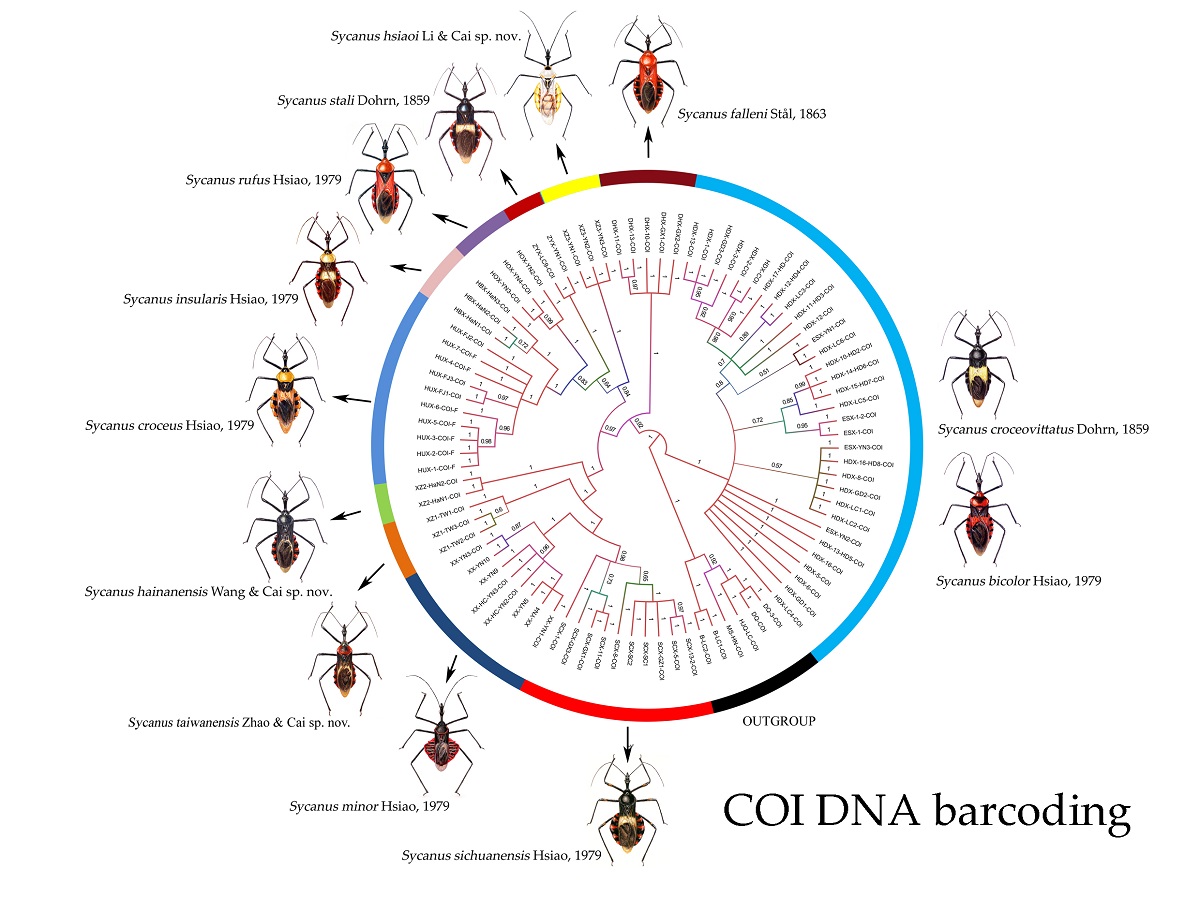
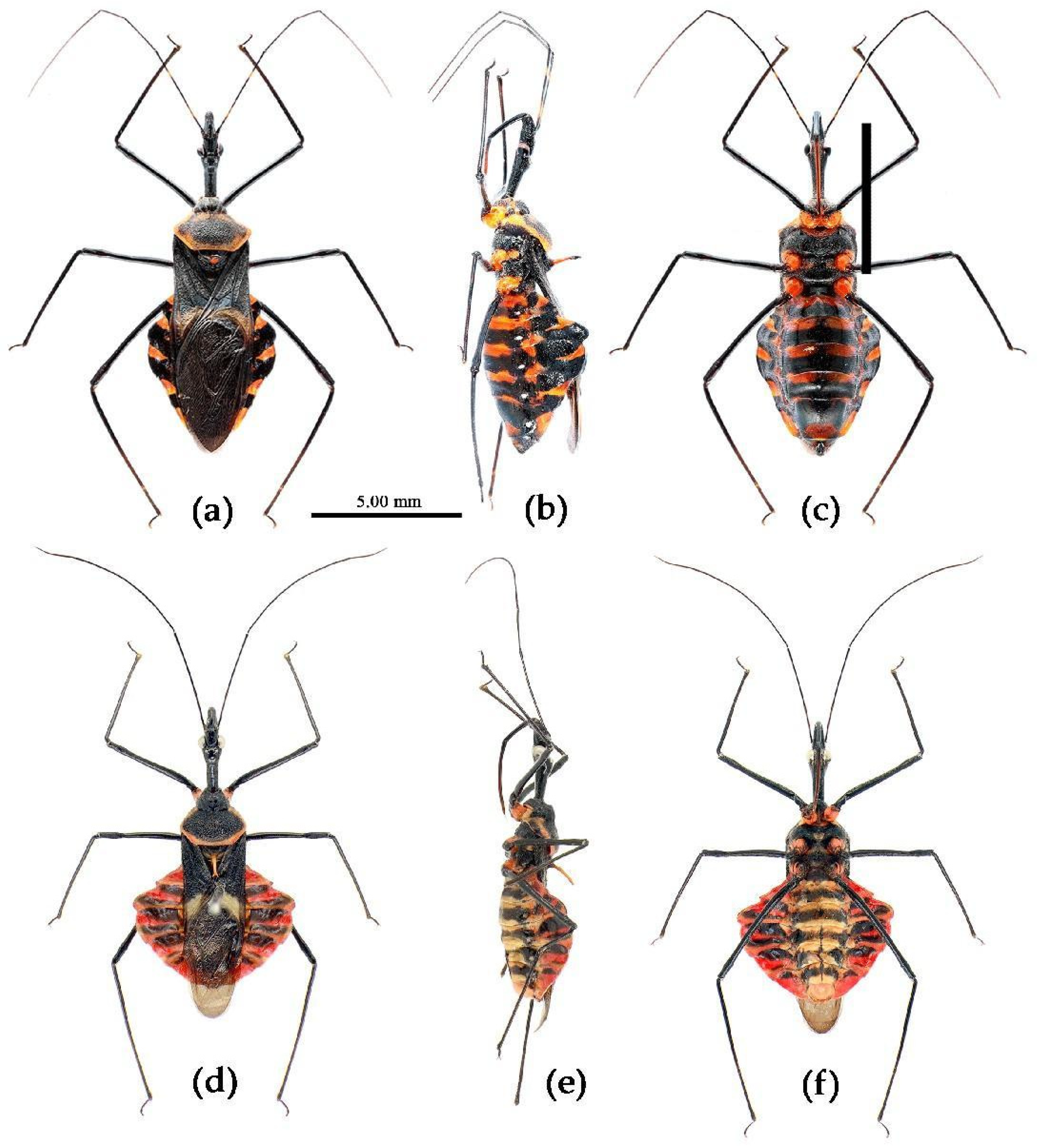
3.3. Systematics
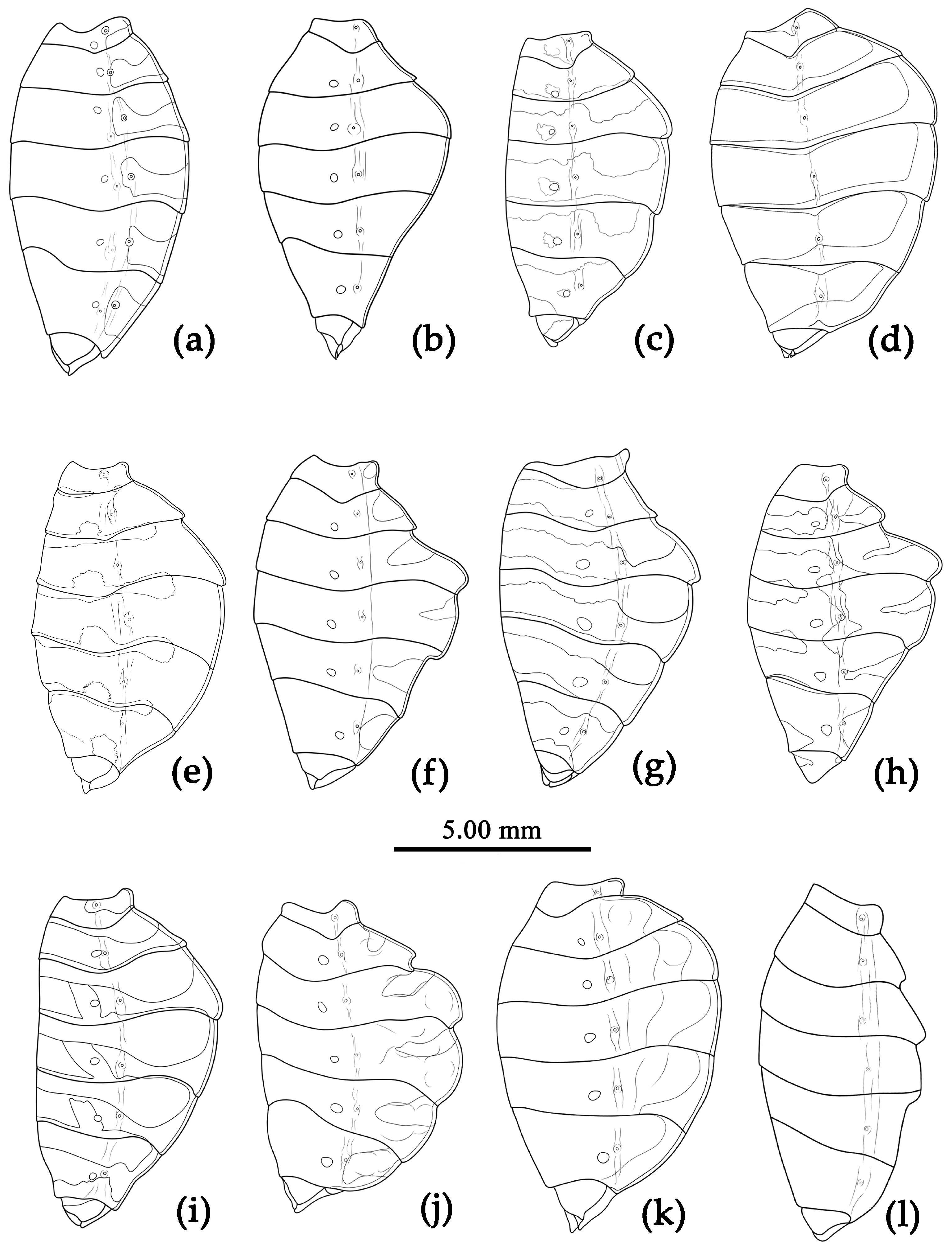
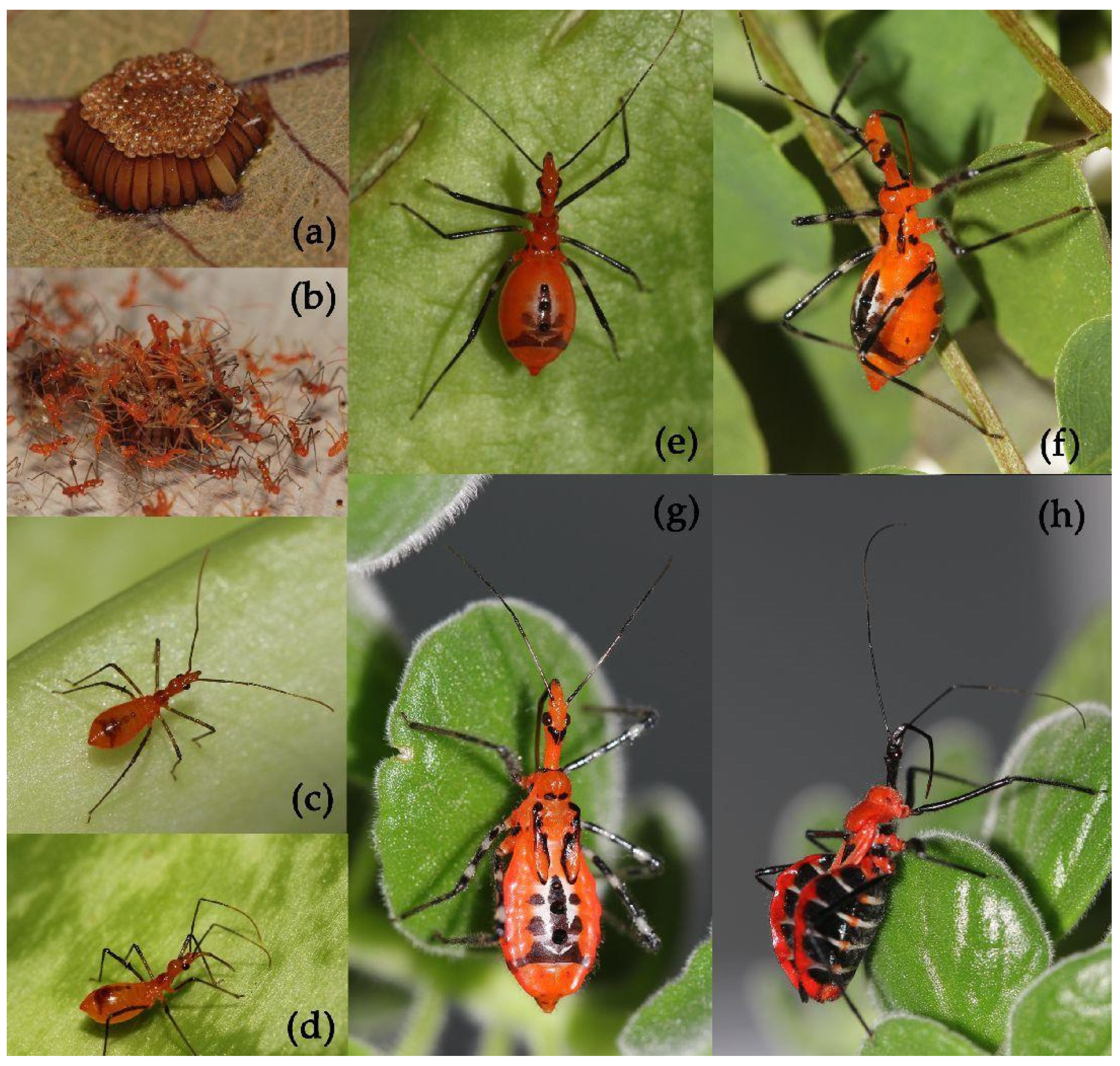
Diagnostic characters. Body of median to large size, mostly large in size. Head unarmed, spindle-shaped, slender and elongate, and longer than pronotum and scutellum together in length; rostrum slender, second rostral segment distinctly longer than first segment; first antennal segment sub-equal to fore femur in length. Pronotum unarmed and constricted before middle; apical angle of collar round; anterior pronotal lobe small and bulged, shorter than half of posterior lobe in length and width, its median longitudinal sulcus short and deeply depressed; posterior lobe wide, its surface rough or wrinkled; posterior and lateral angles obtuse and rounded; posterior margin nearly straight; scutellum round, apical part with a long spine or tubercle, apex of long spine bi-forked. Macropterous form, hemelytra long and passing abdominal apex; legs lender, femora sometimes sub-nodular; abdomen laterally dilated, connexivum strongly expanded dorsal-laterally, more or less undulated, lateral margins evenly curved.
Distribution. Oriental (79), Madagascar (1).
The key to the Chinese species in the genus Sycanus Amyot & Serville, 1843
- 1.
Pronotum totally black...2
- -.
Pronotum red or bicolor...8
- 2.
Scutellum with short tubercle-shaped spine...Sycanus stali Dohrn, 1859
- -.
Scutellum with long spine, apex bi-forked...3
- 3.
Connexivum ventrally and dorsally with red markings…4
- -.
Connexivum totally black…5
- 4.
Corium black, posterior margin yellowish...Sycanus hainanensis Wang & Cai sp. nov.
- -.
Corium yellowish, basal and apical parts black...Sycanus sichuanensis Hsiao, 1979
- 5.
Basal half of hemelytron black, apical half yellow…6
- -.
Most of hemelytron black...7
- 6.
Apical half of corium pale golden-yellow...Sycanus croceovittatus Dohrn, 1859
- -.
Apical half of corium pale stramineous...Sycanus collaris (Fabricius, 1781)#
- 7.
Hemelytron black, apical margin yellow…Sycanus bifidus (Fabricius, 1787)#
- -.
Hemelytron greyish yellow, only basal part black…Sycanus fuscirostris Dohrn, 1859#
- 8.
Apical spine of scutellum long, apex biforked…9
- -.
Apical spine of scutellum very short, apex un-biforke…14
- 9.
Pronotum and corium of hemelytron totally red…Sycanus falleni Stål, 1863
- -.
Pronotum and corium of hemelytron partly red…10
- 10.
Posterior pronotal lobe red, median part with black markings; fourth and sixth abdominal connexiva laterally expended…11
- -.
Posterior pronotal lobe yellow or greyish yellow; abdomen laterally roundly expended…12
- 11.
Abdomen and connexivum red or yellow with black markings...Sycanus minor Hsiao, 1979
- -.
Abdomen totally black, connexivum bicolor...Sycanus taiwanensis Zhao & Cai sp. nov.
- 12.
Corium of hemelytron totally yellowish...Sycanus hsiaoi Li & Cai sp. nov.
- -.
Basal part of corium of hemelytron black, apical half yellow...13
- 13.
Posterior pronotal lobe and median transversal markings of hemelytron pale grayish yellow…Sycanus insularis Hsiao, 1979
- -.
Posterior pronotal lobe orange, most of corium of hemelytron (except inner side and most of clavus black) yellow or orange…Sycanus croceus Hsiao, 1979
- 14.
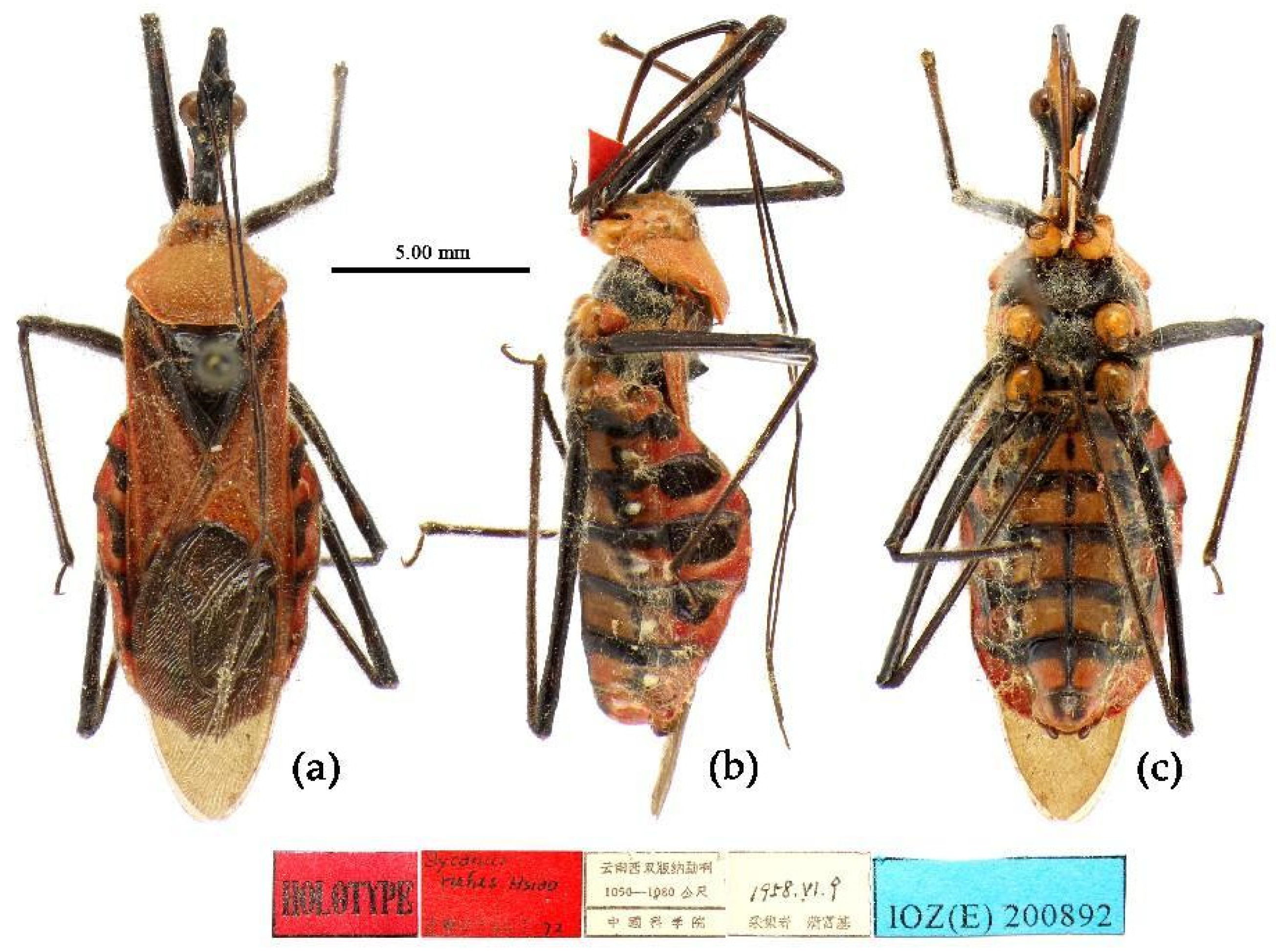
Pronotum and corium of hemelytron totally red; ventral surface of head paler; abdominal sterna yellow, inter-segment with black stripe and lateral side with black round markings; black markings of sixth and seventh connexival segments not extending to outer margin...Sycanus rufus Hsiao, 1979
- -.
Pronotum and corium of hemelytron bicolor, black and red; ventral surface of head black; abdominal sterna black; black markings of connexivum totally extending to outer margin......Sycanus bicolor Hsiao, 1979
#Notes. Sycanus collaris (Fabricius, 1781), Sycanus bifidus (Fabricius, 1787) and Sycanus fuscirostris Dohrn, 1859 have been recorded in China, but we have not examined the specimen for the three species. They are not included in this study.
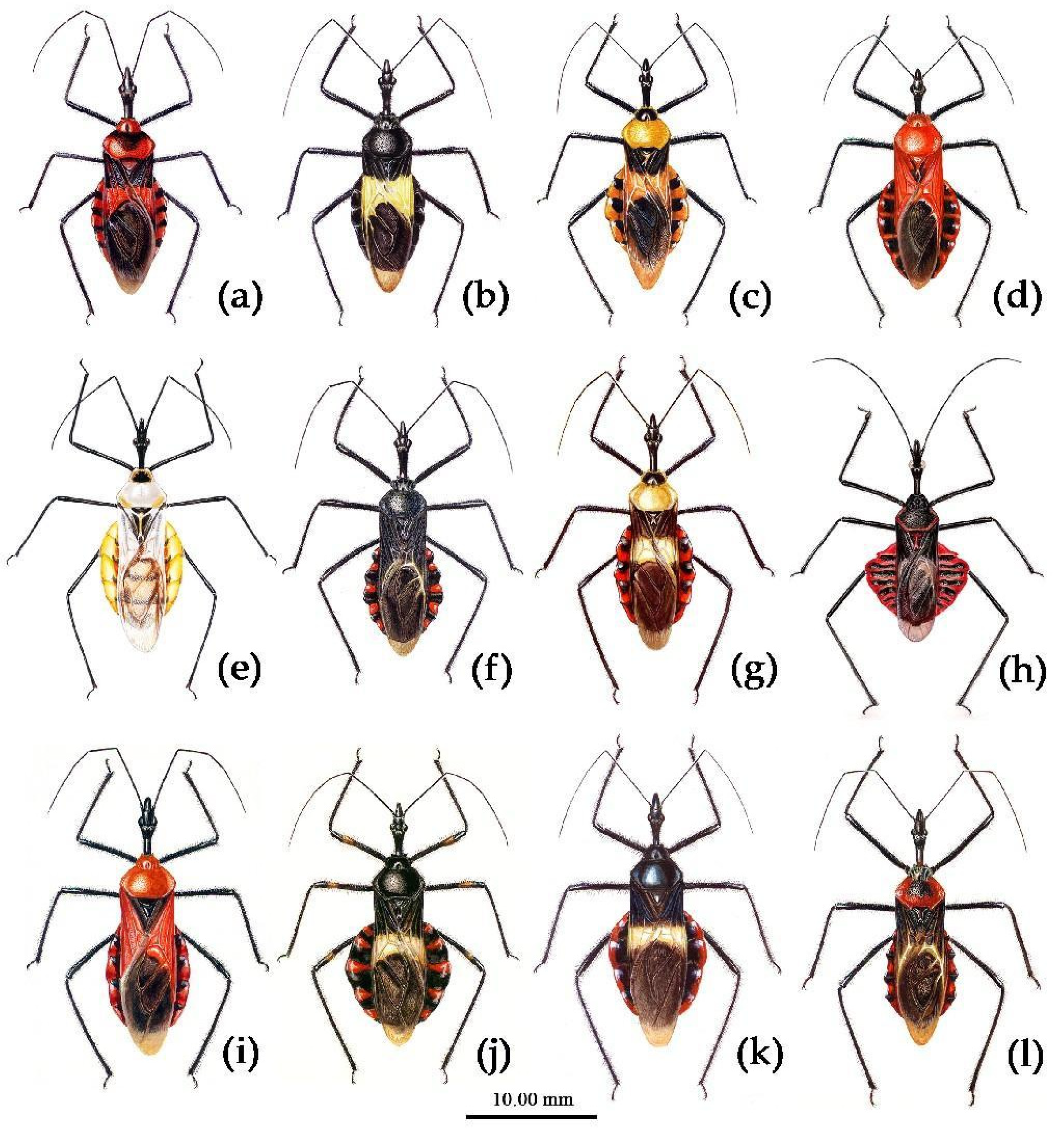
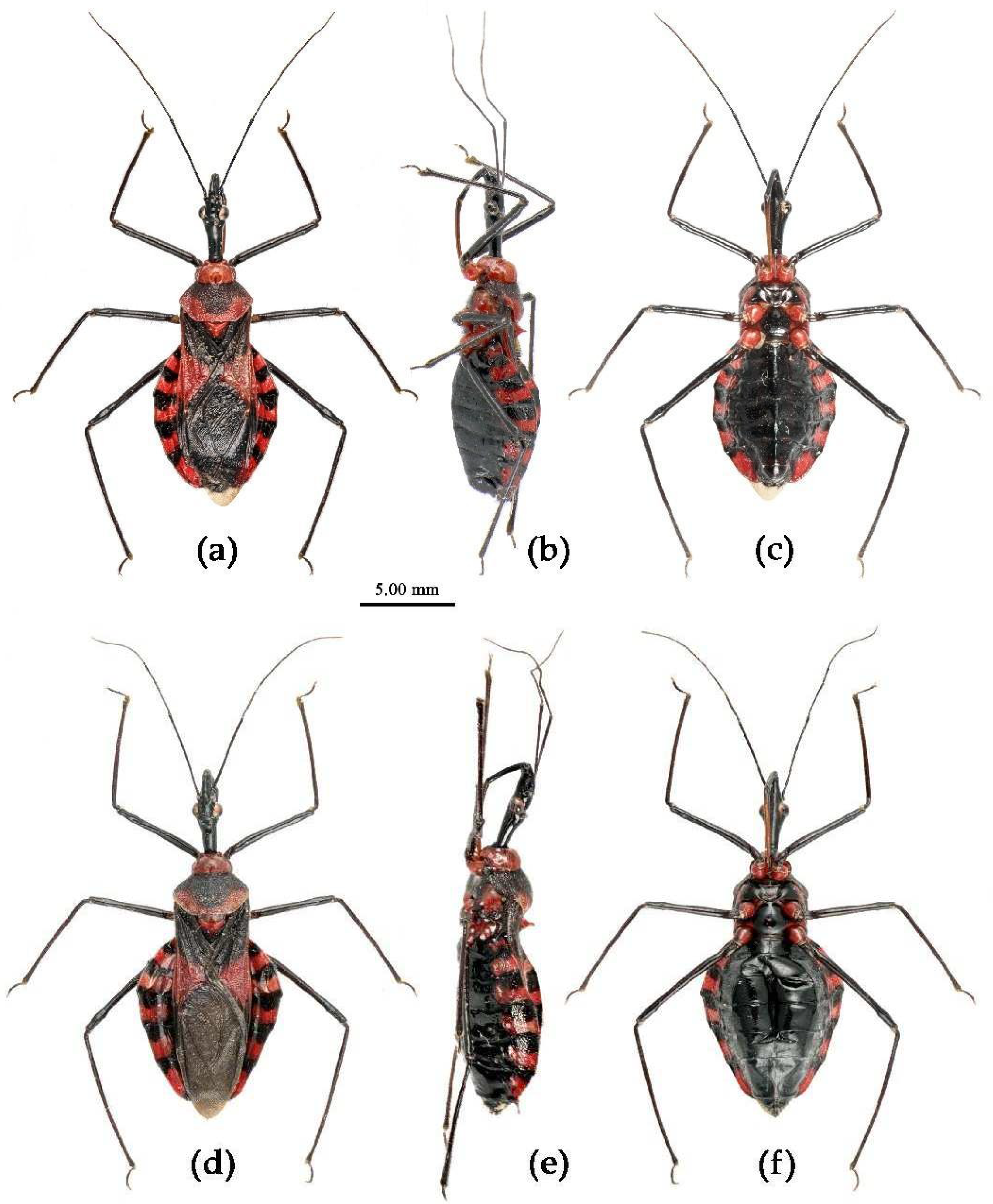
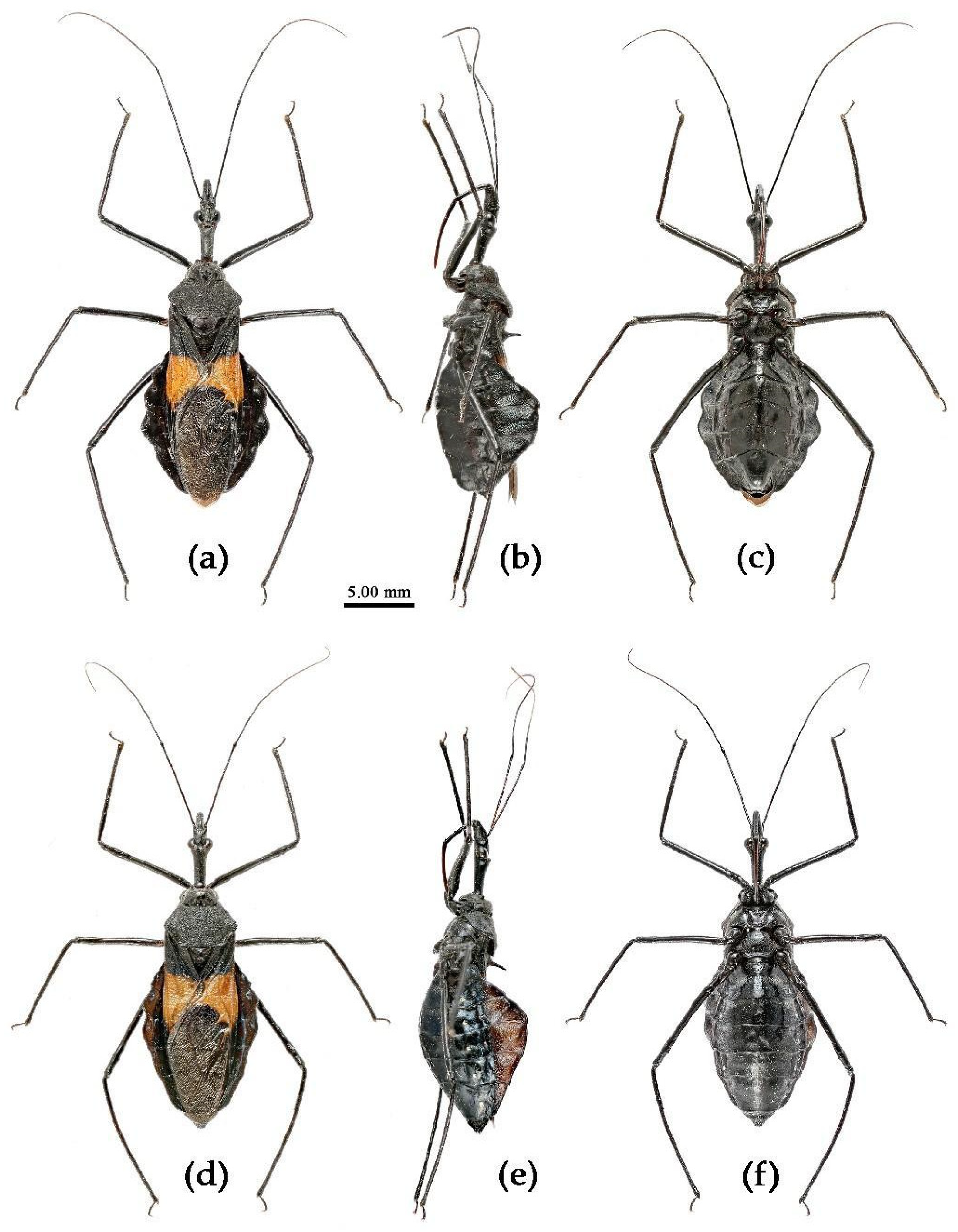
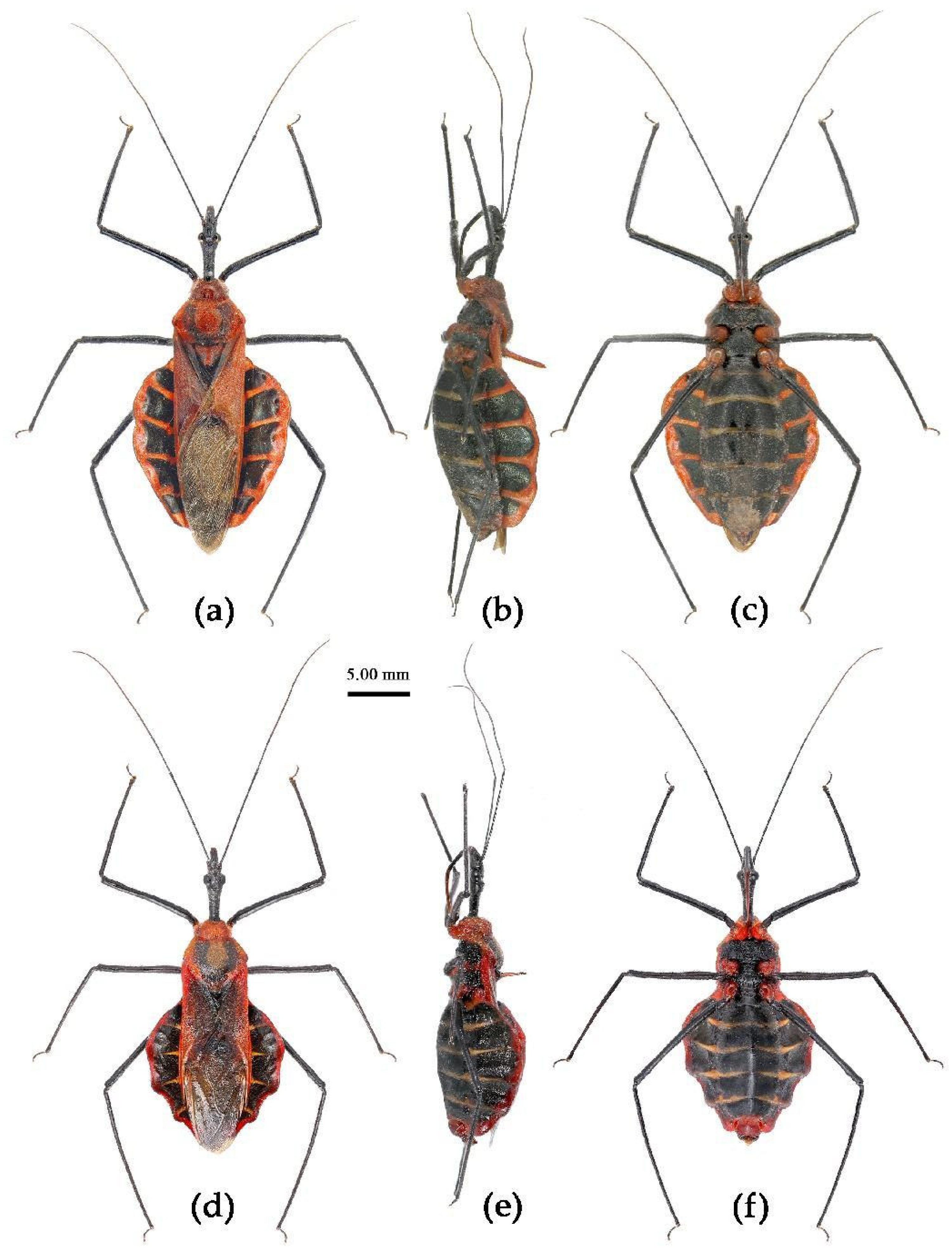
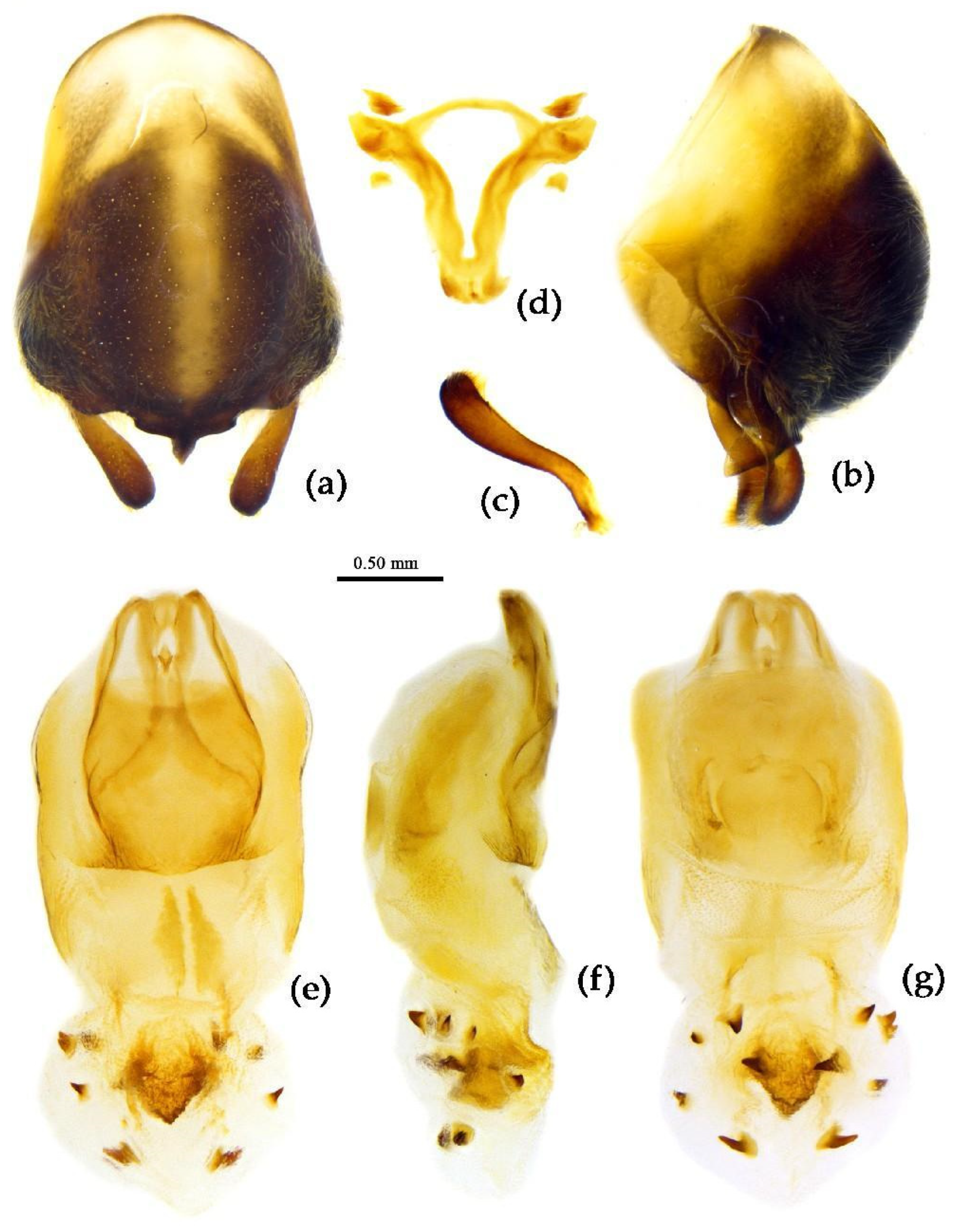
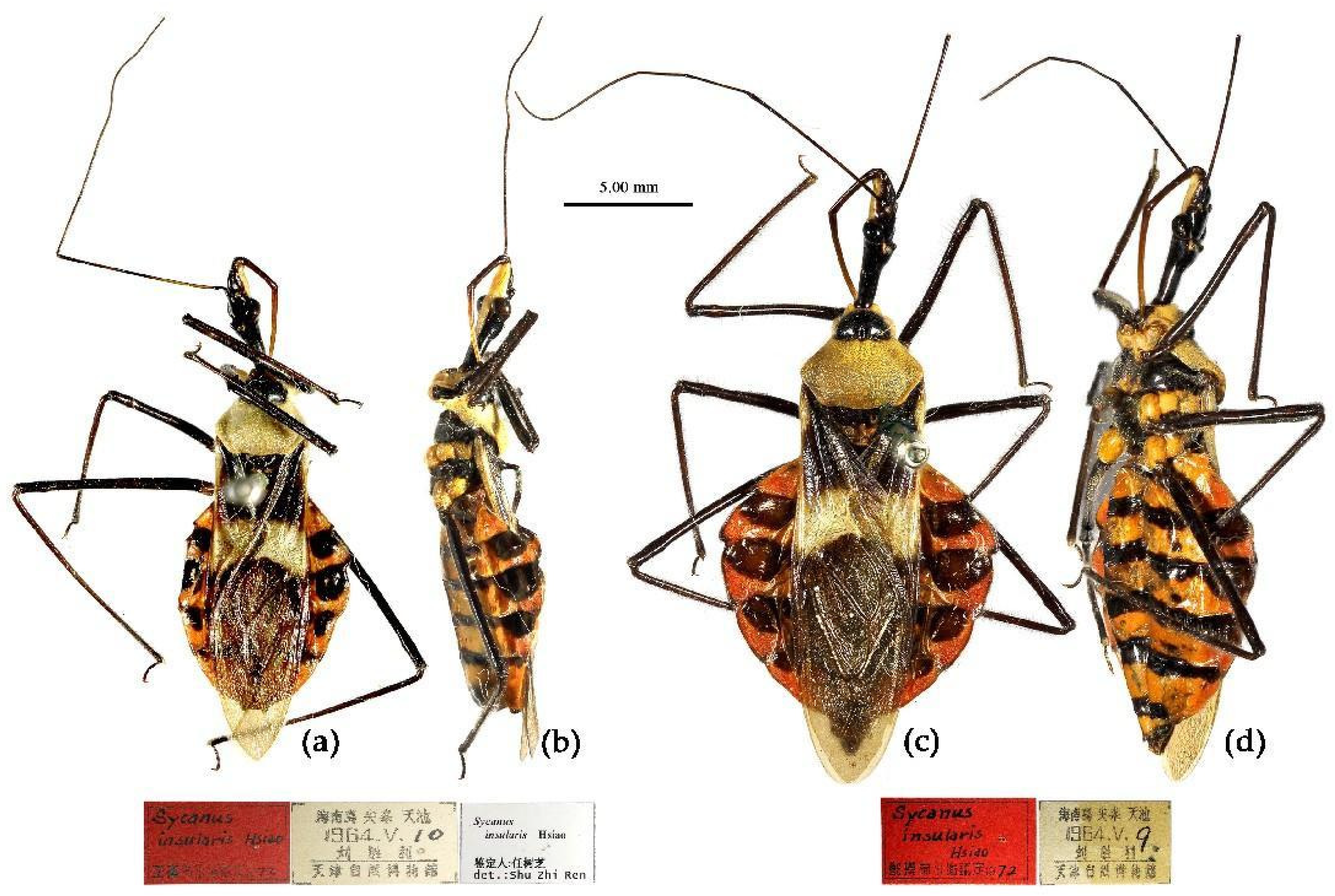
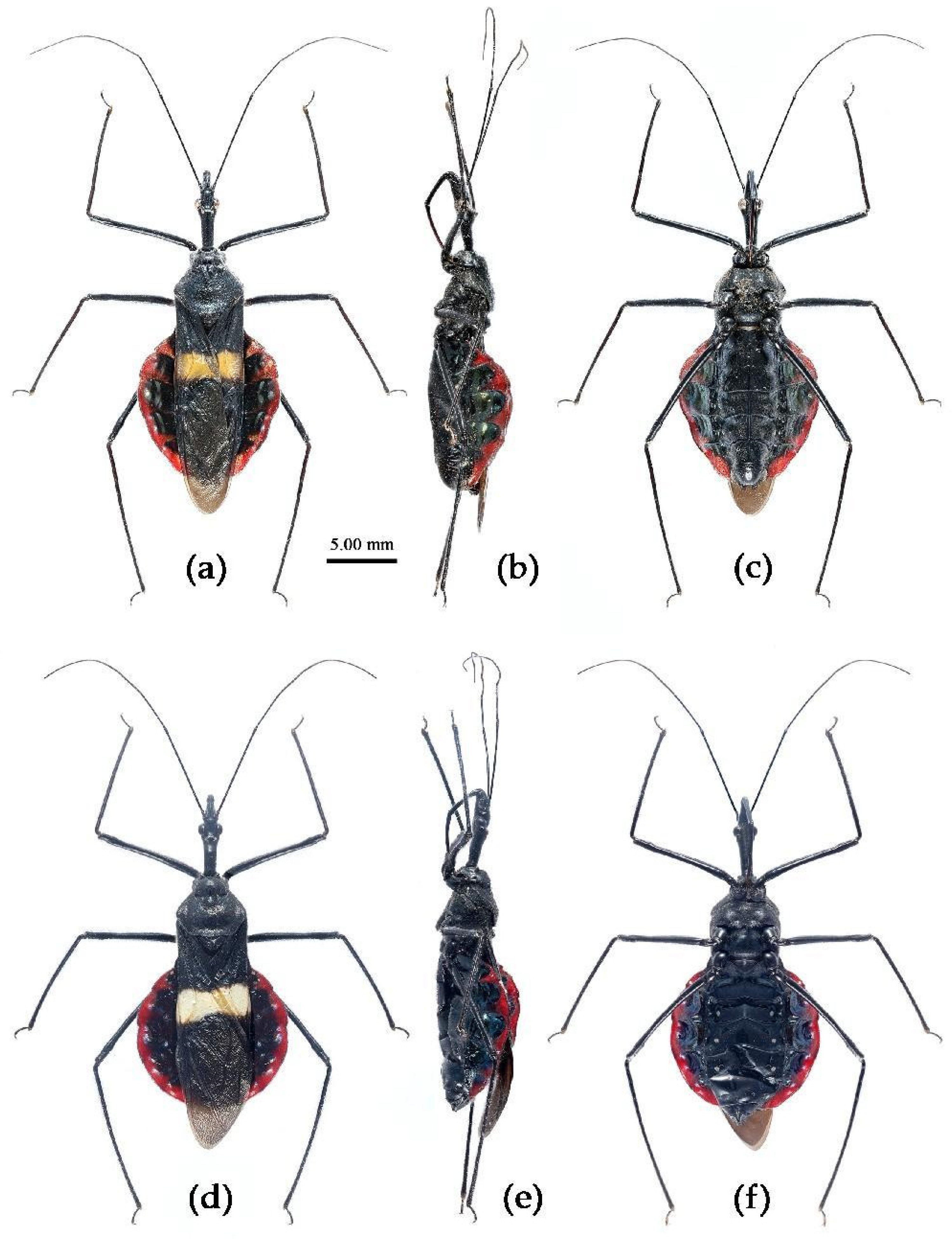
(1) Sycanus bicolor Hsiao, 1979二色犀猎蝽
(Figures 2a, 3a, 4a , 7, 8, S4)
Sycanus bicolor Hsiao, 1979: 141, 154[
30]; Hsiao & Ren 1981: 523[
31]; Maldonado-Capriles 1990: 310[
24]; Putshkov & Putshkov 1996: 259[
32].
Redescription. Coloration. Body black, with red markings (
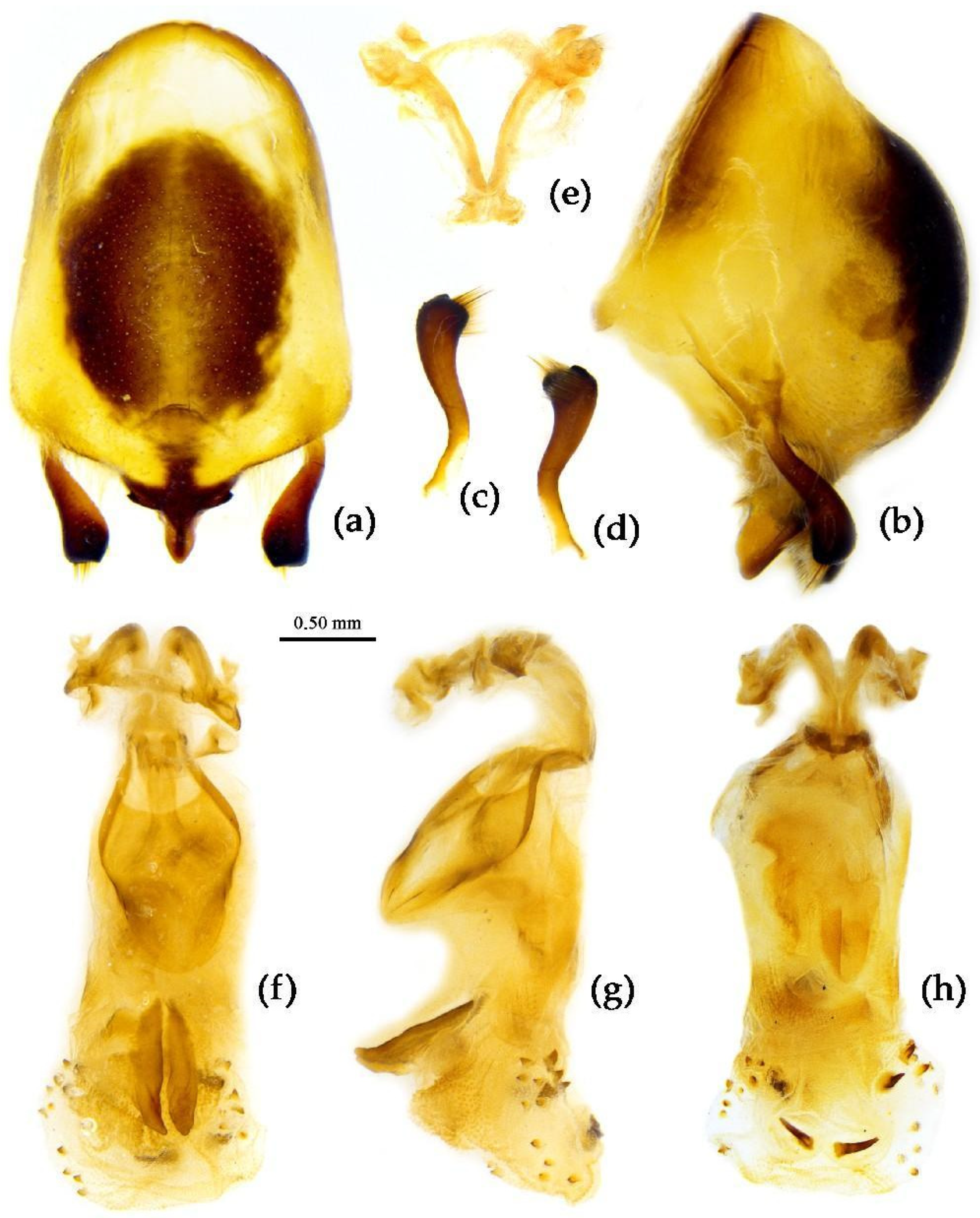
Figure 7). Anterior pronotal lobe, propleural episternum, posterior 1/3 of posterior pronotal lobe, scutellum (except posterior margin), apical half of corium of hemelytron (except apical angle black), most of meso- and meta- pleura, coxae, posterior half of each connexival segment red; abdomen black, abdomen ventrally totally black,or sometimes laterally with red markings at segmental sutures; rostrum black, posterior half of second and third segments gradually paler, brown.
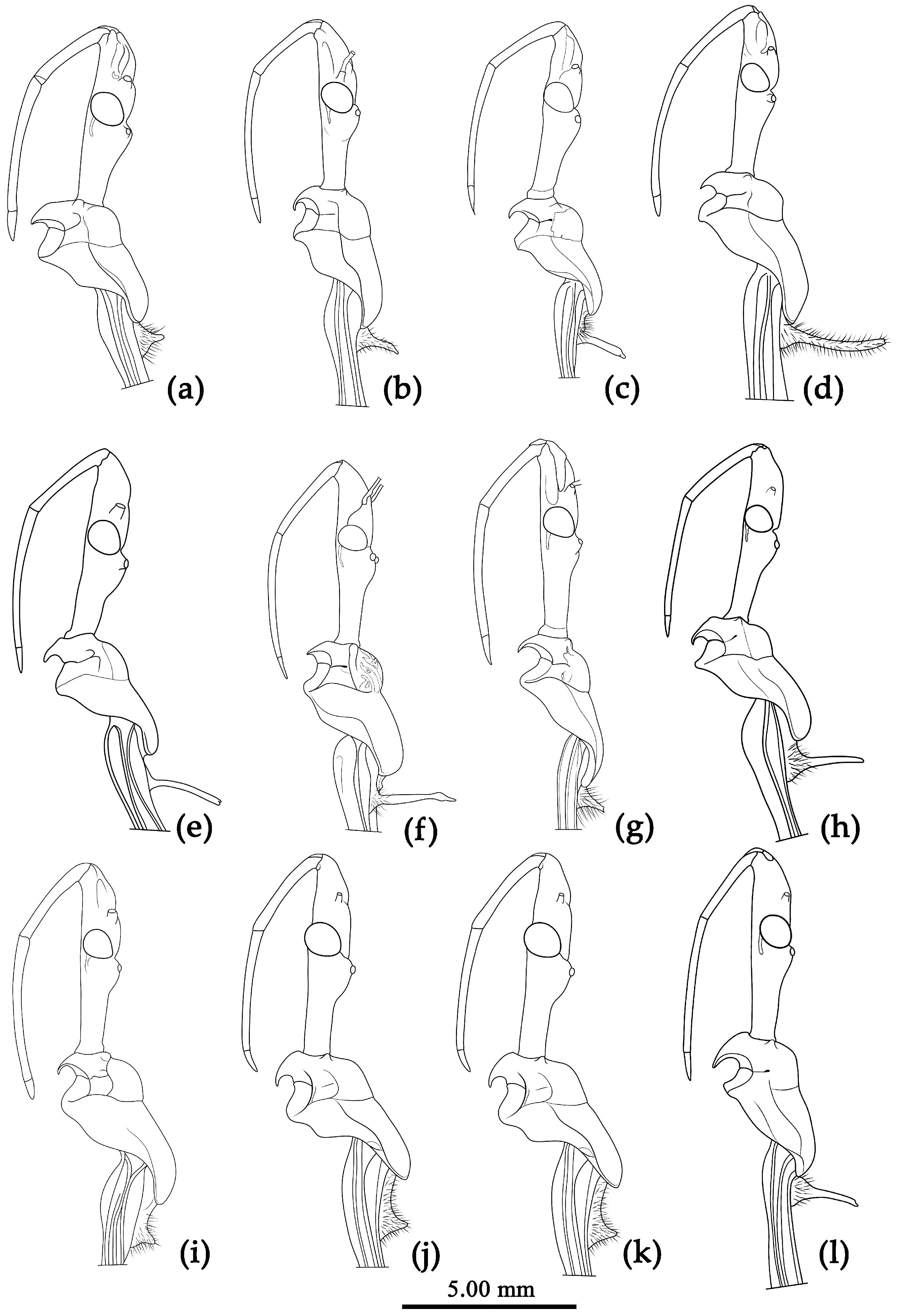
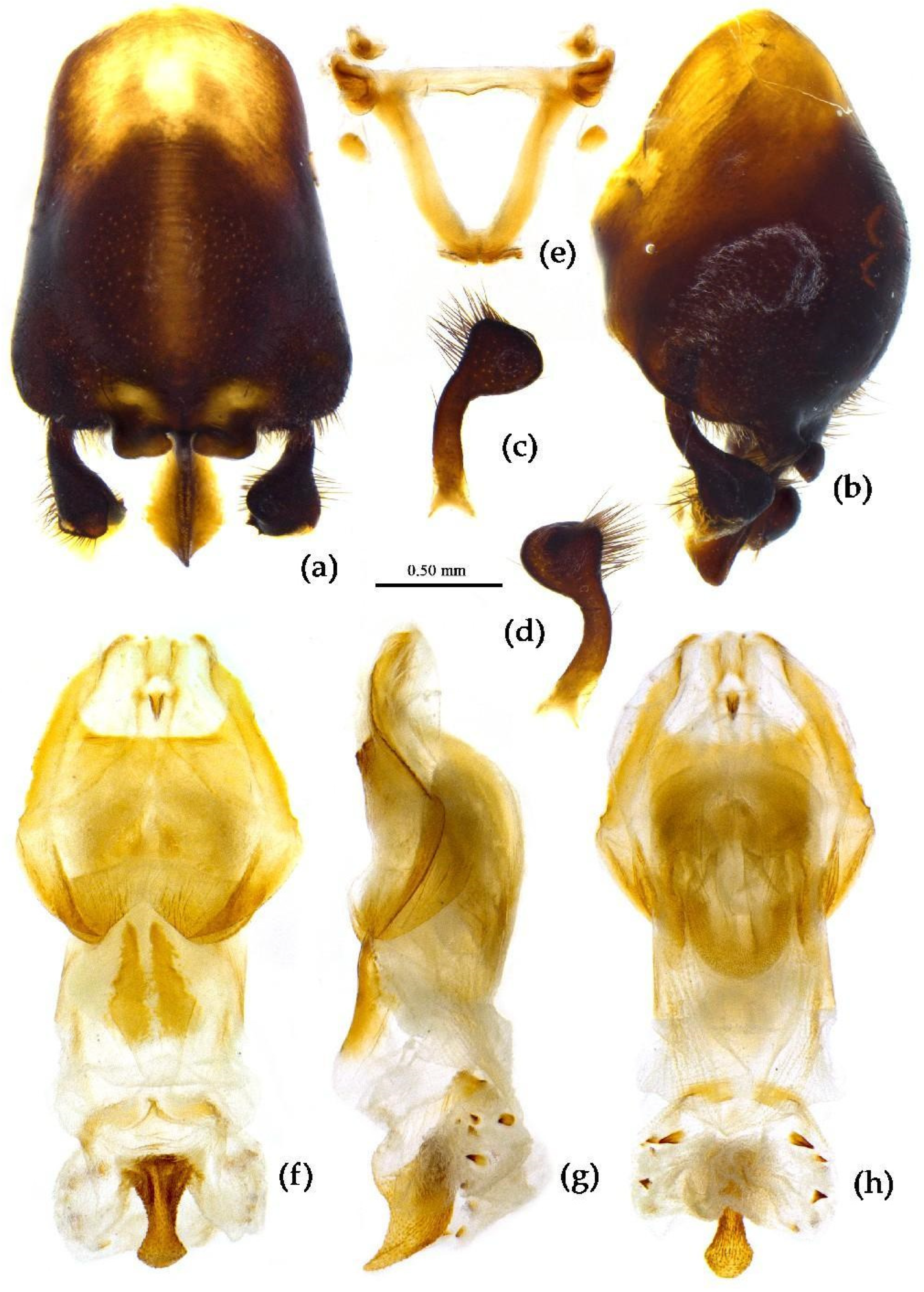
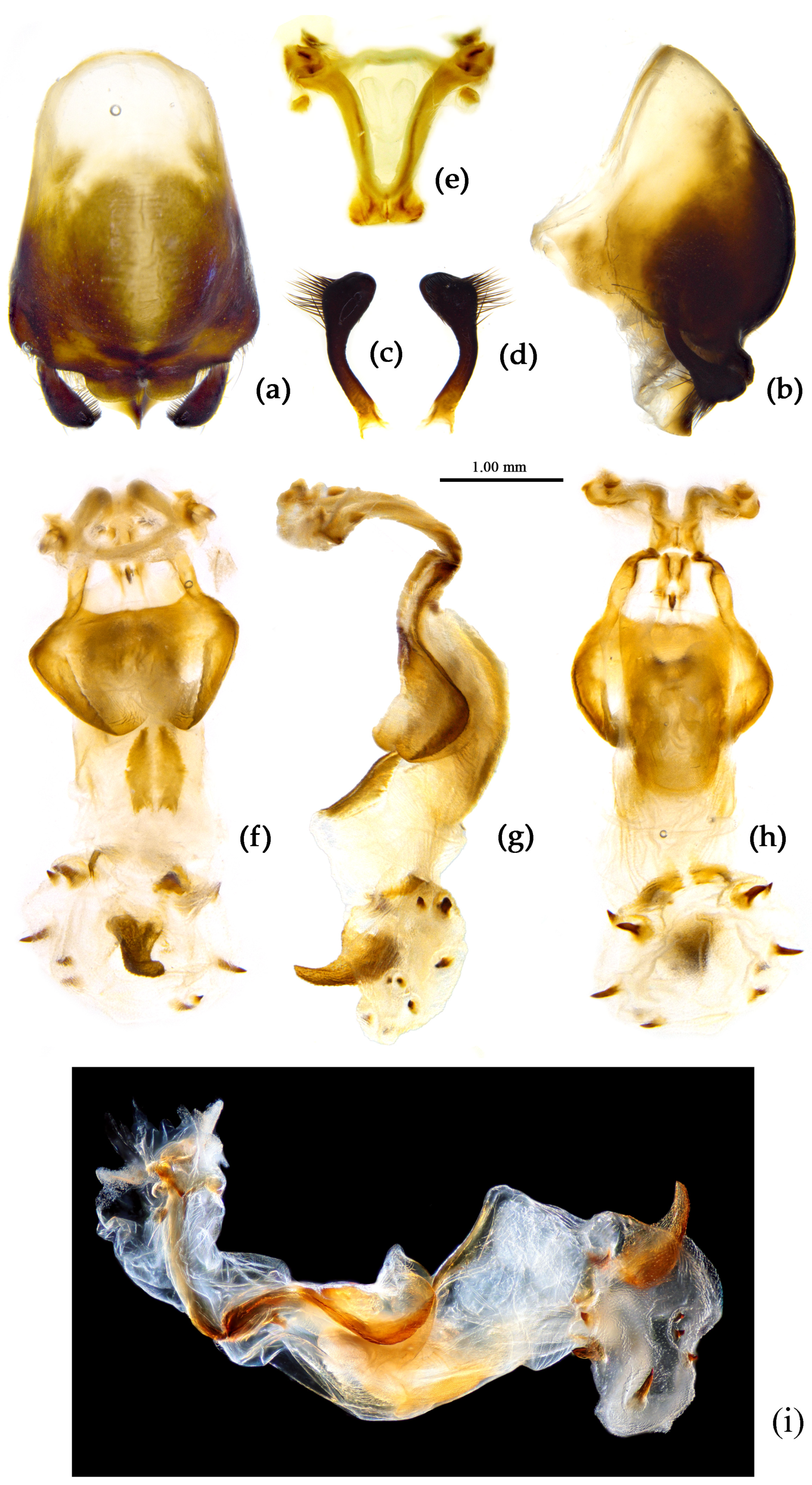
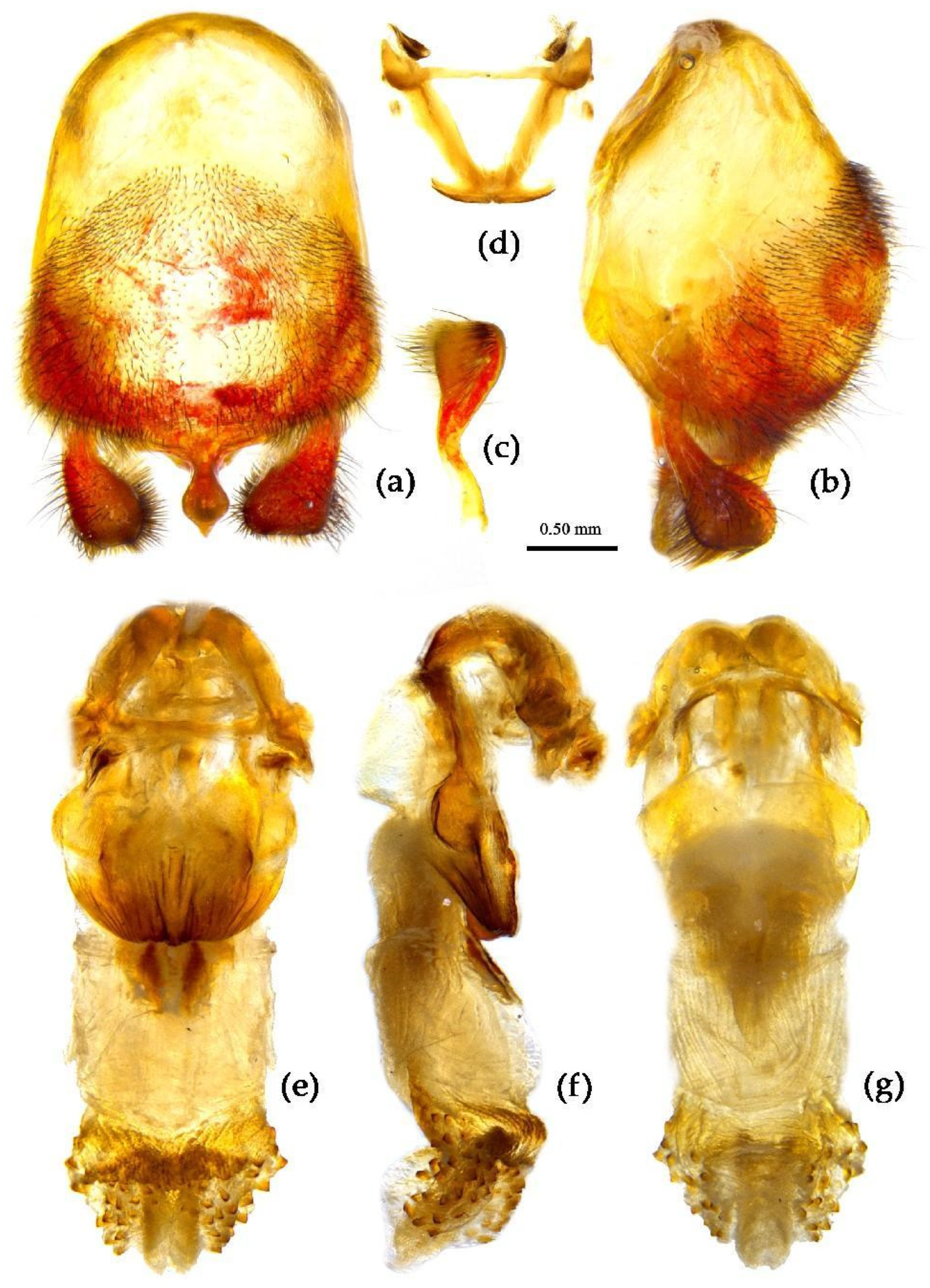
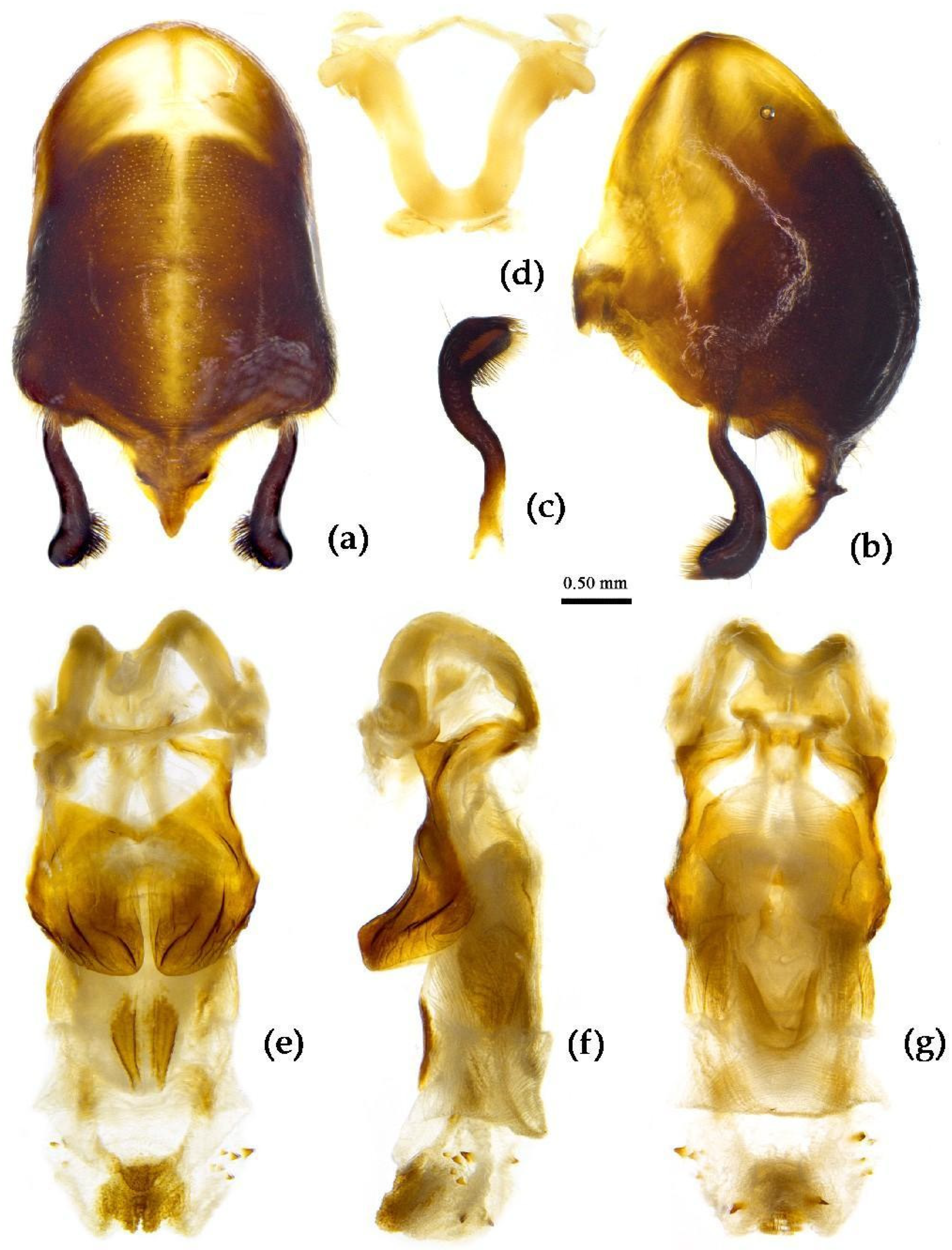
Structure. Body large-sized. Head beneath, pronotum, pleura and sterna of thorax, corium clothed with procumbent white short setae; head (except ventral surface), pronotum and propleuron, scutellum, legs, stetna of abdomen clothed with longer setae; first antennal segment sparsely with oblique short setae, second segment densely with short setae, third and fourth segments with procumbent short setae. Head longer than pronotum, constricted in middle, anterior lobe sub-equal to 1/2 of posterior lobe in length; post-ocular area of head round, then posteriorly thinned, and longer than anti-ocular area; ocelli separated, distance between ocelli longer than distance between ipsolateral ocellus and eyes. Anterior pronotal lobe hemispheric, smooth, median longitudinal sulcus short and deep; posterior pronotal lobe with irregular wrinkles; lateral pronotal angle round and obtuse; posterior and posterior-lateral margins of pronotum nearly straight; apical spine of scutellum situated at middle part, thick and short, produced upwards (
Figure 3a, 7b). Hemelytron extending beyond tip of abdomen. Abdomen laterally dilated. Pygophore elliptic, median pygophore process shown in
Figure 8a, b; paramere clavate, apical half swelled with thick setae, middle part bent (
Figure 8c, d). Struts triangular (
Figure 8e); phallosoma elliptic; dorsal phallothecal sclerite sclerotized; endosoma apically with a horned process and laterally with about 5 pairs of small spines (
Figure 8f-h).
Measurement [♂(n=4)/♀(n=3), in mm]. Body length 17.21–18.47 / 20.75–23.09, maximal width of abdomen 5.70–7.41 / 7.41–7.70; Head length 4.56–4.85 / 5.13–5.42; length of ante-ocular part 1.60–1.80 / 1.82–2.00; length of post-ocular part 1.82–2.11 / 2.34–2.39; length of synthlipsis 0.68–0.80 / 0.86; distance between ocelli 0.40–0.46 / 0.46-; length of antennal segments I–IV = 4.62–5.70 / 4.85–5.24, 2.28–2.30 / 2.00, 1.03–1.10/ 0.97, 7.41–7.50/ 3.99–5.13; length of visible rostral segments I–III = 2.00–2.39 / 2.39–2.51, 3.14–3.71 / 3.99–5.42, 0.63–0.68 / 0.71–0.80; length of anterior pronotal lobe 1.14–1.25 / 1.31–1.37; length of posterior pronotal lobe 1.73–2.00 / 2.57–2.68; maximal width of pronotum 3.53–3.93 / 4.85; length of scutellum 0.68–1.03 / 1.43–1.48; length of hemelytron 10.83–11.40 / 13.00–13.68.
Type material. Holotype, ♂, CHINA, Yunnan, Xishuangbanna, Mengzhe, 1400m, 15-VI-1958, Zhu Zhibin leg.(IOZ).
Allotype, ♀, same as holotype, 15-VI-1958, Cheng Hanhua leg. (TJNHM).
Paratypes (
Figure S1), 2♂, 3♀, CHINA, Yunan, Xishuangbanna, Xiaomengyang, Mengzhe, Damenglong, Luxi and river valleys in west area of Nujiang River (IOZ); 1♀, CHINA, Yunan, Ruili, 1400 m, 6-VI-196, Zhou Bentao leg. (IOZ).
Specimens examined. CHINA: 2♂, Yunan, Yuanjiang, 20-VII-1976, Xiong Shaoda leg. (CAU); 1♀, Yunnan, Yongdeng, Hongcheng, 4-X-1980, Xi Gengsi leg. (CAU); 3♂, 3♀, Yunan, Mengla, Yaoqu, 2005-V-9, 850 m, N21°43′, E101°32′, Bai Xiaoshuan & Cui Jianxin leg. (CAU); 1♀, Yunan, Mengla, Shangyong, 2005-V-18, Cui Jianxin leg. (CAU); 1♂, 1♀, Yunnan, Mengla, 1979-IX-18, 650 m, Zheng Leyi leg. (NKU); 1♂, Yunnan, Xishuangbanna, Xiaomengyang, 1957-IX-12, Wang Shuyong leg. (IOZ); 2♂, 1♀, Yunnan, Mangshi, 1000m, 1956-VI-3-4, 1959-VI-29, Huang Tianrong & Zhou Benshou leg. (NKU); 1♂, 1♀, Yunnan, Jingdong, 1170 m, 1958-VI-16, 1958-VI-14 (NKU); 1♂, Yunnan, Zhenyuan, 1400 m, VI-23 (IOZ); 1♂, 1♀,Yunnan, Xishuangbanna, Mengzhe, 1958-VI-13, 1200 m, Pu Zhelong leg. (IOZ); 2♂, Yunnan, Xishuangbanna, Mengzhe, 1400m, 1958-VI-14 (NKU); 1♀, Yunnan, Xishuangbanna, Menglong, 650 m (IOZ); 1♂, Yunnan, West Nujianghegu, 800 m, 1955-V-9 (NKU); 1♂, Yunnan, Longkou, Longjiang, Mengqiao, 1979-6-15, 1650 m (NKU); 1♂, Yunnan , Lianghe (NKU).
Distribution. China (Yunnan [Luxi, river valleys in west area of Nujiang River, Yuanjiang, Jingdong, Hongcheng, Mangshi, Mengla, Menglong, Mengzhe, Xiaomengyang, Damenglong, Lianghe, Zhenyuan, Yongdeng, Ruili]).
Remark. After we examined the syntype of
Sycanus miles Walker, 1873 (Penang) kept in the Natural History Museum, London, we found that
Sycanus bicolor Hsiao, 1979 is similar to
Sycanus miles in body color and structure. However Distant have assigned
Sycanus miles as a synonym of
Sycanus versicolor Dohrn, 1859 (India) [
27,
44,
45]. We haven’t examined the type of
S. versicolor, so this species needs further study.
(2) Sycanus bifidus (Fabricius, 1787)
Reduvius bifidus Fabricius, 1787: 312; Zimsen, 1964: 339 [
46]. Syntypes, 3 specimens, China, Hong Kong.
Cimex bifidus: Gmelin, 1790: 2200 [
47].
Zelus bifidus: Fabricius, 1803: 285 [
48].
Harpactor bifidus: Westwood, 1842: 50.
Sycanus bifidus: Dohrn, 1859: 97[
27]; Distant 1904: 353[
29]; Putshkov & Putshkov 1996: 259[
32]; Maldonado-Capriles 1990: 310[
24].
Remark. We examined the specimens of
Sycanus bifidus (Fabricius, 1787) collected from Malaya and kept in British Museum. But we didn’t examine type species of
Sycanus bifidus. Syntypes of the species are kept in ZMUC (Zoological Museum. University of Copenhagen, Copenhagen. Denmark), 3 specimens, collected from China, Hong Kong. In the basis of Distant’s description [
29], the three species,
Sycanus bifidus Fabricius, 1787,
Sycanus collaris (Fabricius, 1781) and
Sycanus croceovittatus Dohrn, 1859 are similar in body coloration. We collected many specimens of
Sycanus croceovittatus from Hong Kong, China, and doubted that the species distributed in Hong Kong is
Sycanus croceovittatus. Then
Sycanus croceovittatus Dohrn, 1859 may be synonym of
Sycanus bifidus (Fabricius, 1787).
Distribution. China (Hong Kong); Java, Borneo, India, Bengal.
Distribution. Philippines, Malay Archipelago, Malay Peninsula, Sri Lanka,Thailand, Malacca, Sarawak, India, Assam, China?.
Remark. In the world catalogue of the assassin bugs [
24], the type species of genus
Sycanus,
Sycanus collaris (Fabricius, 1781), has been recorded to be distributed within China, but we have consulted a large number of documents and has no records in China. They are mainly distributed in Southeast Asian countries. We have doubts about the distribution of this species in China, but listed them in the paper.
(4) Sycanus croceovittatus Dohrn, 1859
(Figures 2b, 3b, 4b, 9, 10, S8c,d)
Sycanus croceovittatus Dohrn 1859: 97[
27], Syntypes: 5 speceis (♀/♂), China, kept in Zoologisches Museum, Humboldt University, Berlin, Germany; Hsiao & Ren 1981: 519[
31], China; Maldonado-Capriles 1990: 310[
24], Federated Malay States; Putshkov & Putshkov 1996: 259[
32], China, Burma, India.
Sycanus leucomesus Walker 1873: 84[
44], Burma, China, kept in BMNH; Putshkov & Putshkov 1996: 259[
32], China, Burma.
Redescription. Coloration. Body black, slightly shiny; apical half of corium of hemelytron yellow or orange; membrane of hemelytron pale brown, semitransparent; spot between ipsolateral eye and ocellus yellowish brown; each sternum of abdomen laterally with two round white spots (
Figure 9).
Structure. Body large-sized. Body mostly clothed with black long or short setae and yellow procumbent short setae; first antennal segment sparsely with long setae, second segment densely with short setae, third and fourth segments densely with procumbent short setae; anterior margin of hemelytron with brown bent short setae; legs with black long setae; each segment of sternum of abdomen laterally with two white setae floccus. Anterior lobe of head sub-equal to posterior lobe, anti-ocular part distinctly shorter than post-ocular part; rostrum incurved, long and slender. Collar indistinct; anterior pronotal lobe small, hemisphered and bulged, deeply depressed at base; posterior pronotal lobe rugose, lateral pronotal angle obtuse and round, posterior margin nearly straight, posterior angle nearly absent; scutellum with an erect upward, thick, bi-forked spine (
Figure 9). Femora nearly of equal thickness, apical part somewhat thickened; hemelytron extending beyond tip of abdomen. Fourth and sixth connexival segments of abdomen laterally expended. Pygophore elliptic, median pygophore process shown in
Figure 10a, b; paramere clavate, apical half swelled with thick setae, middle part bent (
Figure 10c, d). Struts triangular (
Figure 10e); endosoma apically with a horned process and laterally with about 7 pairs of small spines (
Figure 10f–i).
Measurement [♂(n=21)/♀(n=19), in mm]. Body length 22.31–23.00 / 21.8–24.52; maximal width of abdomen 6.83–8.70 / 6.83–9.80. head length 5.25–5.41 / 5.36–6.14; length of ante-ocular part 1.84–1.94 / 2.00–2.21; length of post-ocular part 2.36–2.52 / 2.36–3.05; distance between ocelli 0.39–0.50 / 0.45–0.53; length of synthlipsis 0.79–0.89 / 0.84–0.95; length of antennal segments I–IV= 6.46–6.93 / 6.20–7.51, 2.21–2.52 / 2.26–2.36, 1.21–1.73 / 1.21–1.42, 7.35–7.46 / 7.88–9.19; length of visible rostral segments I–III= 2.52–2.68 / 2.84–3.36, 3.89–4.10 / 3.83–5.07, 0.74–0.79 / 0.74–0.89; length of anterior pronotal lobe 1.31–1.37 / 1.13–1.50; length of posterior pronotal lobe 2.31–2.78 / 2.63–3.57; maximal width of pronotum 4.31–4.78 / 4.73–6.46; length of scutellum 1.31–1.42 / 1.10–1.21; length of hemelytron 13.39–15.12 / 14.18–16.80.
Specimens examined. CHINA, Yunnan: 1♀, Yunan, Mengyang, 1991-VI-8, 800m, Cai Wanzhi leg.(CAU); 2♂, Yunnan, Menglun, 1991-V-18, Cai Wanzhi leg.(CAU); 1♀, Yunnan, Mengla, 1991-V-28, Cai Wanzhi leg.(CAU); 1♂, Yunnan, Menghai, 1991-V-30, Cai Wanzhi leg.(CAU); 1♀, Yunan, Mengla, Yaoqu, 2005-V-9, Bai Xiaoshuan leg.(CAU); 1♂, 1♀,Yunnan, Wenshan, Malipo, 2005-VIII-17(CAU); 4♂, 1♀, Yunnan, Lvchun, Huanglian mountain, 2012-V-10, Cai Wanzhi & Niu Xinwei leg.(CAU); 2♂, 1♀, Yunnan, Banna, Jinghong, Jinuo, 1053m, 2021-VII-25, Chen Zhaoyang & Liu Qinpeng leg.(CAU); 1♀, Yunnan, Honghe, Jinping, Fenshuiling National Nature Reserve, 2012-IX-24(CAU); 1♀, Yunnan, Honghe, Yuanjiang, 2014-VIII-18(CAU); 1♂, Yunnan, Puer, Mojiang, Sinanjiang, Dashaba, 2005-IX-5(CAU); 1♂, Yunnan, Yingpan Town, Fengqing County, Lincang, 2005-VI-20(CAU); 1♂, 1♀, Yunnan, Wenshan, Malipo, 2005-VIII-17(CAU); 2♂, 1♀, Yunnan, Banna, Jinghong, Jinuo, 1053m, 2021-VII-25(CAU); 6♂, 2♀, Yunnan, Hekou, 2011-V-20(CAU); 1♂, Yunnan, Xishuangbanna, Mengla, Yaoqu, 2013-III-24(CAU).
CHINA, Guangxi: 5♂, 8♀, Guangxi, Shangsi, Shiwandashanm, 2006-VIII-28, 1♂, 1♀, 2006-IX-2, Huang Xia leg.(CAU); 6♂, 4♀, Guangxi, Ningming, Longrui, 2006-V-13–22, Huang Xia & Shi Zhongting leg.(CAU); 1♂, Guangxi, Huaping, 1963-VI-6, Yang Jikun leg.(CAU); 2♀, Guangxi, Nanning, Fusui, 2004-VIII-18/20, 240m, Zhang Kuiyan leg.(CAU); 2♂, Gungxi, Longzhou, Nonggang, 1982-V-19, 240m, Wang Xinli leg.(CAU); 1♂, Guangxi, Jinxiu, 1982-V-10, 720 m, Wang Xinli leg.(CAU); 1♂, Guangxi, Ningming, Longrui, 1984-V-25, 180 m, Li Fasheng leg.(CAU); 1♂, Guangxi, Baise, Napo, Baisheng, 2020-V-26, Zhao Ping leg.(NNU); 5♂, 7♀, Guangxi, Baise, Napo, 2020-V-26(NNU); 1♀, Guangxi, Nanning, Wuming, Daming Mountain, 2016-VIII-8–10, Zheng Yuchen leg.(CAU); 1♂, Guangxi, Baise, Leye, 1200m, 2017-V-24(CAU); 4♂, 4♀, Guangxi, Ningming, Longrui Nature Reserve, 2006-V-17, Huang Xia & Shi Zhongting leg(CAU).; 1♀, Guangxi, Nanning, Fusui, 2004-VIII-18, 200m, Zhang Kuiyan leg.(CAU); 5♂, 7♀, Guangxi, Baise, Napo, 2020-V-26(NNU); 3♂, 1♀, Guangxi, Chongzuo, Longzhou, Zhubu, 2020-V-26(NNU); 4♂, Guangxi, Fangchenggang, Shangsi, Shiwandashan, 2006-VIII-28, Huang Xia leg.(CAU).
CHINA, Guangdong: 1♂, Guangdong, Meixian, 1981-IX-6 (CAU); 1 ♂, Guangdong, Yangjiang, Yangchun, Mashui, 2002-IV-30 (CAU); 1♂, Guangdong, Huizhou, 2004-VIII-19 (CAU); 1♂, Guangdong, Meixian, 1981-IX-6 (CAU); 2♀, Hong Kong, 2019-V-21–22 (CAU).
CHINA, Hainan: 1♂, 3♀, Hainan, Baisha, Yinggeling, 2008-X-8, Zhang W.J. leg.(CAU); 1♀, Hainan, Wuzhishan, Fanyang, Bulun, 2008-X-26, Zhang W.J. leg.(CAU); 3♂, Hainan, Baisha, Nankai, Mohao, 2008-V-30, Zhang W. J. leg.(CAU); 1♂, Hainan, Ledong, Jianfengling, 2007-V-10, Zhang W.J. leg.(CAU);1♂, Hainan, Baisha, Nankai, 2008-IV-28 Zhang W.J. leg.(CAU).
Distribution. CHINA (Guangdong <Baiyun Mountain, Gaoyao, Guangzhou, Meixian>, Guangxi <Fangcheng Fulong, Shangsi, Nanning, Napo, Ningming, Jinxiu, Longzhou, Huaping>, Yunnan <Jinping, Hekou, Xishuangbanna: Mengban, Menghai, Menglun, Mengyang, Mengla>, Guizhou <Maolan, Guiyang, Wangmo, Guiding>, Hunan, Fujian <Fuzhou, Hua’an>, Hainan<Jianfengling>, Hong Kong); MYANMAR, INDIA, VIETNAM, MALAYSIA ? (Federated Malay States).
Remark.Sycanus croceovittatus is set up by Dohrn in 1859 with Syntypes, 5 speceis (♀/♂) collected from China and kept in Zoologisches Museum, Humboldt University, Berlin, Germany. We have examined the type species of
Sycanus leucomesus Walker, 1873, and
Sycanus leucomesus is a synonym of
Sycanus croceovittatus or
Sycanus collaris (Fabricius, 1781) [
24,
32].
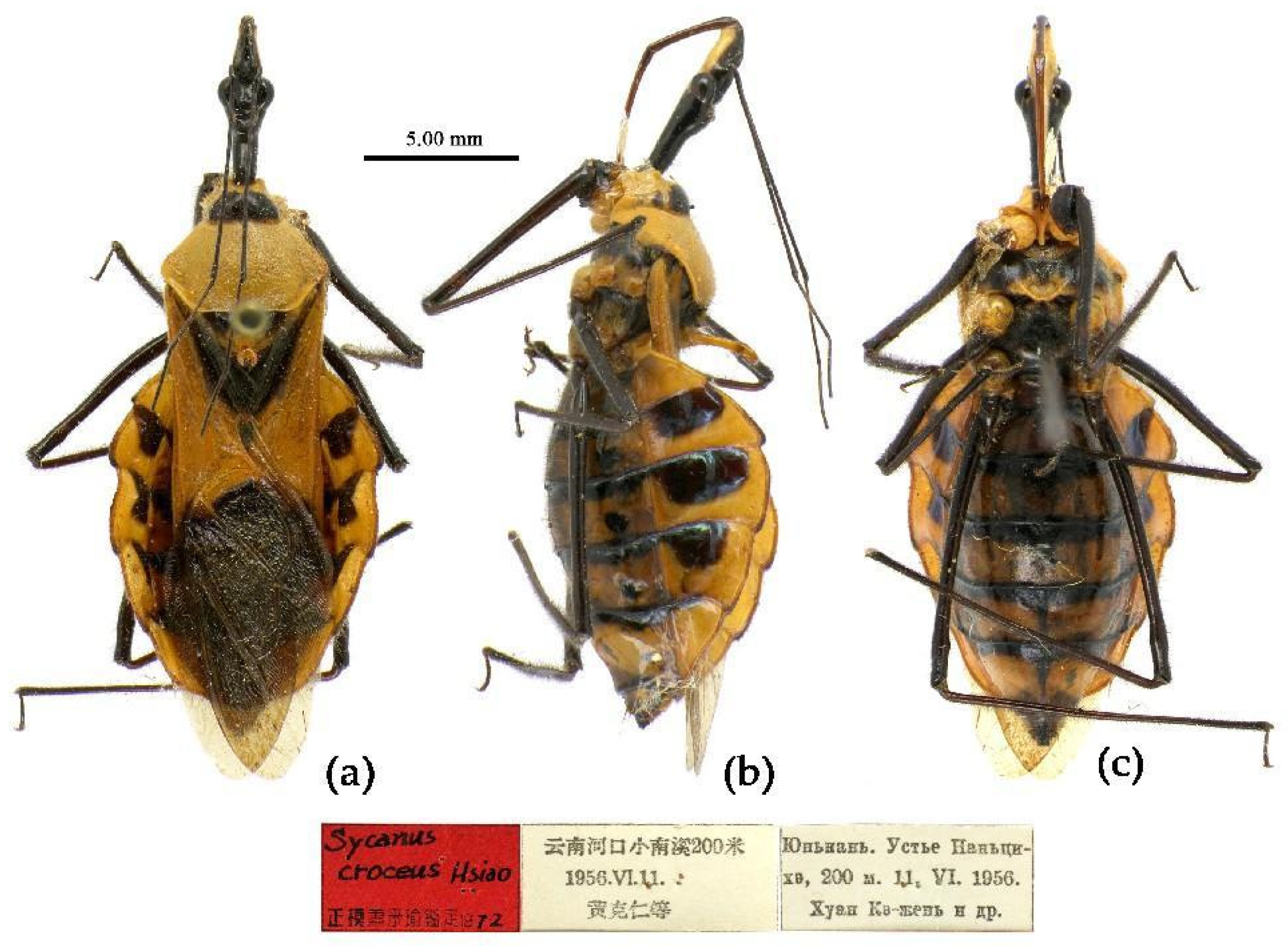
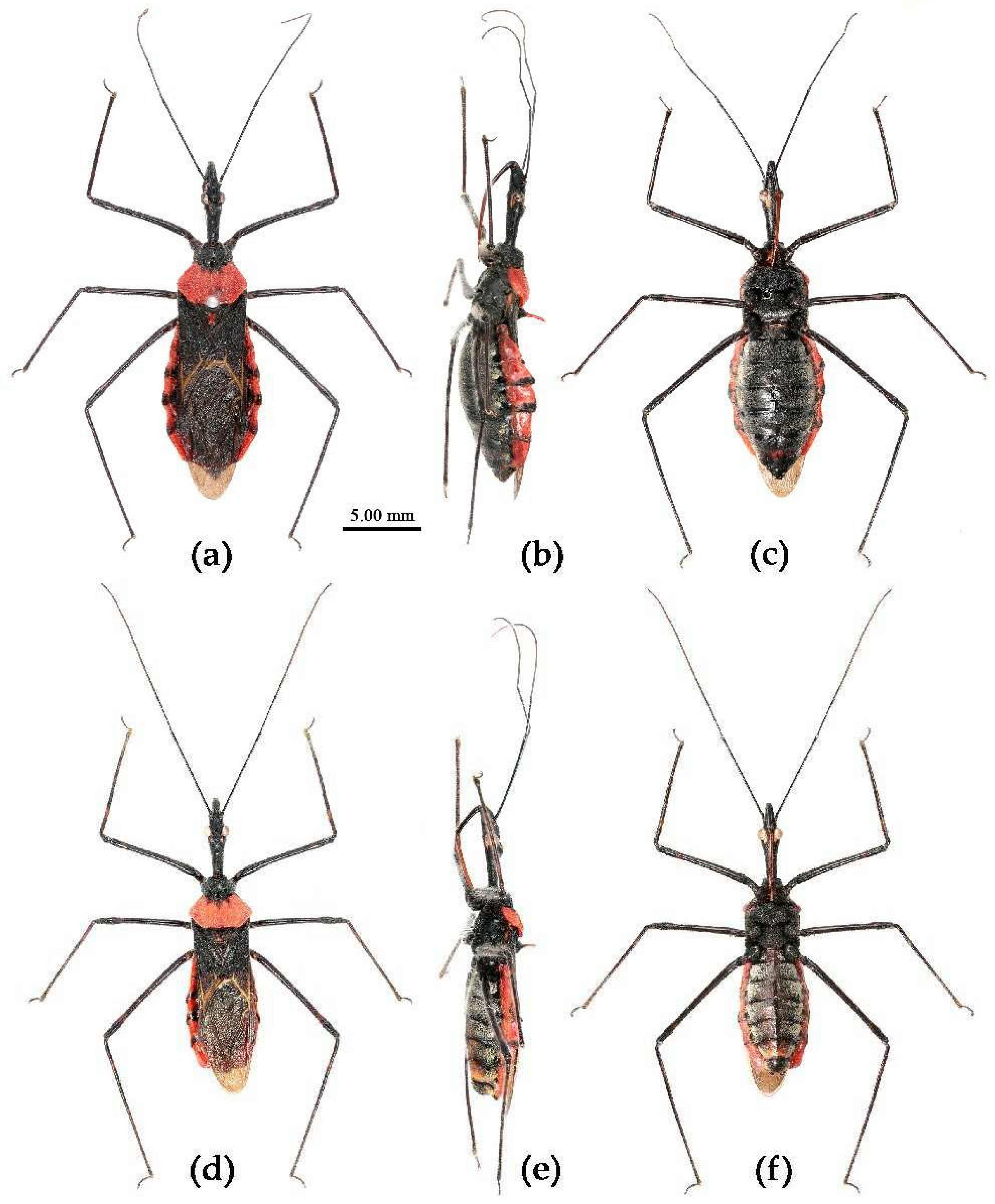
(5) Sycanus croceus Hsiao, 1979
(Figures 2c, 3c, 4c, 5, 11, 12, S5)
Sycanus croceus Hsiao 1979: 145, 154[
30]; Hsiao & Ren 1981: 520[
31]; Maldonado-Capriles 1990: 311[
24]; Putshkov & Putshkov 1996: 259[
32].
Redescription. Coloration. Body orange, with black markings (
Figure 11). Head (except ventral surface and markings around ocellus orange), antennae, eyes, anterior pronotal lobe, scutellum (except apical spine and posterior margin orange), clavus (except basal part yellow), inner side and apical angle of corium, leg (except coxae yellow), meso-sternum (except anterior and posterior margins yellow), meta-sternum, anterior half of meso- and meta-pleuron, basal part of scutellum, segmental strip of abdomen, large markings of connexivum, discontinuous medial longitudinal strip and small round spots laterally on ventral surface of abdomen black; membrane of hemelytron pale brown, semitransparent, with metallic-shiny; first visible rostral segment and basal part of second black, most of second and third brown.
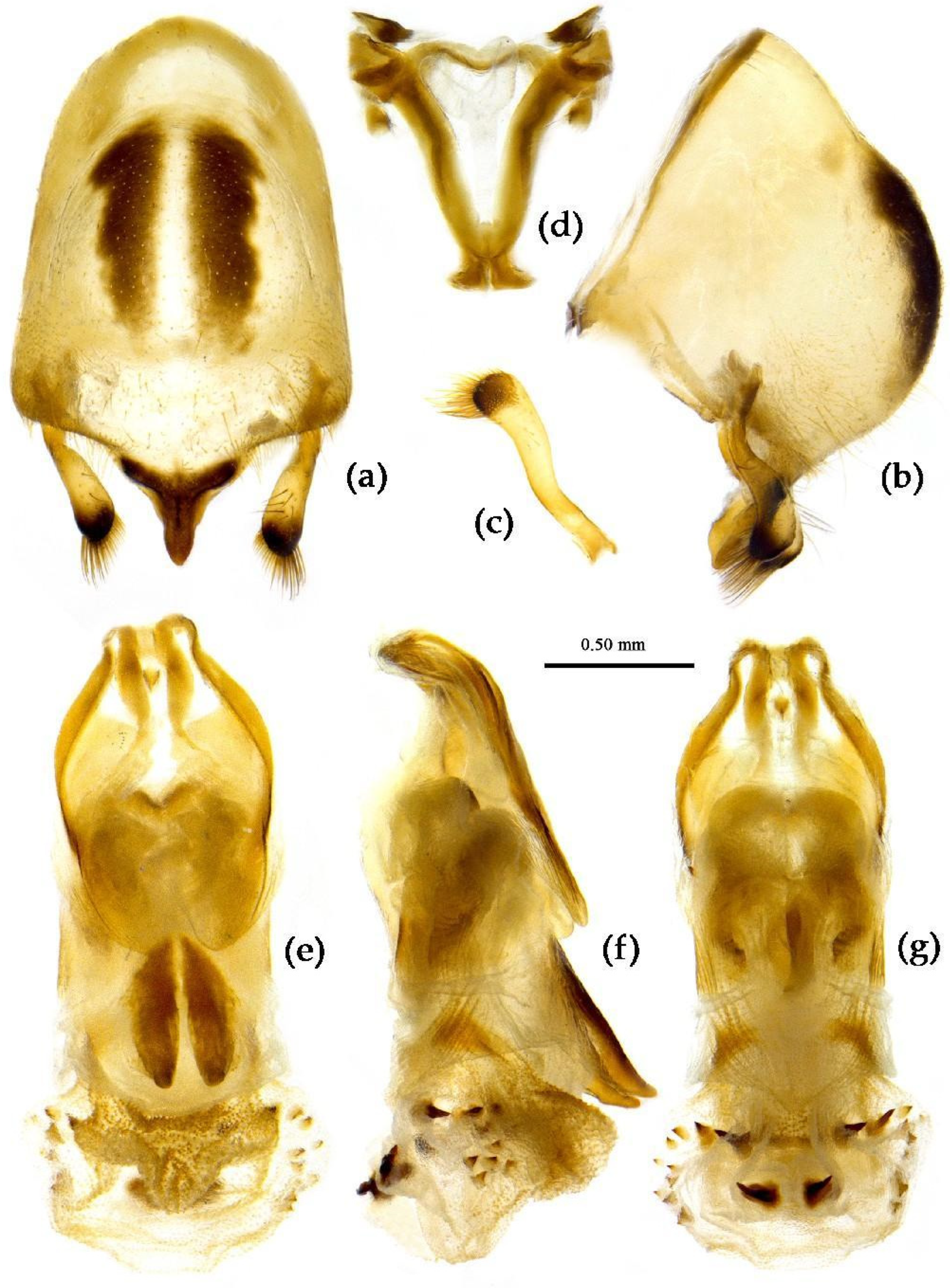
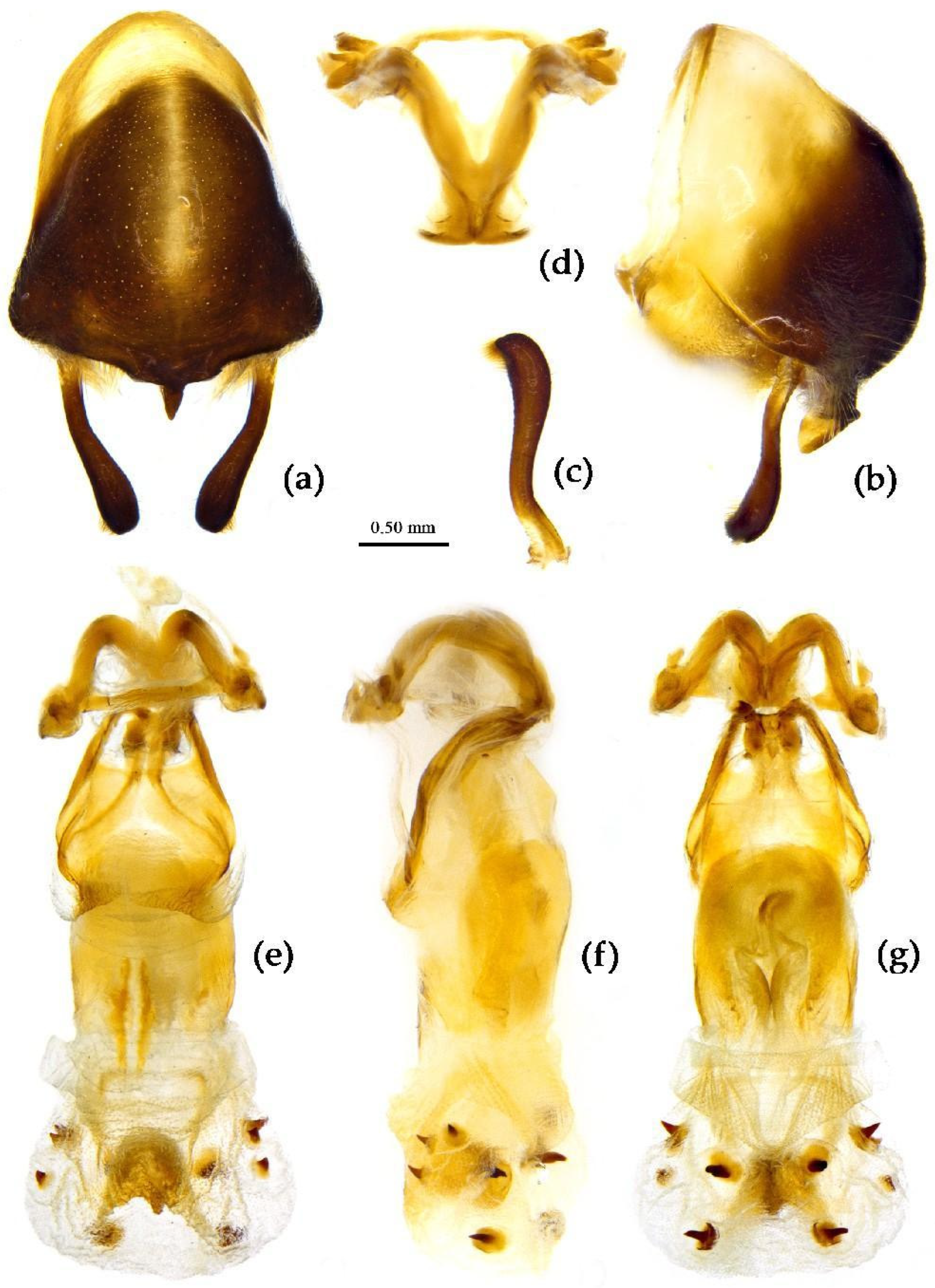
Structure. Body median to large-sized. Body clothed with yellowish short setae; legs with vertical long setae; thorax densely with short setae (
Figure 11). Post-ocular area of head longer than anti-ocular area; antennae long and slender, first segment longest, third shortest, fourth slightly shorter than first; rostrum long, bent, first extending beyond middle part of eyes, second segment longest. Anterior angle of pronotum round; anterior pronotal lobe small, hemisphere, posterior part with short longitudinal sulcus; posterior pronotal lobe rough, lateral angle round; posterior margin of scutellum round, apical spine sub-vertical, apex bi-forked (
Figure 11a); legs slender; hemelytron extending beyond apex of abdomen; fourth to sixth connexival segments roundly laterally dilated (
Figure 11). Pygophore elliptic, median pygophore process “Y”-shaped (
Figure 12a, b); paramere clavate, apical part with thick setae, middle part slightly bent (
Figure 12a-c). Struts triangular (
Figure 12d); middle part of endosoma with two horn-shaped processes (
Figure 12e, f) and apical part laterally with about 11 pairs of small spines (
Figure 12e-g).
Measurement [♂(n=3)/♀(n=3), in mm]. Body length 19.64–20.74 / 20.91–25.20; maximal width of abdomen 7.27 / 8.18. Head length 4.73 / 5.09–5.46; length of anteocular part 1.63–1.64 / 1.82–1.84; length of postocular part 2.36–2.52 / 2.55–2.84; distance between ocelli 0.47–0.55 / 0.47–0.55; length of synthlipsis 0.79 / 0.72–0.84; length of antennal segments I–IV = 6.73–6.83 / 6.18–7.30, 2.21 / 2.36–2.63, 1.64–1.94 / 1.64–2.10, 6.55 / 5.09–6.56; length of visible rostral segments I–III = 2.26–2.36 / 2.55–2.68, 3.41–3.64 / 3.04–4.04, 0.55–0.71 / 0.36–0.74; length of anterior pronotal lobe 1.21–1.27 / 1.27–1.37; length of posterior pronotal lobe 2.36–2.55 / 2.55–3.07; maximal width of pronotum 3.82–4.15 / 4.55–5.36; length of hemelytron 13.64–13.65 / 14.18–17.06.
Holotype, ♀, CHINA, Yunnan, Hekou, Xiaonanxi, 200m, 11-VI-1956, Huang Keren et al. leg. Allotype, same as Holotype, 10-VI-1956. Paratype, 1 ♀, CHINA, Guangxi, Baishou; 1 ♀, Yunnan, Hekou (ZOI).
Specimens examined.
CHINA, Guangxi: 1♂, 1♀, Guangxi, Jinxiu, 1983-VI-13 (NKU); 1♀, Guangxi, Longsheng, Huaping, 1983-VI-13 (NKU); 1♂, 1♀, Guangxi, Jinxiu, 1990-VI-12, 800 m, Li Xinzheng leg. (NKU); 1♀, Guangxi, Jinxiu, 2005-VII-24, Huang Xia leg. (CAU); 1♂, Guangxi, Laibin, Jinxiu, Dayaoshan, 1250m, 2015-V-7 (CAU); 1♀, Guangxi, Jinxiu, Dayaoshan, Luoyingou, 1100m, 2016-V-24, Zhao Jintang leg. (CAU); 2♀, Guangxi, Laibin, Jinxiu, Dayaoshan, 1100m, 2016-V-31, Zhao Jintang leg. (CAU); 1♀ Guangxi, Jinxiu, Dayao Mountain, 1100m, 2017-VII-20, Zhao J. T. leg. (CAU); 2♀, Guangxi, Jinxiu, Dayaoshan, 600m, 2018-V-30 Zhao J. T. leg. (CAU); 1♂, 1♀, Guangxi, Longsheng, Huaping, 1963-V-1, Liu Sikong leg. (NKU); 1♂, Guangxi, Huaping, Cujiang, 1963-IV-8, Yang Jikun leg.(CAU); 1♀, Guangxi, Huaping, Tianping mountain, 1963-IV-5, Yang Jikun leg.(CAU); 1♂, 1♀, Guangxi, Guilin, Huaping, Tianping mountain, 1963-VI-5–8, Yang Jikun leg. (CAU); 1♂, Guangxi, Longsheng 1992-V-23, Liu Guang leg. (CAU); 1♀, Guangxi, Jinxiu, 2005-VII-23, Zhao Ping leg. (CAU); 2♂, 2♀, Guangxi, Laibin, Jinxiu, Dayaoshan, 900m, 2015-V-14, Lu Yanquan leg. (CAU); 1♂, Guangxi, Longzhou Nonggang, 2019-IV-22, He Zhuqing leg. (CAU); 1♀, Guangxi, Chongzuo, Longzhou, Nonggang Nature Reserve, 2020-V-27 (CAU); 2♀, Guangxi, Laibin, Jinxiu, Fenzhan, 810m, 2020-VI-24 (CAU); 3♂, 5♀, Guangxi, Ningming, Huashan, 2014-VIII-13, 2020-VI (NKU);1♂, 1♀, Guangxi, Baishou, 1957-VI-27 (NKU); 1♀, Guangxi, Xing’an, Jinshi, 2007-VII (CAU); 1♀, Guangxi, Baise, Jingxi, Bangliang, Renzhuan, 2010-VIII-1, Zhou S.Y. leg. (CAU); 1♂, Guangxi, Baise, Napo, 2020-V-26 (NNU); 3♀, Guangxi, Hezhou, Gupo mountain, 2011-VII (CAU);
CHINA, Yunnan: 1♂, 1♀, Yunnan, Honghe, Hekou, Nanxi, Huayudong, 200m, 2016-IV-24, Wang Yutang & Yang Xiaodong leg. (CAU); 1♂, Yunnan, Xishuangbanna, Mengla, Mengxing, 1981-V-23, Yang Pingzhi leg. (CAU); 1♀, Yunnan, Xishuangbanna, Menghai, 1984-VI-21, Xiong Jiang leg. (CAU); 1♀, Yunnan, Menghai, 1984-VI-21, Xiong Jiang leg (CAU).; 1♂, Yunnan, Mengxing, 1981-V-23, Yang Pingzhi leg.(CAU); 1♂, Guangxi, Ren Shuzhi leg. (NKU);
CHINA, Guangdong: 2♀, Guangdong, Guangzhou, Conghua, Liuxihe, 2004-VI-20 (CAU); 1♀, Guangdong, Shixing, Chebaling Nature Reserve, 2003-IX-15 (CAU); 1♂, Guangdong, Nanling Nature Reserve, 2017-VI-18, Zhao Yisheng leg. (CAU); 1♀, Guangdong, Shaoguan, Wujiang, 2016-V-28, Ge Zhentai leg. (CAU); 1♂, 1♀, Guangdong, Shaoguan, Qujiang, Xiaokeng National Forest Park, 2013-V-1, Zheng Chaowu leg. (CAU); 1♂, 3♀, Guangdong, Shaoguan, Qujiang, Xiaokeng National Forest Park, 2013-X-1, Zheng Chaowu leg. (CAU); 3♂, 2♀, Guangdong, Nanling Nature Reserve, 2017-VI-18, Zhao Yisheng leg. (CAU);
CHINA, Jiangxi: 1♀, Jiangxi, Ganzhou, Longnan, Hengkeng, Jiulian Mountain, 500m, 2020-VII-05, Zheng Yuchen leg. (CAU); 1♂, 1♀, Jiangxi, Ganzhou, Chongyi, Yangling National Forest Park, 2014-VI-18, Li Yanjing leg. (CAU);
CHINA, Guizhou: 1♂, Guizhou, Rongjiang, Pingyang, Xiaodanjiang, 2005-V-31, Song Yuehua leg. (CAU); 1♂, Guizhou, Rongjiang, Pingyang, 2005-VI-1, Song Yue Hua leg (CAU);
CHINA, Fujian: 2♂, 2♀, Fujian, Quanzhou, Dehua, Shangyong, 2014-VI-25 (CAU); 1♀ Fujian, Wuyi Mountain, 2010-VII-8, Luo X.Y. leg. (CAU); 1♂, 2♀, Fujian, Quanzhou, Dehua, Chishui , 2014-VI-20 (CAU).
Distribution. CHINA (Guizhou <Rongjiang>, Yunnan <Hekou Nanxi, Menghai, Mengxing>, Guangxi <Baishou, Jinxiu, Huaping, Rong’an, Longzhou, Ningming, Du’an, Longsheng, Napo, Fangcheng, Fulong, Longzhou Nonggang>, Guangdong <Conghua, Chebaling, Shaoguan, Nanling>, Jiangxi (Ganzhou), Fujian (Fuzhou, Sanming, Longyan, Dehua, Hua’an, Yongding, Longqishan, Quanzhou); VIETNAM.
(6) Sycanus falleni Stål, 1863
(Figures 2d, 3d, 4d, 13, 14, S6, S8a,b)
Sycanus falleni Stål 1863: 34[
28]; Distant 1904: 354[
29]; Hsiao 1979: 146[
30]; Hsiao & Ren 1981: 520[
31]; Maldonado-Capriles 1990: 311[
24]; Putshkov & Putshkov 1996: 259[
32].
Redescription. Coloration. Body red, with black markings. Head, antennae, legs (except coxae), rostrum (except apical part brown), most of meso- and meta-pleuron (except posterior part red), sternum of meso- and meta-thorax, sterna and connexiva of abdomen (except inter-segmental stripe of abdominal sterna yellow, and inter-segmental stripe and lateral margins of connexiva red), posterior half of clavus of fore wing, basal part of scutellum black, and with blue shiny; pronotum and pro-pleuron, pro-sternum (except middle part), posterior half of meso- and meta-pleuron, scutellum, coxae, inter-segmental stripe and lateral margins of connexivum, apical part of abdomen red; corium of hemelytron red, clavus black (except basal part red), membrane pale brown and semitransparent (
Figure 13).
Structure. Body large-sized. Body clothed densely with yellowish short setae and depressed short setae; legs with vertical longer setae; thorax, pronotum densely with short setae. Head longer; post-ocular area of head longer than anti-ocular area; antennae long and thin, fourth segment longest and sub-equal to first, third shortest and sub-equal to second; rostrum long, bent, first extending to middle part of eyes, second segment longest. Anterior angle of pronotum round and indistinct; anterior pronotal lobe small, hemisphere, posterior part with short longitudinal sulcus; posterior pronotal lobe rough, lateral angle round; posterior margin of scutellum round, apical spine sub-vertical, apex bi-forked; legs slender; hemelytron extending beyond apex of abdomen; fourth to sixth connexival segments dilated laterally (
Figure 13). Pygophore elliptic, median pygophore process shown in
Figure 14a, b; paramere clavate, apical half swelled with thick setae, middle part bent (
Figure 14a-c). Struts triangular (
Figure 14d); endosoma apically laterally with more than 30 pairs of small spines and medianly with a pair of sclerite (
Figure 14e-g).
Measurement [♂(n=10)/♀(n=10),in mm]. Body length 23.63–24.46 / 24.94–26.62; maximal width of abdomen 6.56–8.66 / 8.14–8.40. head length 5.46–5.51 / 5.51–6.20; length of ante-ocular part 1.79–1.84 / 2.10–2.15; length of post-ocular part 2.78 / 2.36–2.89; distance between ocelli0.58 / 0.53–0.58; length of synthlipsis0.84–0.89 / 0.89–1.00; length of antennal segments I–IV = 7.09–7.35 / 6.83–7.25, 2.52–2.57 / 2.52–2.63, 2.15–2.21 / 2.00–2.31, 7.35–7.61 / 6.83–7.35; length of visible rostral segments I–III = 2.63–2.78 / 2.84–3.05, 4.10–4.41 / 4.46–4.73, 0.84–0.89 / 0.79–0.89; length of anterior pronotal lobe1.31–1.37 / 1.50–1.58; length of posterior pronotal lobe2.52–2.78 / 3.15–3.57; maximal width of pronotum4.67–4.99 / 5.57–6.41; length of scutellum1.42–1.47 / 1.47–1.58; length of hemelytron15.23–16.28 / 16.80.
Specimens examined. CHINA: 10♀, 2♂, Guangxi, Ningming, Longrui, 2006-V-18, Huang Xia & Shi Zhongting leg. (CAU); 1♀, Guangxi, Ningming, Longrui, 1984-V-20, Lu Xiaolin & Wu Zheng Liangcai leg.(CAU); 2♀, Guangxi, Longrui, 1984-V-23, Ren Shuzhi leg. (NKU); 1♀, Guangxi, Pingxiang, Daqingshan (NKU); 1♂, 1♀, Guangxi, Pingxiang, 1964-VII-26, Wang Liangchen leg.(NKU); 1♂, Yunan, Pingbian, Lincang, 800-1300 m, 1956-VI-27, Huang Keren leg. (NKU); 1♀, Guangxi, Ningming, Longrui, 1984-V-18, Ren Shuzhi leg. (NKU).
Distribution. CHINA (Guangxi <Longsheng, Ningming, Pingxiang, Napo, Fangcheng Fulong, Longzhou Nonggang, Longrui>, Yunnan <Pingbian>); MYANMAR, CAMBODIA, INDIA, VIETNAM.
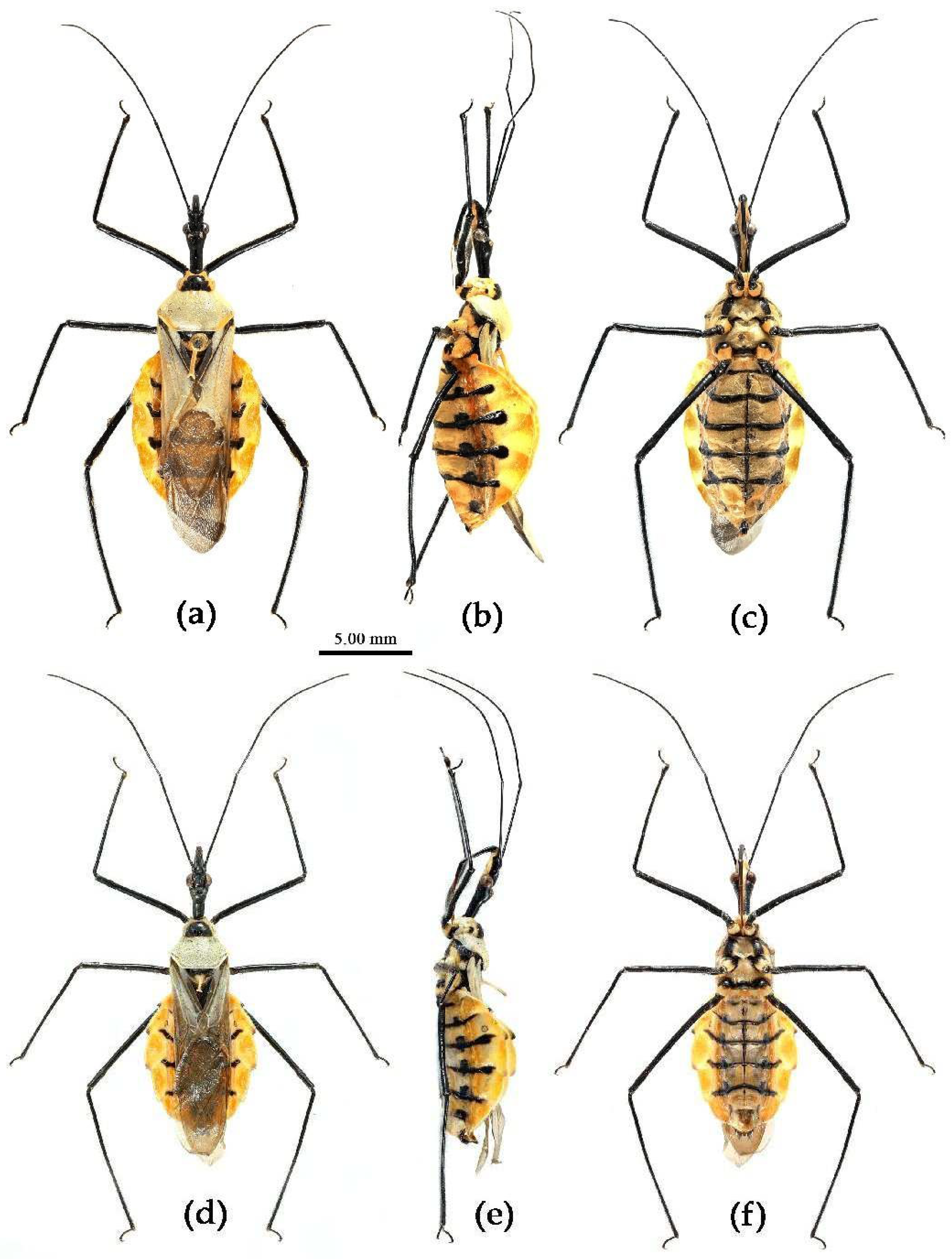
(6) Sycanus hsiaoi Li & Cai sp. nov.
(Figures 2e, 3e, 4e, 15, 16)
Diagnosis. The new species resemble to Sycanus croceus in the body shape and the body coloration. However in the new species, the coxae of leg is yellow, and its basal part is black; the sterna of meso- and meta-thorax are yellow, the markings on lateral side of sternum of meso-thorax and posterior margin of sternum of meta-thorax are black; the black marking of connexivum is smaller, and only laterally extending to half of connexivun or nearly reaching to lateral margin; the posterior pronotal lobe and the corium of fore wing are yellowish white (vs. In Sycanus croceus, the coxae is yellow without black markings; the sterna of meso- and meta-thorax are black, the anterior and posterior margins of sterna of meso- and meta-thorax are yellow; the black marking of connexivum is larger, almost laterally extending to the lateral margin of connexivun; the posterior pronotal lobe and the corium of fore wing are yellow).
The male external genitalia of the new species is somewhat similar to that of Sycanus stali Dohrn, 1859 among these Chinese Sycanus species, but there are great differences in body coloration between the two species: the new species is yellowish with some black markings (vs. Sycanus stali is black with red stripes). In addition, the male external genitalia in new species, the median process of pygophore is more distinct, apical part of endosoma laterally is without small spines (vs. In Sycanus stali, the median process of pygophore is smaller, apical part of endosoma laterally is armed with 6 pairs of small spines).
Description. Coloration. Body yellowish with black markings (
Figure 15). Head (except ventral surface yellowish), anterior pronotal lobe (except anterior angles and lateral sides yellowish), upper margin of pleura of meso- and meta-thorax, scutellum (except posterior margin and apical spine yellowish), round markings of two lateral sides and median longitudinal strip and inter-segmental transversal strips of ventral surface of abdomen, legs (except apical half of coxae), antennae, black; posterior pronotal lobe (except post-lateral margin yellowish), corium, clavus, epimeron of propleuron, sternum of thorax (except lateral margin of meso-sternum and anterior margin of meta-sternum black), sternum of abdomen (except black strips), milk-white to yellowish white; ventral surface of head, coxae (except basal part black), anterior angles, lateral margin of anterior lobe and sternum of pro-thorax, episternum of propleuron, pleura of meso- and meta-thorax (except upper margin black), posterior margin and apical spine of scutellum, connexivum (except apical part of post-lateral angle black) yellowish.
Structure. Body median-sized. Body clothed with white setae; legs clothed with longer vertical setae. Head long, anti-ocular area shorter than post-ocular; ocelli separated, distance between ocelli wider than that between ipsolateral ocellus and eyes. Anterior pronotal lobe hemispheric, basal median longitudinal sulcus short; posterior pronotal lobe rough with irregular wrinkles; lateral pronotal angle round; posterior margin of pronotum straight; spine of scutellum situated in middle part, thick and long, produced upwards then backward, apex bi-forked. Hemelytron extending beyond tip of abdomen. Abdomen laterally widely roundly dilated, especially fourth to sixth segmental connexiva, posterior-lateral angle of each connexival segment indistinct, round (
Figure 15). Pygophore elliptic, median pygophore process shown in
Figure 16a, b; paramere clavate, apical part with thick long setae, middle part bent (
Figure 16a-c); strut triangular (
Figure 16d); endosoma apically armed with many small processes (
Figure 16e-g), but laterally without small spines, subapical part with two pieces of narrow and long sclerites.
Measurement [♂(n=2)/♀(n=2), in mm]. Body length 17.27/19.87, maximal width of abdomen 5.82/5.38; Head length 4.55/4.65; length of ante-ocular part 1.64/1.47 ; length of post-ocular part 2.00/2.03; length of synthlipsis 0.73/0.61; distance between ocelli 0.27/0.28; length of antennal segments I–IV =5.09/5.34, 2.18/2.10, 2.18/2.37, 5.45/5.55; length of visible rostral segments I–III =2.00/2.56, 2.73/3.13, 0.73/0.68; length of anterior pronotal lobe 1.09/1.14; length of posterior pronotal lobe 1.64/2.33; maximal width of pronotum 3.27/4.43; length of scutellum 1.27/1.61; length of hemelytron 11.45/14.22.
Holotype, ♀, CHINA, Yunnan, Lancang, Qianliuyizu town, Tianba village, 1375m, 2017-VII-20, Zhou Zhen& Leo Xiaolong leg. (CAU). Paratypes, 1♂, 1♀, CHINA, Yunnan, Lancang, Gengma, 600m, 2019-VII (CAU); 1♂, CHINA, Yunnan, Puer, 2022-VII-4, Zhang Guirong leg. (NNU).
Etymology: The species name is named after the late Professor Hsiao Tsai-Yu in Nankai University, the famous Chinese Entomologist, for his great contribution to the taxonomy of Heteroptera insects.
Distribution. China (Yunnan<Lancang, Puer>).
Note. We didn’t examine type species, and didn’t find the Chinese specimens of this species.
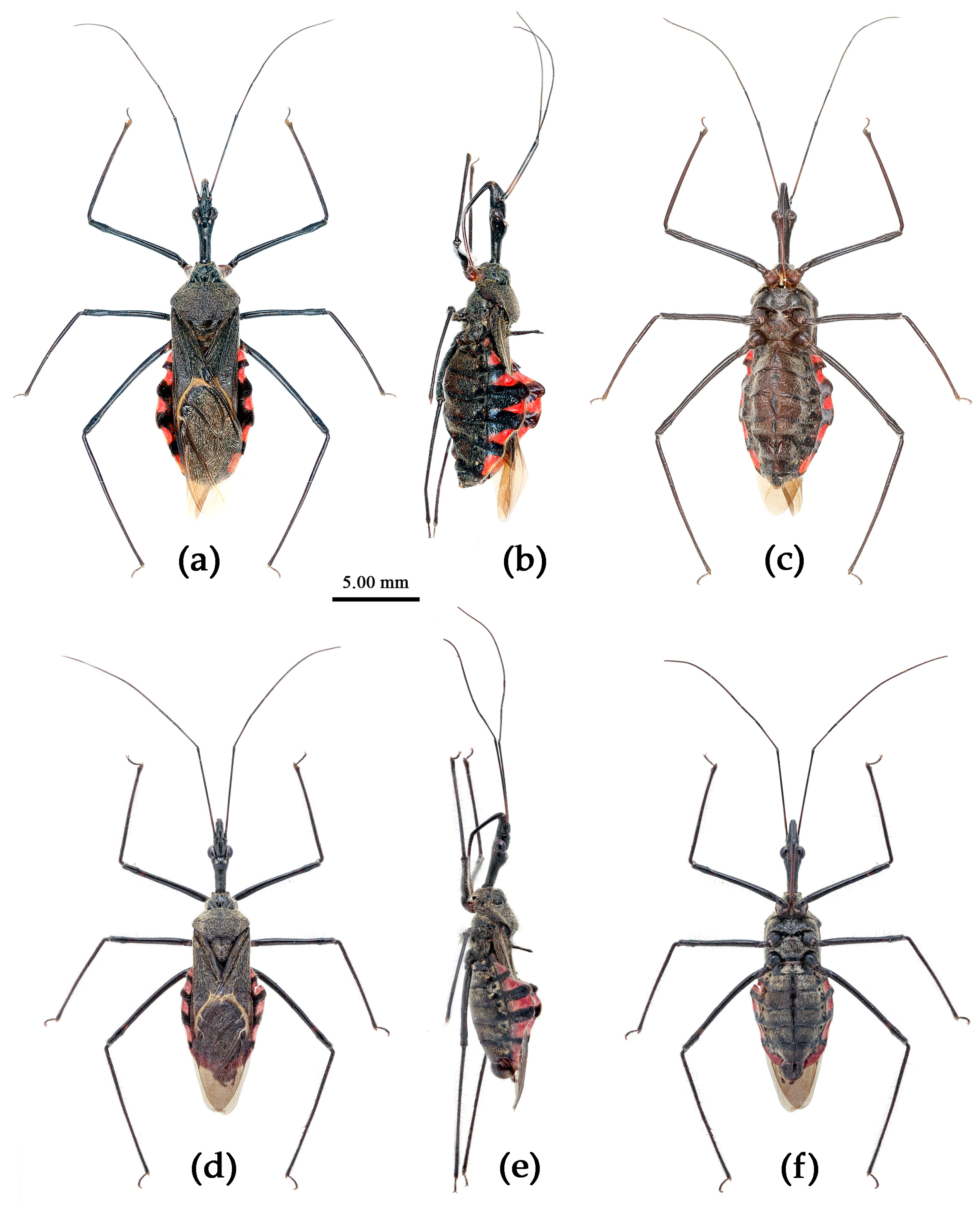
(9) Sycanus hainanensis Wang & Cai sp. nov.
(Figures 17, 18, 2f, 3f, 4f)
Diagnosis: The body coloration and the structure of abdominal connexivum in
Sycanus hainanensis sp. nov. are somewhat different with
Sycanus sichuanensis. In the new species, the body shape is thinner, the posterior margin of corium is yellowish brown (
Figure 13) (vs. In
Sycanus sichuanensis, the body shape is wider, especially the connexivum of abdomen is dilated laterally, most of corium is milk-white to yellowish-white).
Sycanus hainanensis Zhao & Cai
sp. nov. is so much similar to
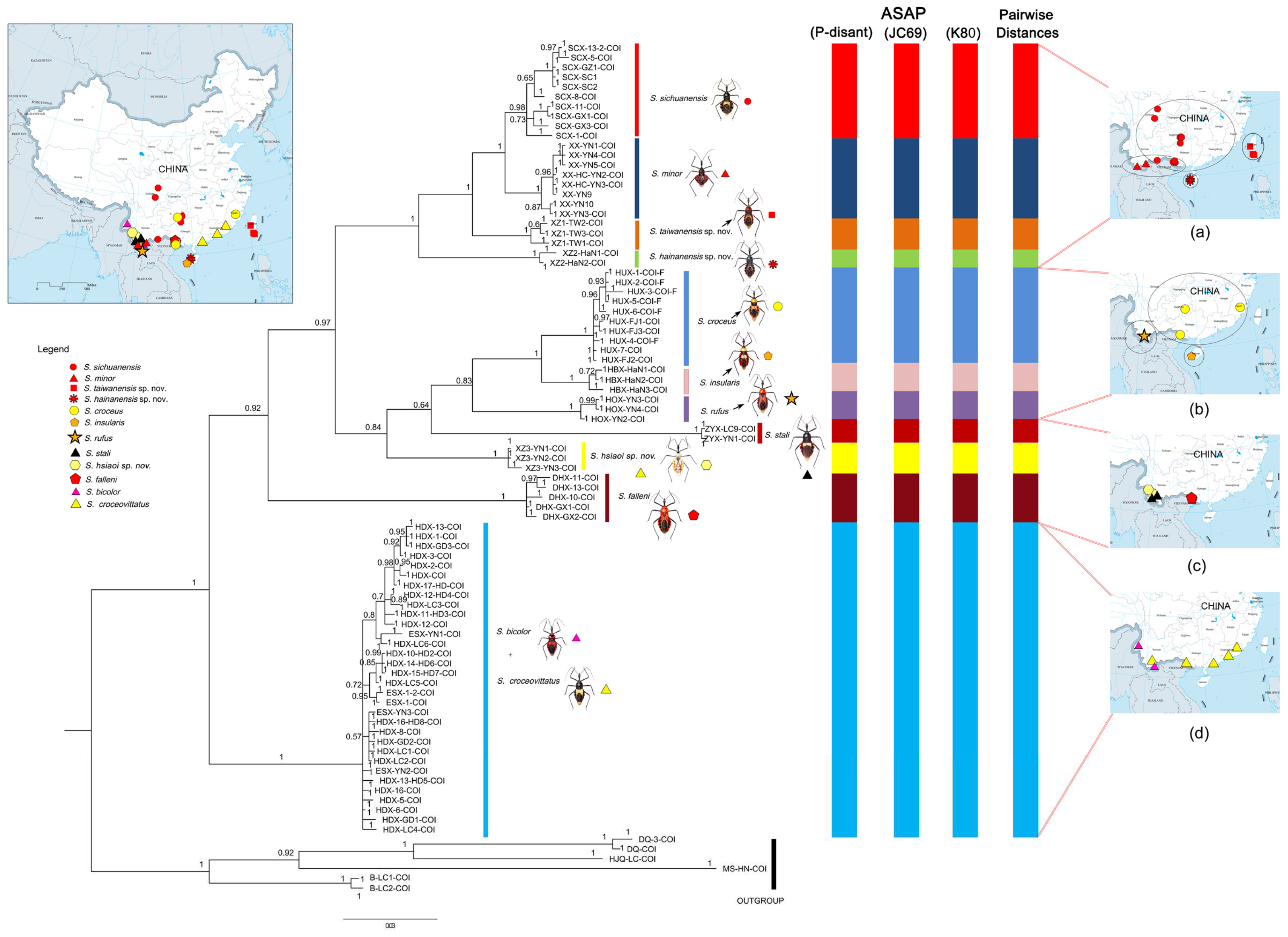
Sycanus sichuanensis Hsiao, 1979 in external morphological characteristics and male external genital structure, so that it is difficultly to distinguish each other. In COI DNA-barcodes molecular analyses, the result shows that the genetic distance between the two species supports two independent species (
Table S3, 9.64) .
Description. Coloration. Body black. Posterior margin of corium of hemelytron yellowish brown, membrane pale brown and semitransparent; connexivum black, transversal markings of each segment of red (
Figure 17).
Structure. Body median-sized. Head, thorax, ventral surface of abdomen, corium of hemelytron clothed with white procumbent short setae; legs with pale vertical setae of different length. Anti-ocular part distinctly shorter than post-ocular part; rostrum in-curved, long and slender. Collar indistinct, tuber-shaped; anterior pronotal lobe small, hemispherical and bulged; posterior pronotal lobe rugose, lateral angle obtuse and round, posterior margin nearly straight; scutellum sub-apically with a vertical long spine, apex of spine bi-forked. Femora nearly of equal thickness, apical part somewhat thickened; hemelytron extending beyond tip of abdomen. Fourth and fifth connexival segments of abdomen laterally distinctly expended (
Figure 17). Pygophore elliptic, median pygophore process “T”-shaped (Figures 18a, b); paramere clavate, sub-basal part somewhat bent, apical part with short setae (
Figure 18a–c). Struts triangular (
Figure 18d); dorsal phallothecal sclerite sclerotized, tongue-shaped; apical part of endosoma with a horned process and laterally with about 5-6 pairs of small spines (
Figure 18e–g).
Measurement [♂(n=3)/♀ (n=7), in mm] Body length 16.38–16.82/18.62–18.91; maximal width of abdomen 4.61–4.94/5.22–6.38; head length 4.25–4.30/4.63–4.79; length of ante–ocular part 1.55–1.56/1.51–1.71; length of post–ocular part 1.99–2.01/2.22–2.24; distance between ocelli 0.31–0.38/0.31–0.39; length of synthlipsis 0.56–0.57/0.52–0.54; length of antennal segments I–IV= 5.19–5.24/5.18–5.40, 1.80–2.05/1.90–2.09, 1.07–1.18/1.16/1.37, 6.05–6.87/6.00–6.02; length of visible rostral segments I–III= 1.81–1.86/1.94–2.29, 2.76–3.24/3.31–3.57, 0.36–0.41/0.42–0.52; length of anterior pronotal lobe 0.76–0.86/1.09; length of posterior pronotal lobe 1.54–1.71/1.89–1.97; maximal width of pronotum 3.23–3.29/3.83–3.89; length of scutellum 1.13–1.24/1.34; length of hemelytron 10.39–10.59/11.85–12.07.
Type species. Holotype, ♀, CHINA, Hainan, Ledong, Jianfengling, Yulin Valley, 680 m, 2022-V-11, Zheng Yuchen leg. (CAU). Paratypes, 3♂, 3♀ (CAU), 1♂, 1♀ (CATAS), CHINA, Hainan, Jianfengling, 2022.5.13, Wang Jianyun & Liu Yinyi leg.; 2♀, CHINA, Hainan, Diaoluoshan, Mengshuichang, 1981-IX-5 (CAU); 1♀, CHINA, Hainan, Wanning, Shimei, 1981-VI-10 (CAU).
Etymology: The specific name hainanensis is named after the type locality.
Distribution. CHINA (Hainan).
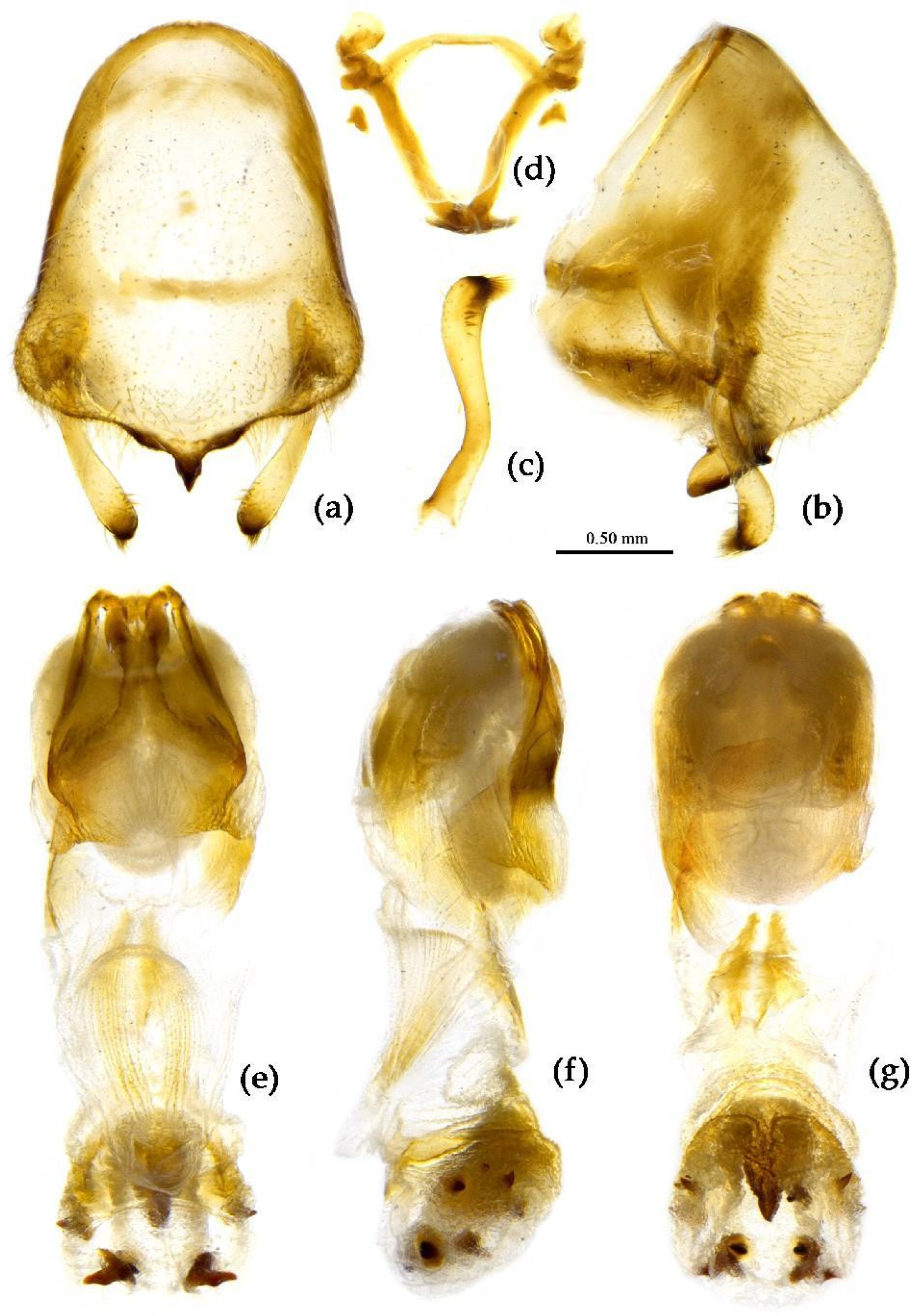
(10) Sycanus insularis Hsiao, 1979
(Figures 2g, 3g, 4g, 19, 20)
Sycanus insularis Hsiao 1979: 146, 154[
30]; Hsiao & Ren 1981: 521[
31]; Maldonado-Capriles 1990: 312[
24]; Putshkov & Putshkov 1996: 259[
32].
Redescription. Coloration. Body black, with yellowish and orange markings. Head (except ventral surface yellow and apical part of rostrum pale brown), antennae (except median two annular markings of first segment brown), legs (except coxae orange), anterior pronotal lobe, episterna of pleura of meso- and meta-thorax, sterna of thorax, segmental strip of abdomen and its expending round markings of connexivum, basal half and apical angle of corium of hemelytron black; pronotum (except anterior lobe), propleuron, anterior-lateral margin of corium, apical half of corium (except apical angle) yellowish; coxa, abdomen (except black inter-segmental stripes), epimera of pleuron of meso- and meta-thorax orange (
Figure 19).
Structure. Body median to large-sized. Body clothed with white short setae; legs, pronotum, scutellum with longer vertical setae. Head longer, post-ocular area of head longer than anti-ocular area; antennae slender and thin, first segment longest and sub-equal to fourth, third shortest and a little shorter than second; rostrum long, bent, first extending to middle part of eyes, second segment longest. Anterior angle of pronotum round and indistinct; anterior pronotal lobe small, hemisphere, posterior part with short longitudinal sulcus; posterior pronotal lobe rough, lateral angle round; posterior margin of scutellum round, apical spine sub-vertical, apex bi-forked; legs slender; hemelytron extending beyond apex of abdomen; fourth to five connexival segments dilated laterally (
Figure 19). Pygophore elliptic, median pygophore process shown in
Figure 20a, b; paramere clavate, apical half swelled with thick setae, middle part somewhat bent (
Figure 20c). Struts triangular (
Figure 20d); phallosoma elliptic; dorsal phallothecal sclerite sclerotized, tongue-shaped; endosoma apically with a horned process and laterally with about 13 pairs of small spines (
Figure 20e–g).
Measurement [♂ (n=4) / ♀ (n=3), in mm]. Body length 19.55 / 23.71–24.85, maximal width of abdomen 7.70 / 10.26–10.83; Head length 4.85 / 5.13–5.42; length of ante-ocular part 1.71/ 1.82–2.00; length of post-ocular part 2.28 / 2.28–2.85; length of synthlipsis 0.68 / 0.80–0.86; distance between ocelli 0.46 / 0.46–0.57; length of antennal segments I–IV = 6.16 / 6.84–7.13, 2.28 / 2.57–2.68, 1.71 / 1.82, 6.10 / 8.55–6.27; length of visible rostral segments I–III = 2.28 / 2.74–2.85, 3.14 / 3.82–3.93, 0.68 / 0.68–0.80; length of anterior pronotal lobe 1.14 / 1.20–1.31; length of posterior pronotal lobe 2.17 / 2.57–3.02; maximal width of pronotum 3.88 / 4.56–5.13; length of scutellum 1.37 / 1.43–1.82; length of hemelytron 12.08 / 15.39–16.82.
Type material. Holotype, ♂, CHINA, Hainan, Jianfengling, Tianchi, 1964-V-10, Liu Shengli leg. Allotype, ♀, same as Holotype, 1964-V-9. Paratype, 3♀, same as Holotpye (kept in NKU, NOT in TJNHM).
Specimens examined. CHINA, Hainan: 1♀, Hainan, Jianfengling Nature Reserve, 1980-IV-10, 900m, Xiong Jiang leg.; 1♂, 2♀, 2023-IV-21, Zhao Ping leg. (CAU); 1♀, Hainan, Jianfengling Nature Reserve, 1982-IV-10, Liu Yuanfu leg. (CAU); 1♂, 2♀, Hainan, Ledong, Jianfengling, Tianchi, 2015-V-07, Cai Nanyi leg. (CAU); 1♀, Hainan, Ledong County, Xingfengling, Fengming Valley, 2015-IV-2, Lu Qiu leg. (CAU); 1♂, Hainan, Ledong, Main peak of Jianfengling, 1412m, 2019-IV-14–16, Song Haitian leg. (CAU); 1♀, Hainan, Ledong, Jianfengling, 2013-IV-12, Sun & Zhang leg. (CAU); 1♀, Hainan, Jianfeng mountain, 1982-Ⅵ-10, Liu Yuanfu leg. (CAU); 1♂, 1♀, Hainan, Jianfengling (CAU); 1♀, Hainan, Lingshui County, Diaoluoshan, 1981-V-6 (CAU); 1♀, Hainan, Lingshui County, Diaoluoshan, 2009-IV-9–12, Hou Xiaohui leg., by light trap (CAU); 1♂, 1♀, Hainan, Wuzhishan Nature Reserve, 2023-IV-18, , Liu Xingyue leg. (CAU).
Distribution. CHINA (Hainan <Jianfengling, Diaoluoshan, Wuzhishan>).
(10) Sycanus minor Hsiao, 1979
(Figures 2h, 3h, 4h, 21, 22, S8e)
Sycanus minor Hsiao 1979: 43, 154[
30]; Hsiao & Ren 1981: 521[
31]; Maldonado-Capriles 1990: 313[
24]; Putshkov & Putshkov 1996: 259[
32].
Redescription. Coloration. Body color changeable greatly, generally dark brown to black, with red markings, sometimes almost totally black (
Figure 21). Head (except apical part of rostrum and markings on outside of ocelli yellowish brown), antennae, pronotum (except lateral and posterior margins of posterior lobe orange, or collar, lateral margin and posterior lobe altogether orange), thorax (except coxal cavity orange, or most of pro-pleuron, markings of meso- and meta-pleura, anterior margin of meso- and meta- sterna orange), scutellum (except apical spine orange, posterior margin greyish brown), legs (except coxae orange, sometimes a sub-apical annulation of femur and tibiae paler), corium of hemelytron (except sub-apical part yellowish or milk white) black; membrane of hemelytron semitransparent, black; abdomen red to yellowish, median longitudinal stripe, transversal strip of sternum and markings of each connexival segment black (
Figure 21). Sometimes body almost totally black, except posterior margin or transversal tripe of corium of hemelytron yellowish, sub-apical annulations of tibiae and femora milk-white, and third and fourth antennal segments and subapical annulation of first segment milk-white to pale brown.
Structure. Median-sized. Body clothed with white setae and depressed curved short setae, legs with straight setae of different length. Head longer, post-ocular area of head longer than anti-ocular area; antennae slender and thin, first segment longest and sub-equal to fourth, third shortest and distinctly shorter than second; rostrum long, bent, first extending to middle part of eyes, second segment longest. Anterior angle of pronotum round and indistinct; anterior pronotal lobe small, hemispheric, posterior part with short longitudinal sulcus; posterior pronotal lobe rough, lateral angle round; posterior margin of scutellum round, apical spine sub-vertical and produced posteriorly, apex bi-forked; legs slender; hemelytron extending beyond apex of abdomen; fourth to sixth connexival segments dilated laterally (
Figure 21). Pygophore elliptic, median pygophore process shown in
Figure 22a, b; paramere clavate, apical part with setae, middle part somewhat bent (
Figure 22c). Struts triangular (
Figure 22d); phallosoma elliptic, dorsal phallothecal sclerite sclerotized, endosoma apically with a horned process and laterally with about 5 pairs of small spines (
Figure 22e–g).
Measurement [♂ (n=3) / ♀ (n=1), in mm]. Body length 17.20–18.18 /19.27, maximal width of abdomen 8.00–8.80 / 8.18; Head length 4.46–4.73/4.73; length of ante-ocular part 1.47–1.64/1.64; length of post-ocular part 2.15–2.18/2.18; length of synthlipsis 0.43–0.56/0.58; distance between ocelli 0.36–0.55/0.36; length of antennal segments I–IV = 5.20–6.00/5.45, 2.00–2.36/2.00, 0.91–1.10/1.09, 6.00-6.18/5.55; length of visible rostral segments I–III = 1.92–2.18/2.36, 3.15–3.64/3.64, 0.55–0.58/0.55; length of anterior pronotal lobe 0.84–1.09/1.27; length of posterior pronotal lobe1.71–2.00/2.18; maximal width of pronotum 3.31–3.73/4.36; length of scutellum 1.45/1.64; length of hemelytron 9.87–11.82/12.36.
Type material. Holotype, ♂, Yunnan, Xishuangbanna, Banna Menglong, Manbing, 1958-IV-14, Cheng Hanhua leg., 650m, Hsiao Caiyu Det., 1972, kept in Tianjin Natural Museum (TJNHM).
Specimens examined. CHINA, Guangxi: 2♂, Guangxi, Ningming, Huashan, 26-VIII-2019, Zhao Ping leg., (NNU);
CHINA, Yunnan: 1♂, Yunnan, Xishuangbanna, Manbing, 1958-IV-14, Zheng leg., Ren Shuzhi Det., 1972, kept in Nankai University (NKU); 1♂, 1♀, Yunnan, Mengla, Menglu, Cui Jianxin leg. (CAU); 1♂, 1♀, Yunnan, Banna, Jinghong, N21.59955, E100.47813, 530m, 2009-V-3, Cao Liangming leg. (CAU); 5♂, Yunnan, Banna, Jinghong, Jinuo, Jinuo, 2021-VII-22, 1099m, Chen Zhaoyang & Liu Qinpen leg. (CAU); 1♀, Yunnan, Xishuangbanna, Menglun botanical garden, Wild Elephant Valley (CAU); 1♂, 1♀, Yunnan, Banna, Jinghong, Jinuo mountain, National Highway 213,1053 m, 2021-VII-25 (CAU).
Distribution. CHINA (Yunnan <Xishuangbanna>, Guangxi <Ningming>); VIETNAM.
Remark. We didn’t check the type specimen, but we found a male specimens kept in Nankai University with the same information as the type specimen and identified by Pro. Ren Shuzhi.
In addition, the body color of Sycanus minor is changeable from orange or red with black markings to almost completely black. The three specimens from Mengla and Jinghong, Xishuangbanna, Yunnan, China, the body color is black (except the posterior margin of corium of fore wing is yellow to milk-white, and the annular markings of sub-apical part of femur is milk-white), and through the DNA barcoding molecular analysis, the result showed that the three specimens should belong to the species Sycanus minor.
(12) Sycanus rufus Hsiao 1979
(Figures 2i, 3i, 4i, 23, 24)
Sycanus rufus Hsiao 1979: 141, 154,
Figure 2[
30]; Hsiao & Ren 1981: 522[
31]; Maldonado-Capriles 1990: 313[
24]; Putshkov & Putshkov 1996: 260[
32].
Redescription. Coloration. Body red, with black markings. Head (except apical part of rostrum and markings around ocelli yellowish brown), antennae, sterna and pleuron of meso- and meta-thorax (except coxal cavity orange), scutellum (except apical spine orange, posterior margin greyish brown), markings of anterior pronotal lobe, legs (except coxae orange, sometimes a sub-apical annulaion of femur and tibiae paler), basal part of corium of hemelytron blackish brown; membrane of hemelytron semitransparent, blackish brown; abdomen red to yellowish red, median longitudinal stripe, basal transversal strip and lateral markings of sternum, and basal round large markings of each connexival segment formed by which basal transversal strip of 3rd o 7th sterna laterally extended (round markings of sixth and seventh not extending to margin of connexivum) black; ventral surface of head, pronotum (except markings of anterior lobe black) and pro-pleuron (except posterior margin black) , corium of hemelytron (except basal half), coxae, coxal cavity red , or orange (
Figure 23).
Structure. Body large-sized. Ventral surface of head, pronotum, pleura and sterna of thorax, corium of hemelytron clothed with yellow procumbent short setae; head (except ventral surface), pronotum and propleuron, scutellum, legs, ventral surface of abdomen with pale setae of different length. Anticular part distinctly shorter than post-ocular part; rostrum in-curved, long and slender. Collar indistinct and round; anterior pronotal lobe small, hemisphered and bulged, deeply depressed at base; posterior pronotal lobe rugose and reticulated, lateral angle obtuse and round, posterior margin nearly straight, posterior angle nearly absent; scutellum sub-apically with an erect upward, thick short spine, tuber-shaped in male, short cone-shaped in female. Femora nearly of equal thickness, apical part somewhat thickened; hemelytron extending beyond tip of abdomen. Fourth to sixth connexival segments of abdomen moderately expended (
Figure 23). Pygophore elliptic, median pygophore process shown in
Figure 24a, b; paramere clavate, apical part with setae, middle part somewhat bent (
Figure 24c, d). Struts triangular (
Figure 24e); phallosoma elliptic, dorsal phallothecal sclerite sclerotized, endosoma apically with a horned process and laterally with about 5 pairs of small spines (
Figure 24f–h).
Measurement [♂ (n=4) / ♀ (n=3), in mm]. Body length 20.29–20.63/24.85–25.88, maximal width of abdomen 5.81–5.99/8.44–10.15; Head length 4.67–4.96/5.24–5.47; length of ante-ocular part 1.71/1.94–2.05; length of post-ocular part 2.28/2.45–2.57; length of synthlipsis 0.68–0.73/0.80–0.86; distance between ocelli 0.46/0.51; length of antennal segments I–IV = 5.89–6.38/6.95–7.01, 2.51–2.85/2.85–3.02, 2.17–2.45/2.37–2.57, 5.53/5.87–6.67; length of visible rostral segments I–III = 2.28/2.85, 3.42/3.99, 0.68–0.71/0.74–0.80; length of anterior pronotal lobe 1.03–1.14/1.14–1.20; length of posterior pronotal lobe 2.11–2.17/2.25–3.08; maximal width of pronotum 3.93–3.99/5.47–5.76; length of scutellum 1.14–1.25/1.20–1.25; length of hemelytron 12.83–12.85/16.82–17.67.
Type material. Holotype, ♂, CHINA, Yunnan, Xishuangbanna, Menga, 1050–1080 m, 9-VI-1958, Fu Fuji leg. Allotype, ♀, same as Holotype, 6-VI-1958. Paratype, 2♂, 1♀, CHINA, Yunnan, Lancang, Xishuangbanna, kept in IOZ.
Specimens examined. CHINA, Yunnan: 1♂, Yunnan, Mengla, V-29, 650m (CAU); 1♀, Yunnan, Xishuangbanna, Mengla, Yaoqu, 2006-VI-5, Wang Hesheng leg. (CAU); 1♀, Yunnan, Banna, Jinghong, Jinuo, Yunfenggusi, 1070m, 2021-VII-20, Chen Zhaoyang & Liu Qinpeng leg. (CAU); 3♂, 1♀, Yunnan, Xishuangbanna, Mengla, Bubeng, 2012-VII-10, Zhao & Chen leg. (CAU); 1♀, Yunnan, Banna, Jinghong, Jinuo, 1100m, 2018-V-31 (CAU); 1♀, Yunnan, Pu’er, Simao, 1436m, 2018-VI-20 (CAU); 1♀, Yunnan, Xishuangbanna, Jinghong Guanping, 2008-V-27, Huang Xinyong leg. (CAU).
Distribution. CHINA (Yunnan <Lancang, Pu’er, Xishuangbanna: Mengla, Meng’a, Xiaomengyang, Mengzha>).
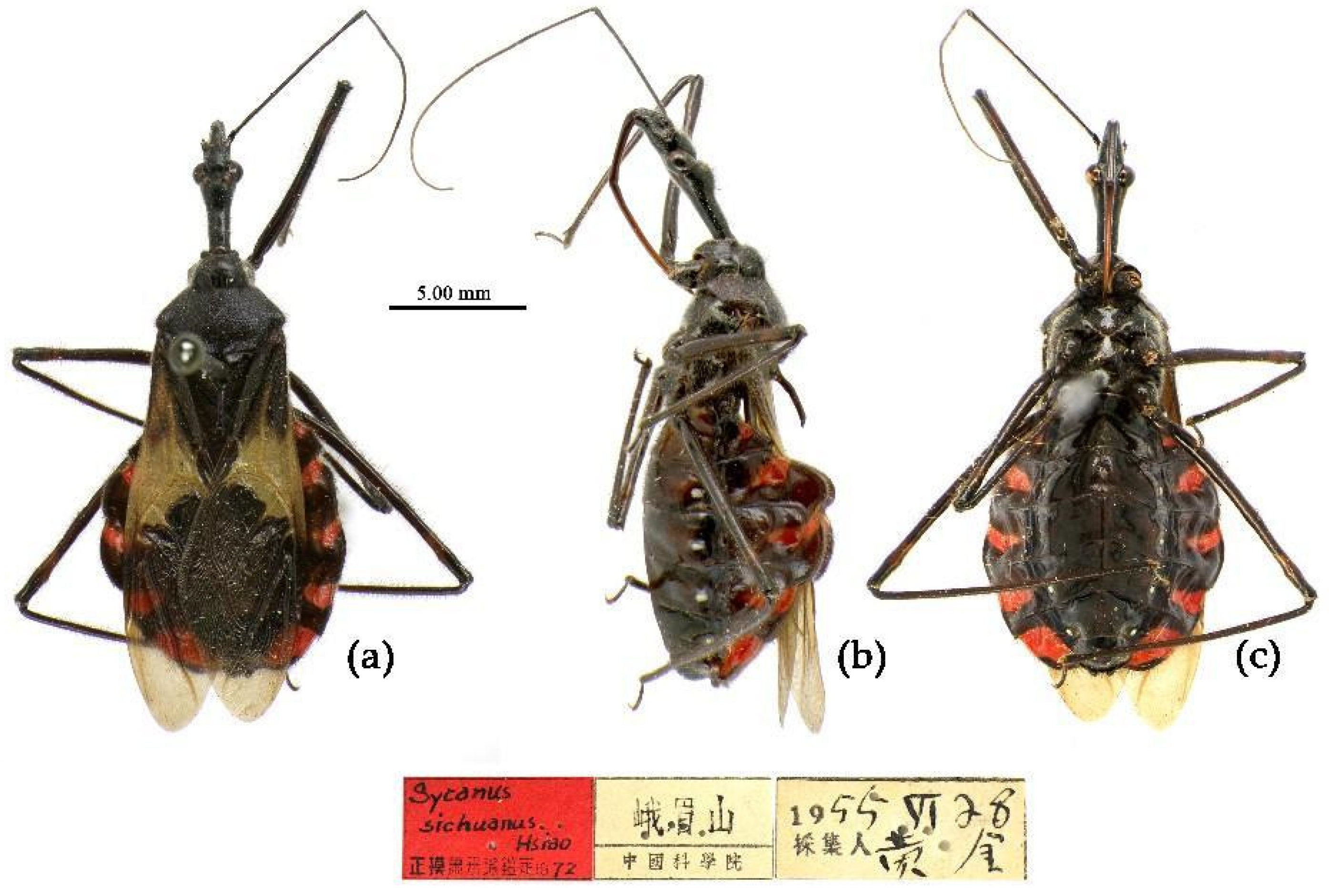
(13) Sycanus sichuanensis Hsiao 1979
(Figures 2j, 3j, 4j, 25, 26, S8f)
Sycanus sichuanensis Hsiao 1979: 153, 154[
30]; Maldonado-Capriles 1990: 313[
24]; Putshkov & Putshkov 1996: 260[
32].
Sycanus szechuanus (sic): Hsiao & Ren 1981: 520[
31].
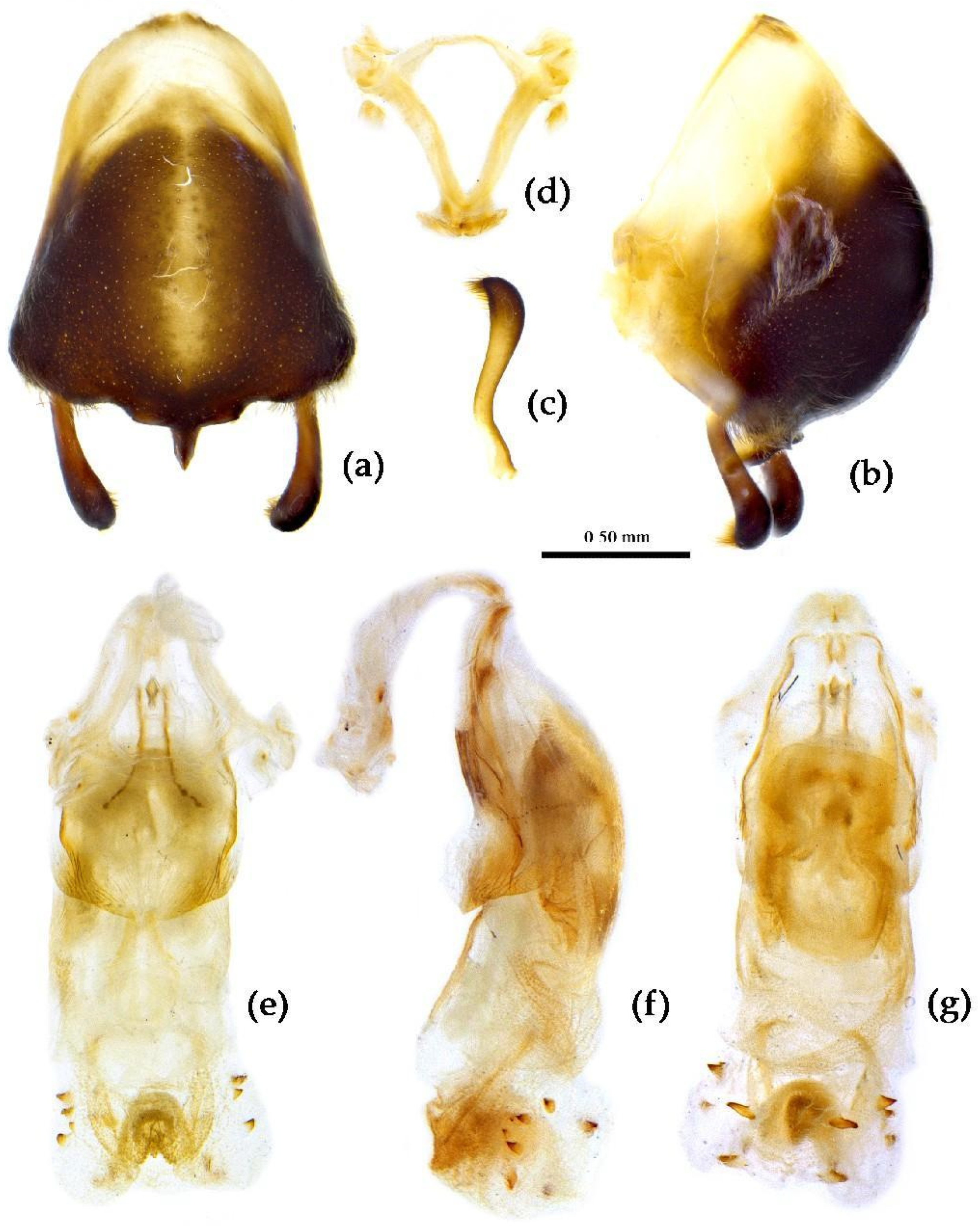
Redescription. Coloration. Body dark brown to black, shiny. Corium of hemelytron (except basal part and apical angle), basal of membrane, yellowish; transversal markings of 3rd to 7th connexival segment red; membrane semitransparent, brown; sub-apical par of femur paler brown o white; marking around ocelli; apical part of rostrum brown (
Figure 25) .
Structure. Body medain to large-sized. Head, thorax, corium of hemelytron clothed with yellow procumbent short setae and vertical short setae of different length; legs with vertical setae of different length. Ante-ocular part distinctly shorter than post-ocular; rostrum in-curved, long and slender. Collar indistinct and round; anterior pronotal lobe small, hemisphered and bulged, deeply depressed at base; posterior pronotal lobe rugose and reticulated, lateral angle obtuse and round, posterior margin nearly straight, posterior angle nearly absent; scutellum sub-apically with an long spine, apical spine produced posteriorly, apex bi-forked. Femora nearly of equal thickness, apical part somewhat thickened; hemelytron extending beyond tip of abdomen. Abdomen distinctly roundly laterally expended (
Figure 25). Pygophore elliptic, median pygophore process “T”-shaped shown in
Figure 26a, b; paramere clavate, apical half somewhat swelled with thick setae, middle part somewhat bent (
Figure 26c). Struts triangular (
Figure 26d); phallosoma elliptic, dorsal phallothecal sclerite sclerotized, endosoma apically with a horned process and laterally with about 5 pairs of small spines (
Figure 26e–g).
Measurement [♂ (n=8)/♀ (n=5), in mm] body length 16.55–18.73/16.73–22.58; maximal width of abdomen 6.36–7.27/6.36–11.16. head length 4.18–4.73/4.18–5.25; length of ante–ocular part 1.45–1.55/1.45–1.84; length of post–ocular part 1.82–2.18/2.00–2.68; distance between ocelli 0.25–0.55/0.29–0.47; length of synthlipsis 0.55–0.73/0.55–2.26; length of antennal segments I–IV= 5.45–6.00/5.82–6.62, /2.18–2.94, 2.27–2.36/1.64–2.26, /6.67–6.91; length of visible rostral segments I–III=1.64–1.82/1.64–2.63, 3.27–3.45/2.73–3.89, 0.64/0.55–0.74; length of anterior pronotal lobe 1.00–1.09/0.91–1.21; length of posterior pronotal lobe 1.82/1.82–2.57; maximal width of pronotum 3.09–4.00/3.36–4.41; length of hemelytron 10.18–11.45/10.36–14.70.
Type material. Holotype, ♂, CHINA, Sichuan, Ermeishan, 1955-VI-28, Huangjin leg. Allotype, ♀, same as Holotype, 1955-VI-23. Paratypes: 3♀, 1♂, CHINA, Sichuan, Ermeishan (IOZ); 1♀, 1♂, CHINA, Sichuan, Ermeishan, 1955-VI-27, 580m, Xie Dabin leg.(IOZ); 1♀, 3♂, CHINA, Sichuan, Er’meishan, Huang Keren & Jin Yintao leg. (IOZ).
Specimens examined. CHINA, Guangxi: 1♂, Guangxi, Longzhou, Longrui, 1984-V-20, Ren Shuzhi leg. (NKU); 1♂, Guangxi, Tianpingshan, 1963-VI-17 (NKU); 1♀, Guangxi, Nonggang, Longzhou, 2003-VIII-15, Zhou Zhihong leg. (CAU); 1♀, Guangxi, Huaping, Tianping mountain, 1963-VI-5, Yang Jikun leg. (NKU); 1♂, 1♀, Guangxi, Jinxiu, 1983-V-27 (NKU); 1♀, Guangxi, Lonhzhou, Nonggang, 1983-V-15 (NKU); 1♀, Guangxi, Pingxiang, Orchid Valley Park, 2014-X-8, Sun & Luo leg. (CAU); 1♀, Guangxi, Guilin, Huaping, Tianping mountain, 1963-VI-5, Yang Jikun leg. (NKU); 1♀, Guangxi, Longzhou, Nonggang, 2003-VIII-15, Zhou Zhihong leg. (CAU); 1♂, 1♀, Guangxi, Jinxiu, 1983-V-27 (NKU); 1♀, Guangxi, Longzhou, Nonggang, 1983-V-15. (NKU).
CHINA, Sichuan: 1♀, Sichuan, Ermeishan, 600m-1200m, 2019-VI-27 (CAU); 1♀, Sichuan, Ermeishan, 580 m, 19-VI, Huang Keren leg. (NKU);1 ♀, Sichuan, Chengdu, Pengzhou, Bailu Town, 800m, 2018-VII-21, Zhou Chao leg. (CAU); 1♀, Sichuan, Leshan, Emeishan, 2014-VII -3, Liang Si leg. (CAU).
CHINA, Guizhou: 1♀, Guizhou, Rongjiang, Pingyang, 693m, 2016-VI-20-24, Wu Shengsheng leg. (CAU); 1♀ Guizhou, Rongjiang, Pingyang, Xiaodanjiang, 2005-VI-3, 920-970m, Zhao Ping leg. (NNU).
CHINA, Yunnan: 1♀, Yunnan, Honghe, Hekou, Huayu cave, 334m, 2016- IV-23, Yang Xiaodong leg. (CAU); 1♂, Yunnan, Honghekou, Huayu cave, 200m, 2016- IV-24, Yu Tang Wang leg. (CAU).
CHINA, Hunan: 1♀, Hunan, Yongzhou, Ningyuan, Jiuyi mountain, 915m, 2021-VI-4–7, Peng Huoliang leg. (CAU);
Distribution. CHINA (Hunan <Xiangxi, Xiangnan, Yonhzhou>, Hubei, Sichuan <Chengdu, Ermeishan, Ya’nan, Xingjing>, Guizhou<Maolan, Rongjiang, Shiqian>, Guangxi<Longsheng, Longzhou, Guilin, Pingxiang, Jinxiu, Huaping>, Yunan <Gengma, Honghe>); VIETNAM.
(14) Sycanus stali Dohrn, 1859
(Figures 2k, 3k, 4k, 27, 28, S7)
Sycanus stali Dohrn, 1859: 96[
27].
Sycanus generosus Stål, 1863: 58[
28].
Sycanus (
Cosmosphodrus)
stalii (sic) var.
generosus: Stål, 1874: 29[
41].
Sycanus marginatus Hsiao, 1979: 143, 154[
30].
Sycanus marginellus Putshkov P.V.: Putshkov, V.G., Putshkov, P.V. & Štys, 1987: 104[
43], new name for
S. marginatus Hsiao 1979.
Sycanus hsiaoi Maldonado-Capriles 1990: 312[
24], new name for
S. marginatus Hsiao 1979. new synonym.
Redescription. Coloration. Body black. Apical half of corium of hemelytron (apical angle) yellowish; margin of connexivum red; apical part of rostrum brown (
Figure 27).
Structure. Body large-sized. Body clothed with black short setae; legs, pronotum, scutellum with longer vertical setae. Head longer, post-ocular area of head longer than anti-ocular area; antennae slender and thin, fourth segment longest and sub-equal to first, third shortest and a little shorter than second; rostrum long, bent, first extending to middle part of eyes, second segment longest. Anterior angle of pronotum round; anterior pronotal lobe small, hemisphere, posterior part with short longitudinal sulcus; posterior pronotal lobe rough, lateral angle round; posterior margin of scutellum round, apical spine with tubercular processes; legs slender; hemelytron extending beyond apex of abdomen; abdomen roundly dilated laterally, fourth to five connexival segments dilated laterally (
Figure 27). Pygophore elliptic, median pygophore process shown in
Figure 28a, b; paramere clavate, apical half swelled with thick setae, middle part distinctly bent (
Figure 28c). Struts triangular (
Figure 28d); phallosoma elliptic, dorsal phallothecal sclerite sclerotized, endosoma apically with a horned process and laterally with about 6 pairs of small spines (
Figure 28e–g).
Measurement [♂ (n=4) / ♀ (n=3), in mm]. Body length 21.27–25.08/26.11–27.27, maximal width of abdomen 8.18–8.55/9.92–10.91; Head length 4.73–5.53/5.70–5.82; length of ante–ocular part 1.63–1.88/2.00; length of post–ocular part 2.18–2.74/2.73–2.74; length of synthlipsis 0.64/0.91; distance between ocelli 0.27/0.33–0.51; length of antennal segments I–IV= 5.82/6.91–6.95, 2.36/2.96–3.09, 2.85/2.28, 6.55/9.09; length of visible rostral segments I–III= 2.18–2.85/2.91–2.96, 3.27–4.39/4.10–4.18, 0.64–0.74/0.80–0.91; length of anterior pronotal lobe 1.09–1.31/1.27–1.43; length of posterior pronotal lobe 2.00–2.85/3.02–3.09; maximal width of pronotum 3.64–4.85/5.42–5.45; length of scutellum 1.43/1.71; length of hemelytron 13.09–16.53/ 17.67–18.18.
Specimens examined. 1♂, 1♀, Yunnan, Menghai, 20-V-1991, 600m, Cai Wanzhi leg. (CAU); 30♂, 30♀, China, Yunnan, Puer, Simao district, Nanping, Baizhi tamper, Wanmu tea Garden, 2022-VI-15, Zhang Guirong leg. (NNU).
Distribution. CHINA (Yunnan<Lancang, Xishuangbanna: Menga, Menghai, Mengzha, Puer>); PHILIPPINES.
Notes. Although we haven’t examined the type of
Sycanus stali Dohrn, 1859[
27], we found the syntype of
Sycanus generosus Stål, 1863 [
28] kept in Swedish Museum of Natural History on the website:
http://www2.nrm.se/en/het_nrm/g/sycanus_generosus.html (
Figure S7a,b). Stål in 1874 regarded
Sycanus generosus Stål, 1863 and
Sycanus generosus Stål, 1863 as two variants of
Sycanus stali Dohrn, 1859[
41]. The type photograph of
Sycanus marginatus Hsiao, 1979 (
Figure S7c–e) is morphologically consistent with the type photograph of
Sycanus generosus Stål, 1863 (
Figure S7a,b). Therefore, we use
Sycanus stali Dohrn, 1859 as the specific name.
(15) Sycanus taiwanensis Zhao & Cai sp. nov.
(Figures 2l, 3l, 4l, 29, 30)
Diagnosis. The male external genitalia of the new species is the most similar to that of
Sycanus sichuanensis, but there are differences in external body color and structure. In the new species, the body is slightly thin and long, the connexivum is laterally dilated, the posterior pronotal lobe is red (except the middle part is black) and posterior margin of corium of fore wing is milk-white (vs. In
Sycanus sichuanensis, the body is posteriorly widen, and the connexivum is wide and round, and the middle part of each segment of connexivum is elevated, the pronotum is totally black and the most of corium of fore wing is white) (
Figure 29).
Description. Coloration. Body black, with red markings. Head (except rostrum and markings around ocelli yellowish brown), antennae, thorax (except lateral and posterior margins of posterior lobe red or totally red), scutellum, legs, corium of hemelytron (except posterior margin yellowish), abdomen (except connexivum) black; membrane of hemelytron semitransparent, brown; connexivum red, markings of 4th to 7th segmental suture black (
Figure 29).
Structure. Body large-sized. Head, thorax, ventral surface of abdomen, corium of hemelytron clothed with white procumbent short setae; legs with pale vertical setae of different length. Ante-ocular part distinctly shorter than post-oculart; rostrum in-curved, long and slender; antennae slender and thin, first segment longest and somewhat longer than fourth, third shortest and a little shorter than second. Collar indistinct; anterior pronotal lobe small, hemisphered and bulged, deeply depressed at base; posterior pronotal lobe rugose and reticulated, lateral angle obtuse and round, posterior margin nearly straight; scutellum sub-apically with an oblique spine, spine indistinctly bi-forked. Femora nearly of equal thickness, apical part somewhat thickened; hemelytron extending beyond tip of abdomen. Fourth and fifth connexival segments of abdomen moderately expended (
Figure 29). Pygophore elliptic, median pygophore process shown in
Figure 30a, b; paramere clavate, apical half somewhat swelled with thick setae, middle part somewhat bent (Figure 39c). Struts triangular (
Figure 30d); phallosoma elliptic; dorsal phallothecal sclerite sclerotized; endosoma apically with a horned process and laterally with about 5 pairs of small spines (
Figure 30e-g), and middle part dorsally with two short and slender sclerites.
Measurement [♂ (n=3) / ♀ (n=3), in mm]. Body length 18.73–20.41/22.8–20.00, maximal width of abdomen 5.24–6.73/6.56–6.91; Head length 4.55–5.13/5.64–6.91; length of ante–ocular part 1.73–1.77/1.88–2.00; length of post–ocular part 2.00–2.57/2.36–2.57; length of synthlipsis 0.69–0.80/0.73–0.80; distance between ocelli 0.36–0.57/0.51–0.57; length of antennal segments I–IV = 5.45–6.56/6.00–6.44, 2.36–2.62/2.62–2.73, 1.54/1.68, 5.00/5.80; length of visible rostral segments I–III = 2.00–2.45/2.18–2.34, 3.45–3.99/3.64–3.93, 0.55–0.74/0.55–0.86; length of anterior pronotal lobe 1.09–1.25/1.09–1.37; length of posterior pronotal lobe 1.82–2.22/2.39–2.45; maximal width of pronotum 3.82–3.99/4.36–4.39; length of scutellum 0.86/1.25; length of hemelytron 11.82–12.56 /12.73–13.97.
Holotype, ♀, CHINA, Taiwan, Pingtung County, Lilong Mountain, 650 m, 2015-I-26 (CAU). Paratypes. 1♂, CHINA, Taiwan, Pingtung County, Lilong mountain, 650 m, 2015-I-26 (CAU); 1♂, CHINA, Taiwan, Pingtung County, Manchu Township, Jialeshui, 2019-XI-08, Liu Xingyue leg. (CAU); 1♀, CHINA, Taiwan, Pingtung County, Manchu Township, Jialeshui, 2010-V-29, Zhou Wenyi leg. (CAU); 1♀, CHINA, Taiwan, Pingtung County, Heng-Chun, Ken-Ting, 400 m, 2016-VIII-23, Wu S.P. & Chung Y.T. leg. (CAU); 1♀, CHINA, Taiwan, Pingtung County, Heng-Chun, Ken-Ting, 2010-X-7, Cai Wanzhi leg. (CAU); 1♂, CHINA, Taiwan, Pingtung County, Fenggang, Lilong Moutain, 2015-I-26, 650m, Zhong Yiting leg. (CAU); 2♂, 4♀, CHINA, Taiwan, Pingtung County, Lilong mountain, 650m, 2015-Ⅷ-17, Y.-T. Chung leg. (CAU); 1♂, 2♀, CHINA, Taiwan, Pingtung County, Lilong mountain, 651m, 2015-Ⅷ-18, Y.-T.Chung leg. (CAU); 1♀, CHINA, Taiwan, Pingtung County, Manchu Township, Gangkou, 2010-VI-13, Zhou Wenyi leg. (CAU); 1♀, CHINA, Taiwan, Pingtung County, Neipu Township, 2001-Ⅵ-23, Wu Shuping leg. (CAU); 1♂, CHINA, Taiwan, Kaohsiung City, Taoyuan, Zhou W.Y. leg. (CAU); 1♀, CHINA, Taiwan, Kaohsiung City, Taoyuan District, Meilong Mountain, 1370m, 2016-Ⅵ-15, Po-Hsin Kuo leg. (CAU); 1♀, CHINA, Taiwan, Kaohsiung, Taoyuan, Xiaoguanshan forest road,1523m, 2016-V-15, Po-Hsin Kuo leg. (CAU); 1♀, CHINA, Taiwan, Kaohsiung, Maolin (CZ), 365m, 2012-X-26, (CAU); 1♀, CHINA, Taiwan, Chiayi County, Alishan Township, Danayi Valley, 2012-VI-23, Song Fan leg. (CAU); 1♀, CHINA, Taiwan, Xinbei City, Sanxia District, Beichatian Mountain, 2016-Ⅶ-19, Wu Shuping leg. (CAU); 1♀, CHINA, Nantou country, Endemic Species Research Institute, 2016-Ⅺ-4, Zhao Yisheng leg. (CAU).
Etymology: The specific name taiwanensis alludes to the locality of the type species in Taiwan province, the Peoples Republic of China.
Distribution. CHINA (Taiwan).