Submitted:
14 December 2023
Posted:
14 December 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
2.1. Phylogeny
2.2. API ZYM
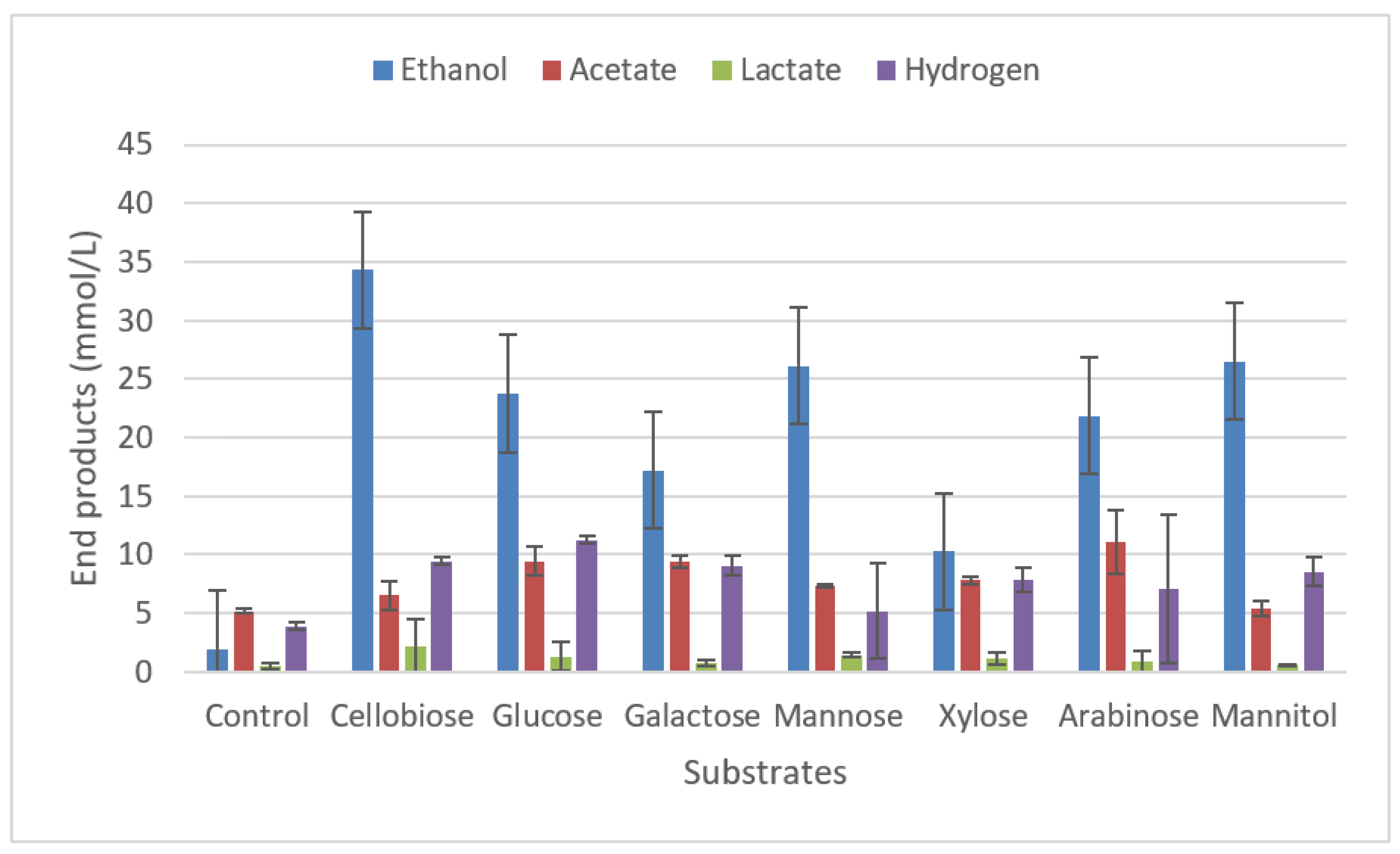
2.3. Substrate spectra
2.3.1. Degradation of carbohydrates
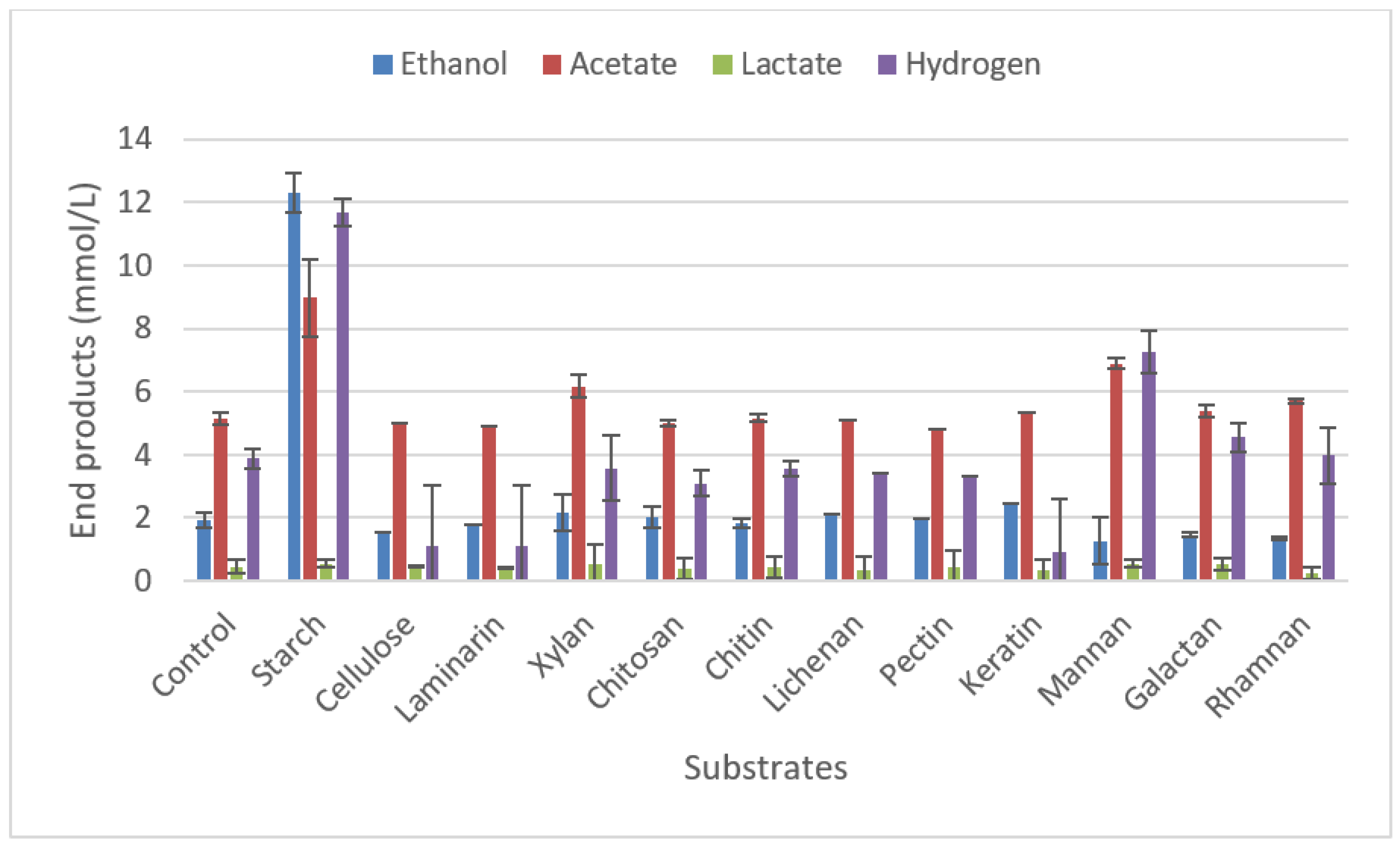
2.3.2. Degradation of polymeric carbohydrate substrates.
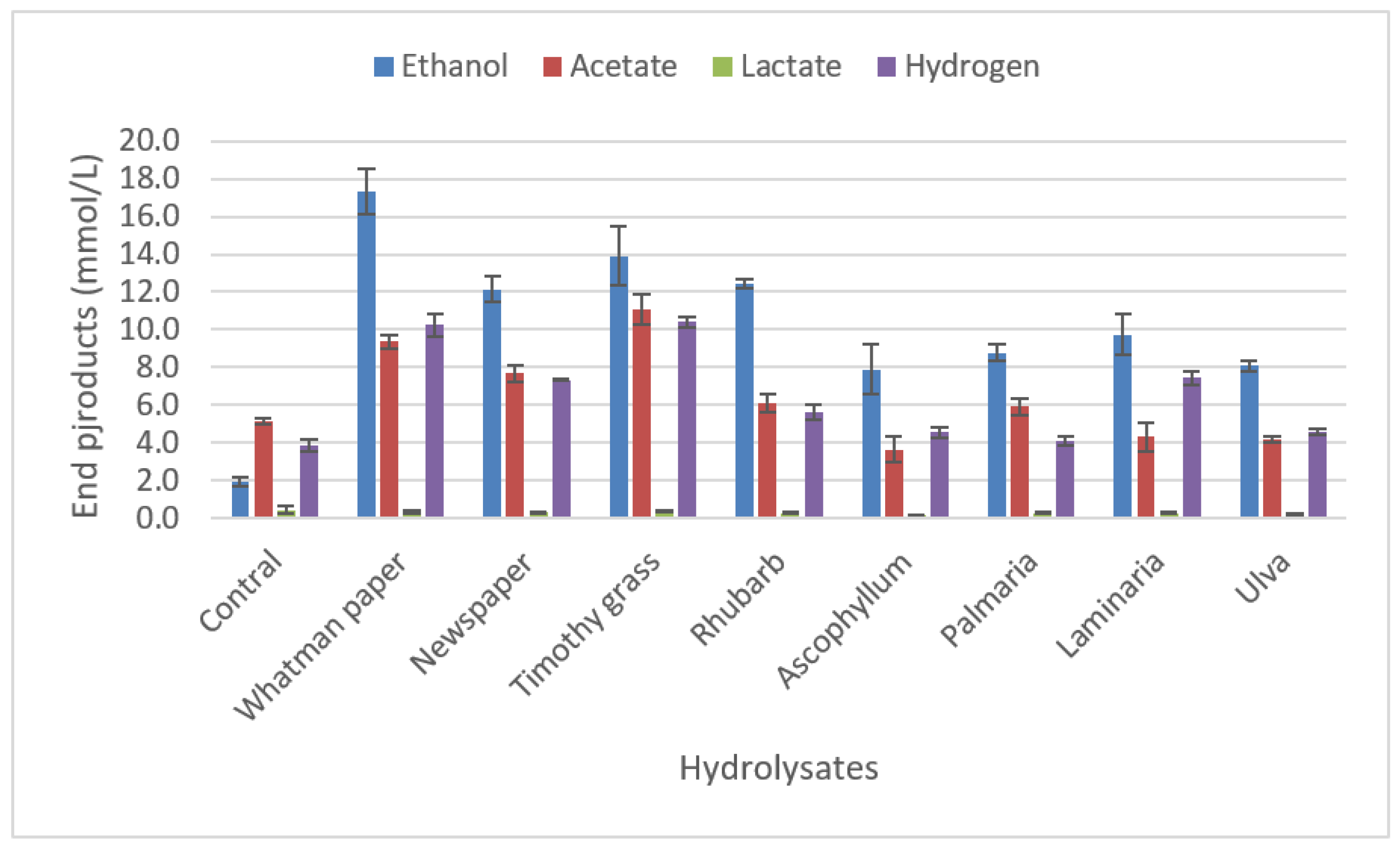
2.3.3. Degradation of biomass hydrolysates
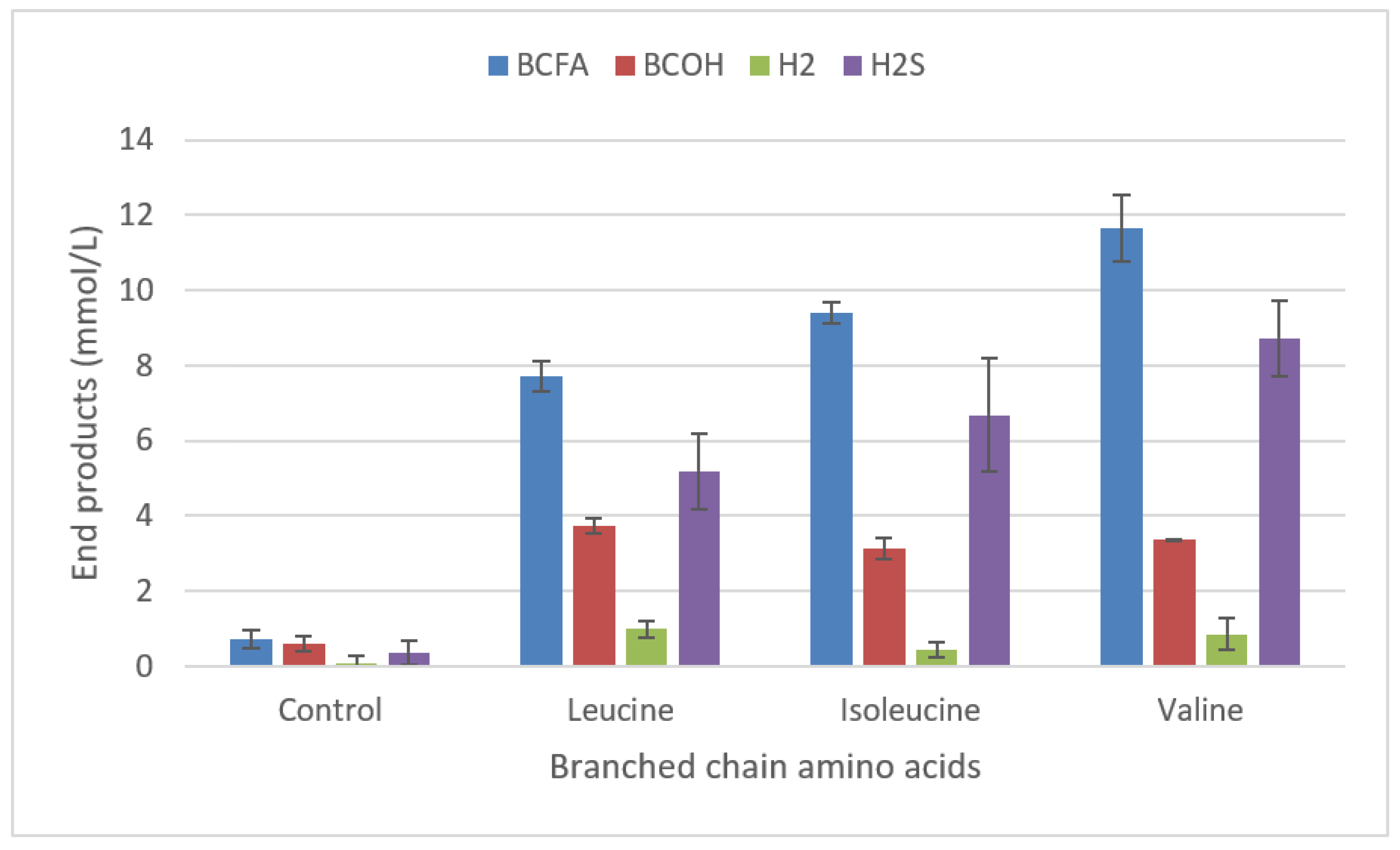
2.3.4. Degradation of amino acids.
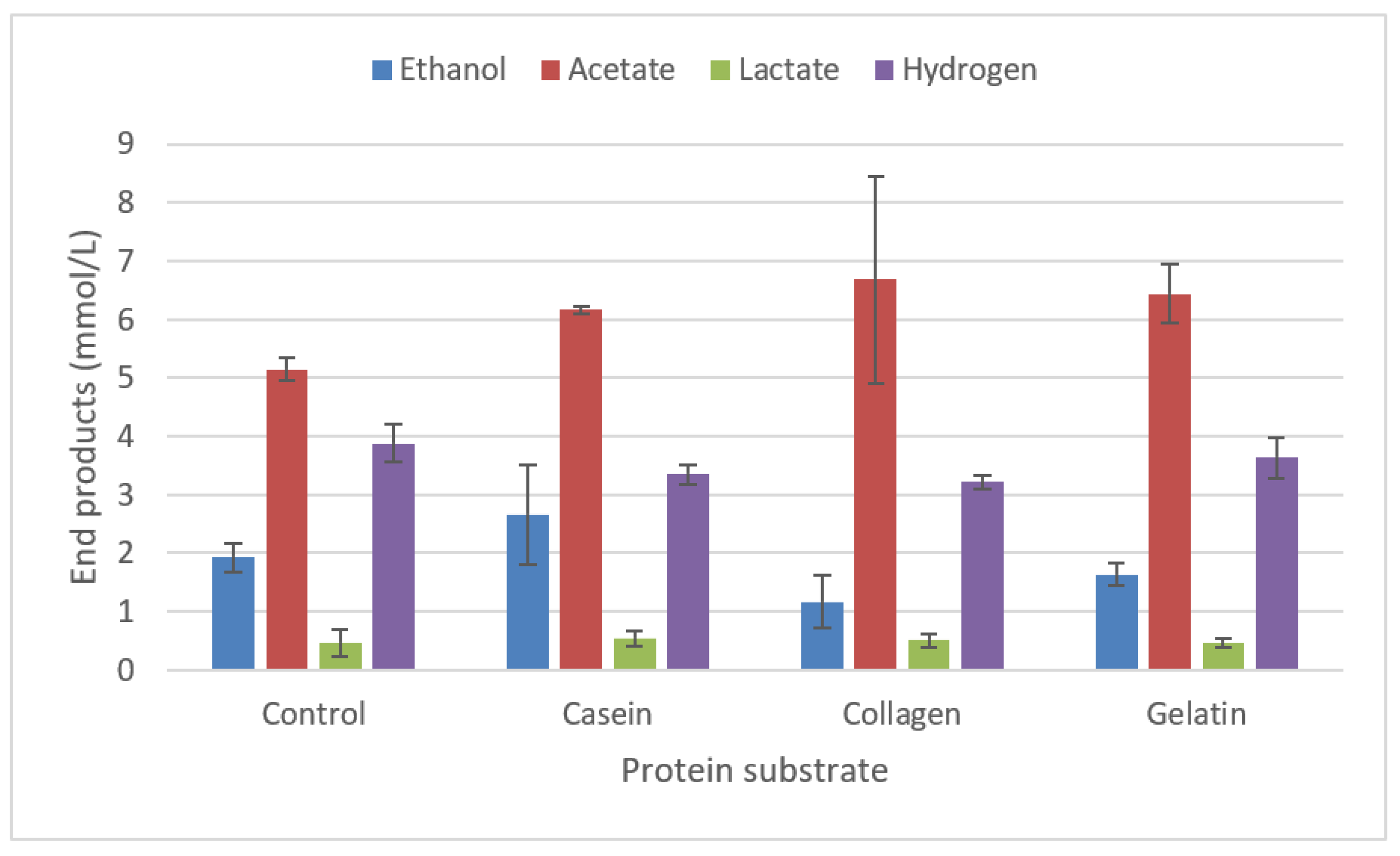
2.3.5. Degradation of proteins
2.3.6. Conversion of fatty acids to alcohols
4. Materials and Methods
3.1. Chemicals
3.2. Culture medium and preparation
3.3. Bacterial strain
3.4. API ZYM test
3.5. Substrate utilization spectrum
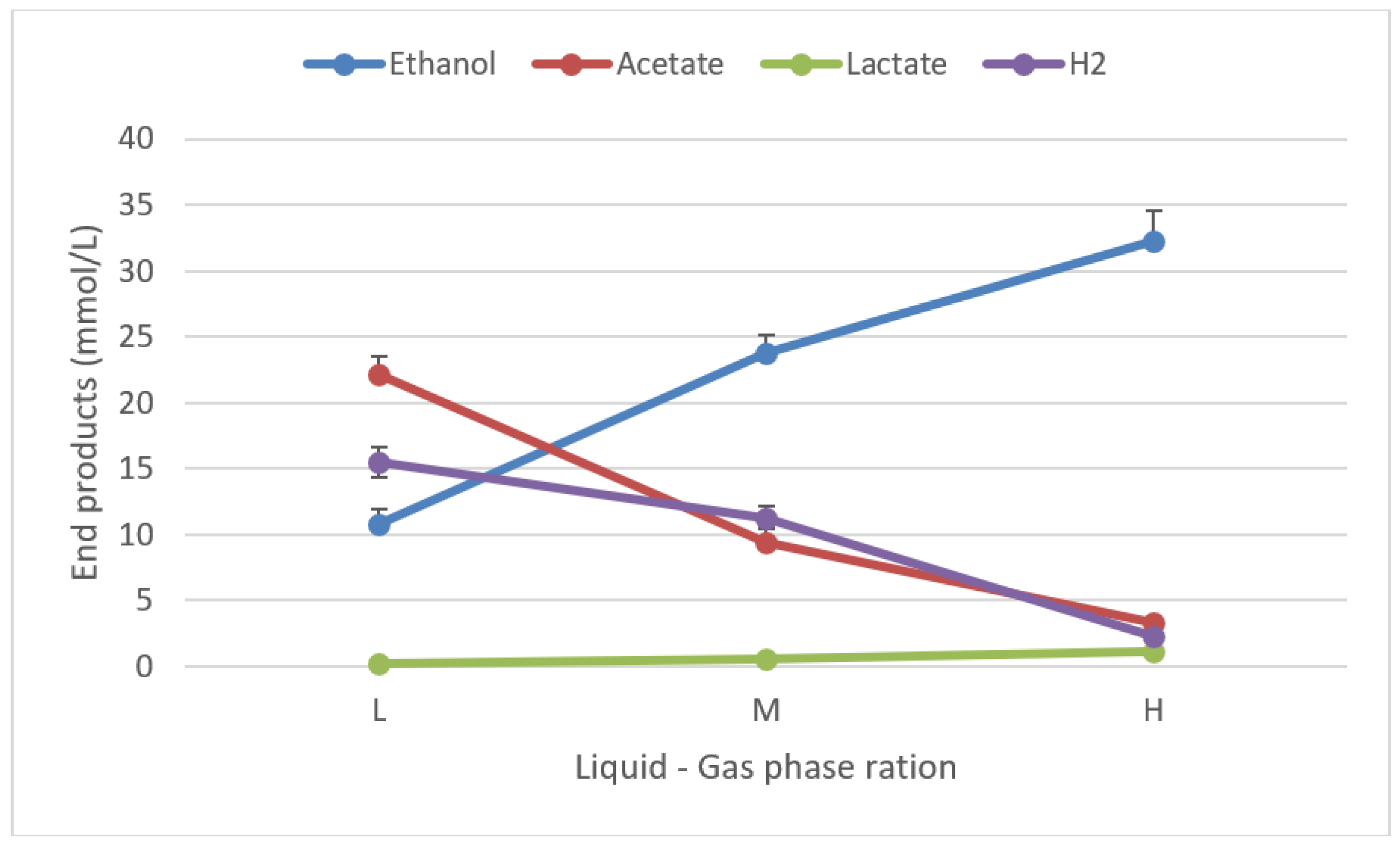
3.6. Influence of liquid-gas phase ratio
3.7. Analytical methods.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demain, A. Biosolutions to the energy problem. J. Ind. Microbiol. Biotechnol. 2009, 36, 319–32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Recent Advances in Second Generation Ethanol Production by Thermophilic Bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Hahn-Hagerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Liden, G.; Zacchi, G. Bioethanol - the future of tomorrow from the residues of today. Trends Biotechnol. 2006, 12, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.S.; Dworjanyn, S. The potential of marine biomass for anaerobic biogas production. 2008. Wrown Estate: London, UK.
- Tedesco, S.; Stokes, J. Valorisation to biogas of macroalgal waste streams: A circular approach to bioproducts and bioenergy in Ireland. Chem. Pap. 2017, 71, 721–728. [Google Scholar] [CrossRef]
- Martin, A.; Murphy, F.H. Propylene glycols. In: Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 17, 4th edition, 1994. Kroschwitz, J.I. (Ed.). Wiley, New York, pp. 715-726.
- Saxena, R.; Anand, P.; Saran, S.; Isar, J.; Agarwal, L. Microbial production and applications of 1,2-propanediol. Ind. J. Microbiol. 2010, 50, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.C.; Cooney, C.L. A novel fermentation: The production of ®-1,2-propanediol and acetol by Clostridium thermosaccharolyticum. Biores. Technol. 1986, 4, 651–654. [Google Scholar] [CrossRef]
- Ingvadottir, E.M.; Scully, S.M.; Orlygsson, J. Evaluation of the genus of Caldicellulosiruptor for producation of 1,2-propanediol from methylpentoses. Anaerobe 2017, 47, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, M.; Lyon, D.; Rainey, F.A.; Wiegel, J. Caloramator viterbensis sp. nov., a novel thermophilic glycerol-fermenting bacterium isolated from a hot spring in Italy. Int. J. Syst. Evol. Microbiol. 2002, 52, 1177–1184. [Google Scholar]
- Scully, S.M.; Iloranta, P.; Myllymaki, P.; Orlygsson, J. Branched-chain alcohol formation by thermophilic bacteria within the genera of Thermoanaerobacter and Caldanaerobacter. Extremophiles 2015, 19, 809–818. [Google Scholar] [CrossRef]
- Stackebrandt, E. The family Thermoanaerobacteraceae. In: The Prokaryotes - Firmicutes and Tenericutes (Rosenberg, E., et al). Springer-Verlag, Berlin, 2014.
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandezgarayzabal, J.; Garcia, P. The phylogeny of the genus Clostridium - proposal of 5 new genera and 11 new species combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Nielsen, P.; Ahring, B.K. Thermoanaerobacter mathranii sp nov, an ethanol-producing, extremely thermophilic anaerobic bacterium from a hot spring in Iceland. Arch. Microbiol. 1997, 168, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Jain, M.K.; Lee, C.Y.; Lowe, S.E.; Zeikus, J.G. Taxonomic distinction of saccharolytic thermophilic anaerobes - description of Thermoanaerobacterium xylanolyticum gen-nov, sp-nov, and Thermoanaerobacterium saccharolyticum gen-nov, sp-nov - reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb-nov, Thermoanaerobacterium thermosulfurigenes comb-nov, and Thermoanaerobacter thermohydrosulfuricus comb-nov, respectively - and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int. J. Syst. Bacteriol. 1993, 43, 41–51. [Google Scholar]
- Slobodkin, A.I.; Tourova, T.P.; Kuznetsov, B.B.; Kostrikina, N.A.; Chernyh, N.A.; Bonch-Osmolovskaya, E.A. Thermoanerobacter siderophilus sp. nov., a novel dissimilarory Fe(III)-reducing, anaerobic thermophilic bacterium. Int. J. Syst. Bacteriol. 1999, 49, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Lacis, L.S.; Lawford, H.G. Ethanol production from xylose by Thermoanerobacter ethanolicus in batch and continuous culture. Arch. Microbiol. 1988, 150, 48–55. [Google Scholar] [CrossRef]
- Avci, A.; Donmez, S. Effect of zinc on ethanol production by two Thermoanaerobacter strains. Process Biochem. 2009, 41, 984–989. [Google Scholar] [CrossRef]
- Jessen, J.E.J.; Orlygsson, J. Production of ethanol from sugars and lignocellulosic biomass by Thermoanaerobacter J1 isolated from a hot spring in Iceland. J. Biomed. Biotechnol. 2012, 1869–1882, 1869–1882. [Google Scholar] [CrossRef]
- Wiegel, J.; Ljungdahl, L. Thermoanerobacter ethanolicus gen. nov., spec. nov., a new, extreme thermophilic, anaerobic bacterium. Arch. Microbiol 1981, 128, 343–348. [Google Scholar] [CrossRef]
- Onyenwoke, R.U.; Kevbrin, V.V.; Lysenko, A.M.; Wiegel, J. Thermoanaerobacter pseudethanolicus sp. nov., a thermophilic heterotrophic anaerobe from Yellowstone National Park. Int. J. Syst. Evol. Microbiol. 2007, 57, 2191–2193. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-chain alcohol formation from branched-chain amino acids by Thermoanaerobacter brockii and Thermoanaerobacter yonseiensis. Anaerobe. 2014, 30, 82–84. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Amino acid metabolism of Thermoanaerobacter strain AK90: The role of electron-scavenging systems on end product formation. J. Amino Acids. 2015, 410492. [Google Scholar] [CrossRef]
- Scully, SM,; Brown, A.E.; Mueller-Hilger, Y.; Ross, A.B.; Orlygsson, J. Influence of culture conditions on the bioreduction of organic acids to alcohols by Thermoanaerobacter pseudethanolicus. Microorganisms. 2021. 9, 162.
- Brynjarsdottir, H.; Wawiernia, B.; Orlygsson, J. Ethanol production from sugars and complex biomass by Thermoanaerobacter AK5: The effect of electron-scavenging systems on end-product formation. Energy and Fuels 2012, 26, 4568–4574. [Google Scholar] [CrossRef]
- Ingvadottir, E.M.; Scully, S.M.; Orlygsson, J. Production of (S)-1,2-prpanediol from rhamnose using the moderate thermophilic Clostridium strain AK1. Anaerobe 2018, 54, 26–30. [Google Scholar] [CrossRef]
- Altaras, N.E.; Etzel, M.R.; Cameron, D.C. Conversion of sugars to 1,2-propanediol by Thermoanaerobacterium thermosaccharolyticum HG-8. Biotech. Progr. 2001, 17, 52–56. [Google Scholar] [CrossRef]
- Chades, T.; Scully, S.M.; Ingvadottir, E, M.; Orlygsson, J. Fermentation of Mannitol Extracts From Brown Macro Algae by Thermophilic Clostridia. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Wagner, I.D.; Zhao, W.; Zhang, C.L.; Romanek, C.S.; Rohde, M.; Wiegel, J. Thermoanaerobacter uzonensis sp. nov., an anaerobic thermophilic bacterium isolated from a hot spring within the Uzon Caldera, Kamchatka, Far East Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Riessen, S.; Antranikian, G. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with karatinolytic activity. Extremophiles 2001, 5, 399–408. [Google Scholar] [CrossRef]
- Georgieva, T.I.; Mikkelsen, M.J.; Ahring, B.K. Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Appl. Biochem. Biotechnol. 2008, 145, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.I.; Ahring, B.K. Evaluation of continuous ethanol fermentation of dilute-acid corn stover hydrolysate using thermophilic anaerobic bacterium Thermoanaerobacter BG1L1. Appl. Microbiol. Biot. 2007, 77, 61–68. [Google Scholar] [CrossRef]
- Almarsdottir, A.R.; Sigurbjornsdottir, M.A.; Orlygsson, J. Effect of various factors on ethanol yields from lignocellulosic biomass by Thermoanaerobacterium AK 17. Biotechnol. Bioeng. 2012, 109, 686–694. [Google Scholar] [CrossRef]
- Trivedi, N.; Reddy, C.R.K.; Radulovich, R.; Jha, B. Solid state fermentation (SSF)-derived cellulose for saccharification of the green seaweed Ulva for bioethanol production. Algal Res. 2015, 9, 48–54. [Google Scholar] [CrossRef]
- Ramachandra, T.V.; Hebbale, D. Bioethanol production from macroalgae: Prospects and challenges. Ren. Sust. Energ. Rev. 2020, 117, 109479. [Google Scholar] [CrossRef]
- Moenaert, A.; Lopez-Contreras, A.M.; Budde, M.; Allahgholi, L.; Xiaoru, H.; Bjerre, A.-B.; Orlygsson, J.; Karlsson, E.N.; Fridjonsson, O.H.; Hreggvidsson, G.O. Evaluation of Laminaria digitata hydrolysate for the production of bioethanol and butanol by fermentation. Fermentation 2023, 9, 59. [Google Scholar] [CrossRef]
- Stickland, L.H. CCXXXII. Studies in the metabolism of the strict anaerobes (genus Clostridium). The chemical reactions by which Cl. sporogenes obtains its energy. Biochem. J. 1934, 28, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Deklevat, M.L.; Dasgupta, B.R. Purification and characterization of a protease from Clostridium botulinum Type A that nicks single-chain type A botulinum neurotoxin into the Di-Chain form. J. Bacteriol. 1990, 172, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G. The end products of metabolism of aromatic amino acids by clostridia. Arch. Microbiol. 1976, 107, 283–288. [Google Scholar] [CrossRef]
- Scully, S.M. Amino acid and related catabolisms of Thermoanaerobacter species. PhD thesis. University of Iceland. 2019.
- Fardeau, M.-L.; Patel, B.K.C.; Magot, M.; Ollivier, B. Utiliation of serine, leucine, isoleucine, and valine by Thermoanerobacter brockii in the presence of thiosulfate or Methanobacterium sp. as electron acceptors. Anaerobe 1997, 3, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Branched-chain amino acid catabolism of Thermoanerobacter strain AK85 and the influence of culture conditions on branched-chain alcohol formation. Amino acids 2019, 51, 1039–1054. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavor profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-chain amino acid catabolism of Thermoanerobacter pseudethanolicus reveals potential route to branched-chain alcohol formation. Extremphiles 2020, 24, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Faudon, C.; Fardeau, M.L.; Heim., J.; Patel, B.; Magot, M.; Ollivier, B. Peptide and amino acid oxidation in the presence of thiosulfate by members of the genus Thermoanaerobacter. Curr. Microbiol. 1995, 31, 152–157. [Google Scholar] [CrossRef]
- Hitschler, L.; Kuntz, M.; Langschied, F.; Basen, M. Thermoanaerobacter species differ in their potential to reduce organic acids to their corresponding alcohols. Appl. Microbiol. Biotechnol. 2018, 102, 8465–8476. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Brown, A.; Ross, A.B.; Orlygsson, J. Biotransformation of organic acids to their corresponding alcohols by Thermoanaerobacter pseudoethanolicus. Anaerobe 2019, 28–31, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Biotransformation of carboxylic acids to alcohols: Characterization of Thermoanaerobacter strain AK152 and 1-propanol production via propionate reduction. Microorganisms 2020, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottir, M.; Baldursson, S.R.B.; Orlygsson, J. Ethanol production from monosugars and lignocellulosic biomass by thermophilic bacteria isolated from Icelandic hot springs. Icelandic Agricult. Sci. 2009, 22, 45–58. [Google Scholar]
- Hungate, R.E. A roll tube method for cultivation of strict anaerobes. In: Norris, J.R.; Ribbons, D.W. (eds). Methods in microbiology, vol 3B. 1969. Academic Press, New York, pp. 117-132.
- Orlygsson, J.; Baldursson, S.R.B. Phylogenetic and physiological studies of four hydrogen-producing thermoanareobes. Icelandic Agric. Sci. 2007, 20, 93–105. [Google Scholar]
- Taylor, K.A.C.C. A simple colorimetric assay for muramic acid and lactic acid. Appl. Biochem. Biotechnol. 1996, 56, 49–58. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oeanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
| Strain AK15 | T. uzonensis | |
|---|---|---|
| Alkaline phosphatase | + | - |
| Esterase (C4) | + | + |
| Lipase esterase (C8) | - | + |
| Lipase (C14) | - | + |
| Leu arylamidase | - | - |
| Val arylamidase | - | - |
| Cys arylamidase | - | - |
| Trypsin | - | - |
| α-Chymotrypsin | - | - |
| Acid phosphatase | + | + |
| Naphthol-AS-BI-phosophohydrolase | + | + |
| α -Galactosidase | - | - |
| β-Galactosidase | - | - |
| β-Glucuronidase | - | - |
| α-Glucosidase | - | - |
| β-Glucosidase | - | + |
| N-Acetyl-β-glucosaminidase | - | + |
| α-Mannosidase | - | - |
| α-Fucosidase | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





