitro antitumour activity and proteomics: MTT reduction assay [
21,
22] has been used to determine the anticancer activity of the drugs alone. The previously reported method was used for determining the activity of drugs in sequenced combination [
7]. In short, stock solutions of cisplatin (1 mM), NH1 (0.5 mM), curcumin (10 mM) and 6-shogaol (10 mM) was prepared by dissolving the respective drug in DMF + H
2O (1:4), DMSO, ethanol and ethanol respectively. For determining the antitumour activity of the drugs alone, different concentrations of drug solutions were made following serial dilution method. For example, concentrations of cisplatin drug solutions were ranging from either 0.8 μM to 100 μM (for A2780, HT-29, CACO-2, MCF-7 and HeLa cell lines) or 0.16 to 20 μM (for A2780
cisR and A2780
ZDO473R cell lines) and NH1 drug solutions were ranging from 0.8 to 100 μM (for all selected cell lines). For combination study, cells were treated with gradually increasing concentrations of selected compounds at fixed ratios of their IC
50 values. The concentration ranges were: NH1: 1.09–17.41 μM and 1.53–24.51 μM; curcumin: 1.36–21.76 μM and 1.94-31.04 μM; 6-shogaol: 1.89–30.24 μM and 1.5–24 μM for A2780 and A2780
cisR cell lines respectively. Prepared drug solutions were then added to the cells contained in 96 well plates and kept into incubator for 72 h under normal growth conditions. Then the plates were taken out from the incubator, medium was removed and 50 μL of MTT solutions were added to each vial of 96 well plates and incubated again for 4 h. Later on, 150 μL of DMSO were added to each well and formation of purple formazan product was detected using iMarkTM Bio-Rad Microplate Reader Version 1.04.02.E. The methodology used for proteomics was the same as described earlier in our published research article [
2,
8].
Experimental animals: Forty-eight Swiss-albino adult male mice (body weight 20 ± 5 g) were obtained from Department of Pharmacy, Jahangirnagar University, Bangladesh. Mice were acclimatized for 5 days before drug administration in cages (580 × 375 × 190 mm), supplied with commercially available food pellet [Rodent diet, ICDDRB, Bangladesh] and drinking water ad libitum. Body weight and other physical changes of each mouse were recorded every morning and evening.
Drug administration and treatment: Since the investigated drug NH1 was completely insoluble in water and very sparingly soluble in ethanol, stock solution of the drug was prepared by dissolving 5 mg of NH1 in 10 mL of DMSO. The drugs and control vehicles had been administered to experimental mice subcutaneously which were randomly categorized into two groups (each group having six mice) for antitumour activity study and six groups (each group having six mice) for preliminary toxicity study. During anticancer activity study, control group A received DMSO at 0.75 mg/kg body weight while treatment group B received investigational drug NH1 at a dose of 0.75 mg/kg body weight. In contrary, during toxicity study C1 group received solvent DMSO at 2.5 mg/kg body weight while C2 group received solvent DMSO at 5 mg/kg body weight; E1 group received compound NH1 at a dose of 2.5 mg/kg body weight while E2 group received compound NH1 at a dose of 5 mg/kg body weight; S1 group received anticancer drug cisplatin at a dose of 2.5 mg/kg body weight while S2 group received anticancer drug cisplatin at a dose of 5 mg/kg body weight respectively.
Transplantation of EAC cell: EAC cells used for this experiment were obtained from cancerous mice of Jahangirnagar University. The EAC cells propagated for 12- 14 days were used in the experiment. The mice were first made unconscious using chloroform. Then the ascitic fluid was collected using a 1mL syringe. Then it was diluted two times using 0.9% NaCl saline. And at last, it was diluted in phosphate buffer solution (PBS) and prepared for cell count under an electron microscope. Each mouse was given 0.5 mL of EAC cells. All the groups were injected with EAC cells (0.5 mL of 2×106 cells/mouse) intra-peritoneally. This was taken as day zero. After twenty hours of EAC administration, drug treatment was started.
Tumour weight, volume and cell count: Tumour growth was observed by recording daily weight fluctuations and host endurance time was recorded. The tumor weight was measured by taking the weight of the mice previously and subsequently the collection of the ascitic fluid from the peritoneal cavity. For measuring tumor volume, the ascitic fluid was collected from the peritoneal cavity of Swiss albino mice and the volume was measured by using a graduated centrifuge tube. The ascitic fluid taken from the peritoneal cavity of the mice was taken with a WBC pipette and diluted 100 times with normal saline and 10 times with PBS. A drop of the diluted cell suspension was positioned on Neubauer’s counting chamber and the numbers of cells in the 64 squares were calculated.
Hematological and biochemical assay: Animal sacrifice, blood and organs collection were conducted following the same procedure as described in previous report [
2]. In brief, blood was collected and centrifuged at 1000 rpm. Later, collected plasma and serum were preserved at – 80 °C for the determination of hemoglobin, RBC, WBC, SGPT, SGOT and creatinine levels in blood. Biochemical investigations were done in an auto analyser (Photometer 5010 V5+, Robert Riely, Berlin) using Sigma Aldrich (Merc) reagent kit. Approval for preliminary toxicity study using mice model was obtained from the Biosafety, Biosecurity and Ethical Committee [Approval Number: BBEC-JU/M2019(12)1 and BBEC-JU/M2022(03)1] of Jahangirnagar University, Savar, Dhaka, Bangladesh. Statistical analysis was done through one-way ANOVA followed by Dunnett’s multiple comparisons for anticancer activity study while for preliminary toxicity study data were analyzed via SPSS based on Satterthwaite's method for equality of variances.
4. Conclusions
Palladium complex [Bis(quinoline)palladium (II) chloride] coded as NH1 has been synthesized, characterized and studied for activity on ovarian and colorectal cancer cell lines. The investigated compound showed better or at least comparable activity against seven different cancer models. Proteomic studies revealed 14 key proteins associated with antitumour activity of NH1. In vivo antitumour activity study on EAC bearing Swiss-albino mice also proved the efficacy of the investigated compound. Moreover, preliminary toxicity study on Swiss-albino mice revealed that NH1 demonstrated lower toxicity compared to standard drug cisplatin. Thus, NH1 is considered to have a great potential to be developed as a new anticancer drug.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, M.N.A. and F. H.; methodology, J. Q. Y., M.A.A., M.N.A., P. B., and N. P.; formal analysis M.N.A., N. P. and J. Q. Y.; investigation, M.N.A.; in vivo antitumour study, F.A.R. and M.S.; preliminary toxicity study, F.A.R. and M.S.; data curation, J. Q. Y. and F. H.; writing—original draft preparation, M.N.A.; writing—review and editing, F. H., M.A.A. and N.P.; supervision, F. H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The mice model preliminary toxicity study was conducted according to the guidelines of Ethics Committee of JAHANGIRNAGAR UNIVERSITY (protocol code: BBEC-JU/M2019(12)1 and date of approval was 7th December 2019). In vivo anticancer activity of NH1 (novel tumour active compound) was also conducted according to the guidelines of Ethics Committee of JAHANGIRNAGAR UNIVERSITY (protocol code: BBEC-JU/M2022(03)1 and date of approval was 6th March 2022).
Data Availability Statement
Not applicable.
Acknowledgments
Md Nur Alam is grateful to Department of Education and Training, Government of Australia for supporting him through ‘Endeavour postgraduate Scholarship’.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; IC50, half maximal inhibitory concentration; NH1, Bis(quinoline) palladium (II) chloride.
References
- Alam, M.N.; Huq, F. Comprehensive review on tumour active palladium compounds and structure–activity relationships. Coord.Chem. Rev. 2016, 316, 36–67. [CrossRef]
- Alam, M. N.; Moni, M. A.; Yu, J. Q.; Beale, P.; Turner, P.; Proschogo, N.; Rahman, M. A.; Hossain, M.; Huq, F. Promising Anticancer Activity of [Bis (1, 8-quinolato) palladium (II)] Alone and in Combination. Int. J. Mol. Sci. 2021, 22, 8471. [CrossRef]
- Alam, M.N.; Yu, J.Q.; Beale, P.; Turner, P.; Proschogo, N.; Huq, F. Crystal Structure, Antitumour and Antibacterial Activity of Imidazo [1, 2-] pyridine Ligand Containing Palladium Complexes. ChemistrySelect 2020, 5, 668–673. [CrossRef]
- Nainwal, L. M.; Tasneem, S.; Akhtar, W.; Verma, G.; Khan, M. F.; Parvez, S.; Shaquiquzzaman, M.; Akhter, M.; Alam, M. M. Green recipes to quinoline: a review. Eur. J. Med. Chem. 2019, 164, 121-170. [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M. R.; Ali, M. R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871-910. [CrossRef]
- Jain, S.; Chandra, V.; Jain, P. K.; Pathak, K.; Pathak, D.; Vaidya, A.Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019, 12, 4920-4946. [CrossRef]
- Alam, M.N.; Yu, J.Q.; Beale, P.; Huq, F. Cisplatin in combination with emetine and patulin showed dose and sequence dependent synergism against ovarian cancer. Synergy 2020, 10, 100060. [CrossRef]
- Alam, M.N.; Yu, J.; Beale, P.; Huq, F. Dose and Sequence Dependent Synergism from the Combination of Oxaliplatin with Emetine and Patulin against Colorectal Cancer. Anti-Cancer Agents Med. Chem. 2020, 20, 264–273. [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. BioMed. Res. Int. 2018, Article ID 4154185. [CrossRef]
- Abadi, A.J.; Mirzaei, S.; Khaksary M.; Mahabady.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A. R.; Hamblin, M. R.; Hushmandi, K.; Zarrabi, A.; Sethi, J. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytotherapy Res. 2021. [CrossRef]
- Liu, Z.-C.; Wang, B.-D.; Li, B.; Wang, Q.; Yang, Z.-Y.; Li, T.-R.; Li, Y. Crystal structures, DNA-binding and cytotoxic activities studies of Cu (II) complexes with 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur. J. Med. Chem. 2010, 45, 5353-5361. [CrossRef]
- Motswainyana, W. M.; Onani, M. O.; Madiehe, A. M.; Saibu, M.; Jacobs, J.; Van Meervelt, L.Imino-quinolyl palladium (II) and platinum (II) complexes: Synthesis, characterization, molecular structures and cytotoxic effect. Inorg.ChimicaActa. 2013, 400, 197-202. [CrossRef]
- Arzuman, L.; Beale, P.; Yu, J.; Huq, F. Synthesis of tris (quinoline) monochloroplatinum (II) Chloride and its Activity Alone and in Combination with Capsaicin and Curcumin in Human Ovarian Cancer Cell Lines. Anticancer. Res. 2016, 36, 2809-2818.
- Warin, R. F.; Chen, H.; Soroka, D. N.; Zhu, Y.; Sang, S. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. J.Agri. Food. Chem. 2014, 62, 1352-1362. [CrossRef]
- Tan, B. S.; Kang, O.; Mai, C. W.; Tiong, K. H.; Khoo, A. S.-B.; Pichika, M. R.; Bradshaw, T. D.; Leong, C.-O. 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor γ (PPARγ). 2013,Cancer. Lett. 336, 127-139. [CrossRef]
- Han, M. A.; Woo, S. M.; Min, K.-j.; Kim, S.; Park, J.-W.; Kim, D. E.; Kim, S. H.; Choi, Y. H.; Kwon, T. K. 6-Shogaol enhances renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated cytochrome c release and down-regulation of c-FLIP (L) expression. Chem.-biol. Interact. 2015, 228, 69-78. [CrossRef]
- Qi, L.-W.; Zhang, Z.; Zhang, C.-F.; Anderson, S.; Liu, Q.; Yuan, C.-S.; Wang, C.-Z. Anti-colon cancer effects of 6-shogaol through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am.J. Chin. Med. 2015, 43, 743-756. [CrossRef]
- Funasaka, T., Haga, A., Raz, A., & Nagase, H. (2002). Tumor autocrine motility factor induces hyperpermeability of endothelial and mesothelial cells leading to accumulation of ascites fluid. Biochemical and Biophysical Research Communications, 293(1), 192-200. [CrossRef]
- Trebukhina, R. V., V. G. Petushok, V. N. Tumanov, and G. N. Mikhal'tsevich. "Level of thiamine diphosphate in the liver of tumor-bearing animals kept on a diet including an excessive amount of vitamin B 1." Voprosy Pitaniia 1 (1986): 63-65.
- Oun, Rabbab, Yvonne E. Moussa, and Nial J. Wheate. "The side effects of platinum-based chemotherapy drugs: a review for chemists." Dalton transactions 47, no. 19 (2018): 6645-6653. [CrossRef]
- Hermersdörfer, H. RI Freshney: Culture of Animal Cells. A Manual of Basic Technique; John Wiley and Sons, Inc.: New York, NY, USA, 1994; Volume 39, pp. 184–185.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).