Submitted:
13 August 2024
Posted:
16 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Bioassay
2.3. RNA and DNA Extraction
2.4. Mutation Survey
2.5. RNA-seq Analysis
2.6. Clean Read Assembly and Unigene Construction
2.7. Functional Annotation
2.8. Differential Gene Expression (DGE) Analysis
2.9. Orthologous Cluster Analysis
3. Results
3.1. Bioassay
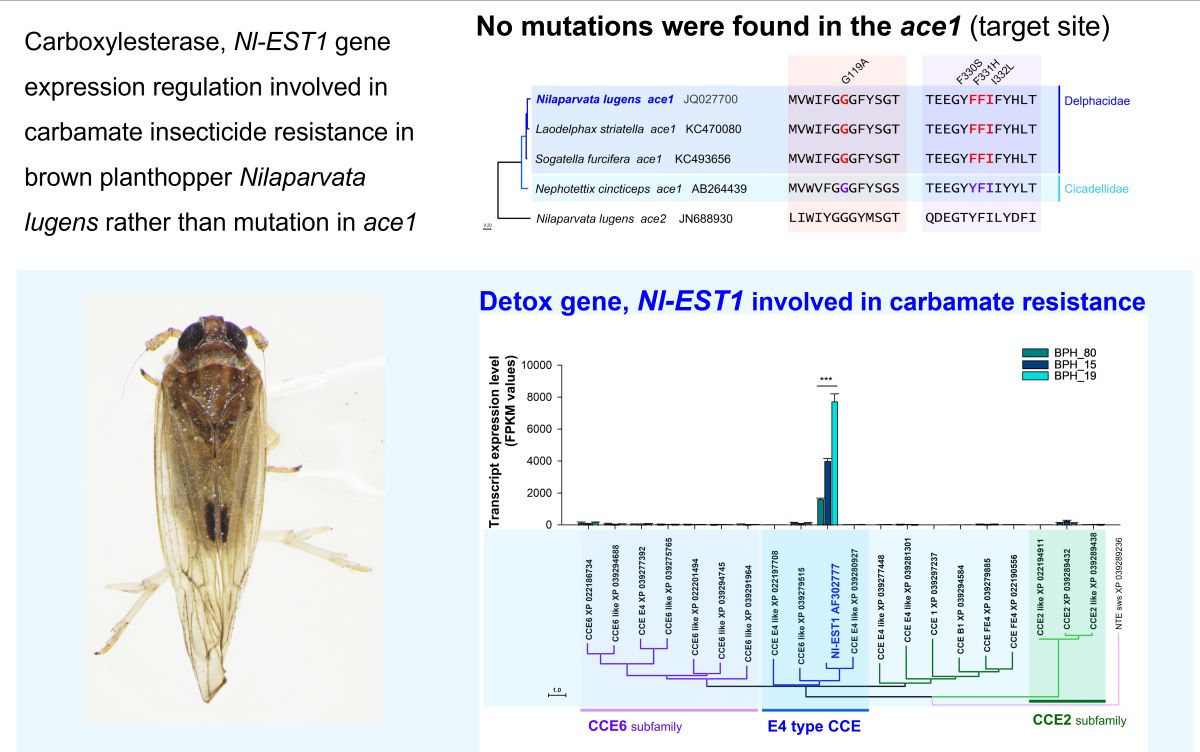
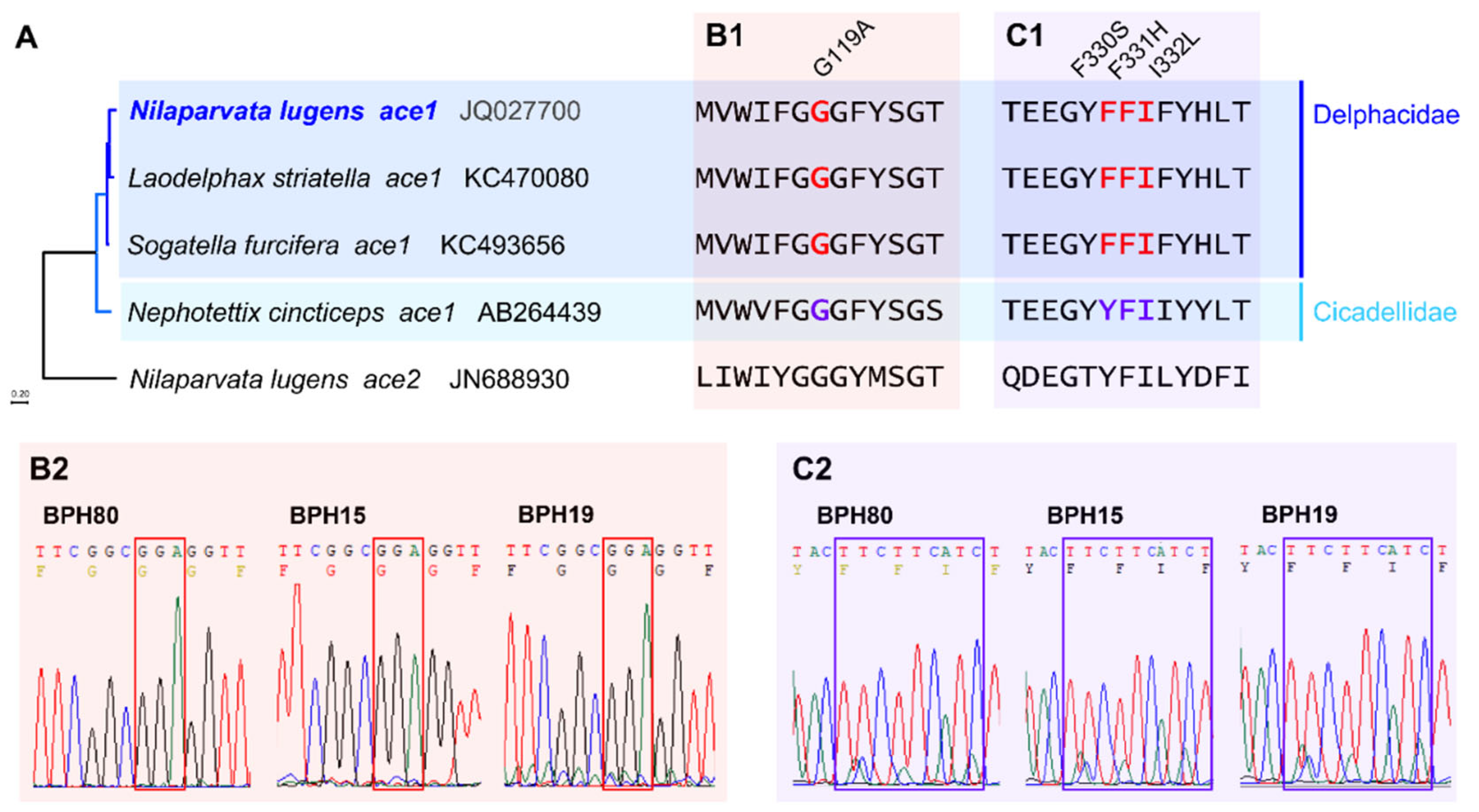
3.2. Mutation Analysis
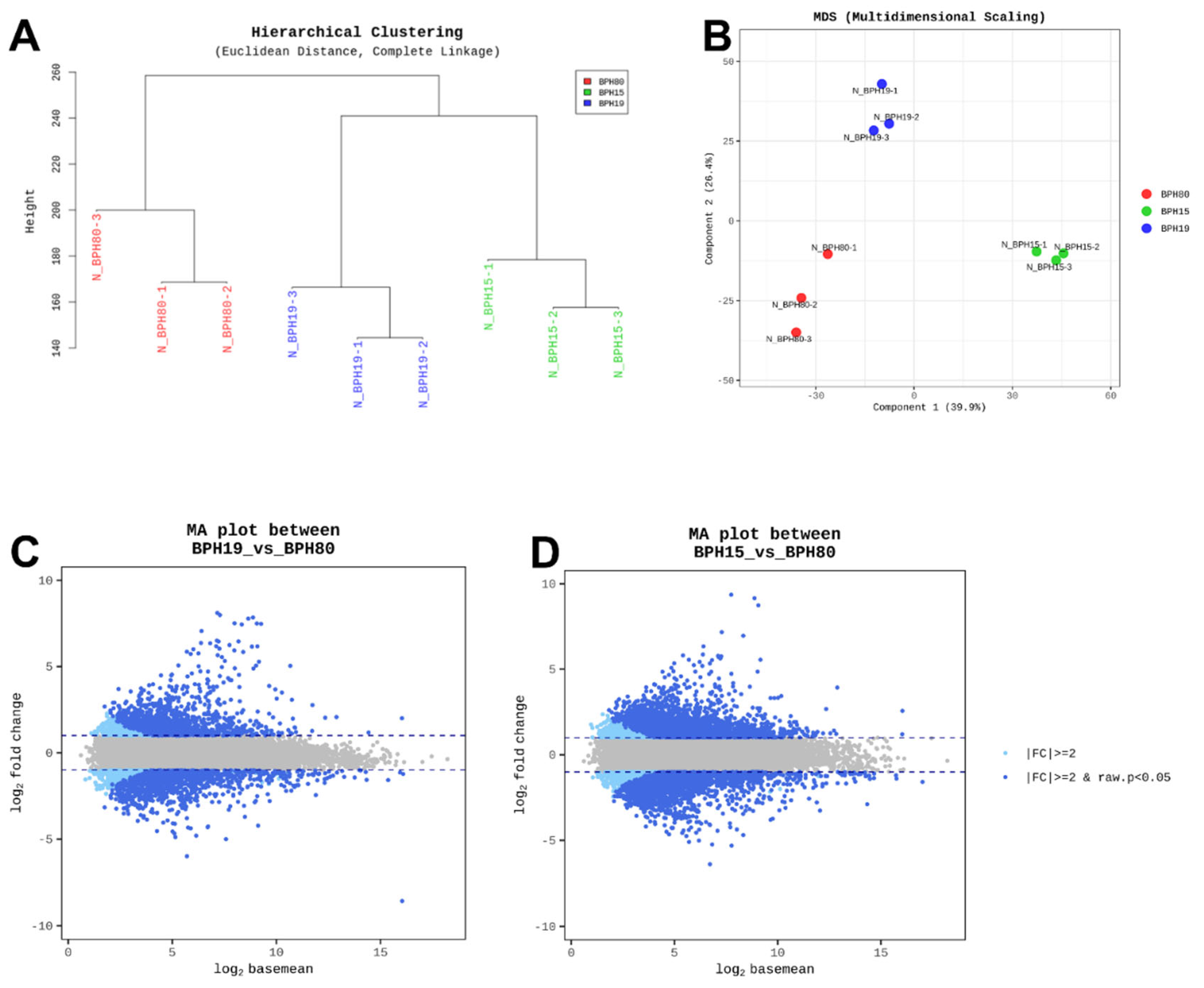
3.3. Raw and Trimmed Data Statistics of RNAseq
3.4. De Novo Assembly of Unigene Sets
3.5. ORF prediction
3.6. Transcriptomic DEG analysis
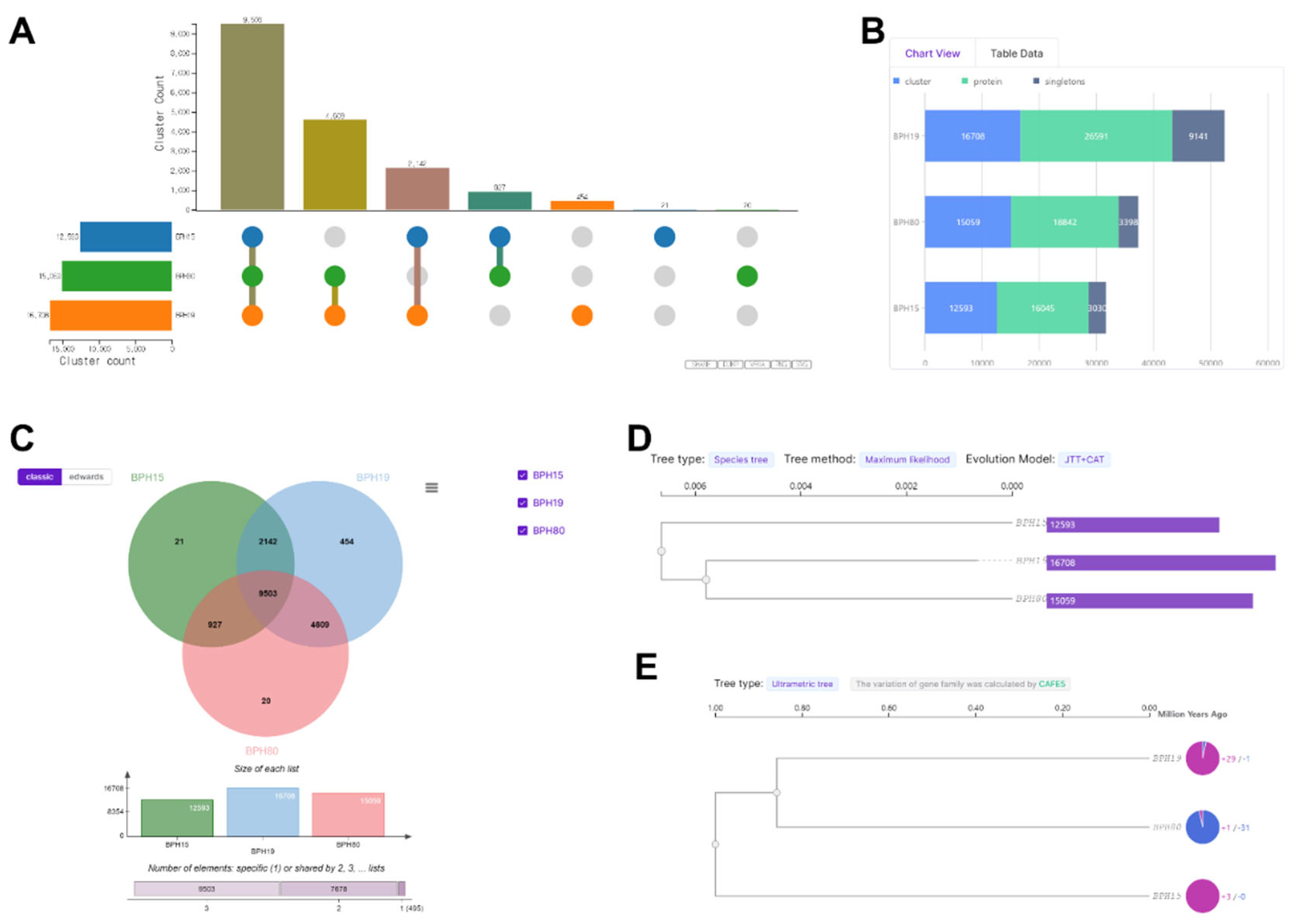
3.7. Orthologous Cluster Analysis
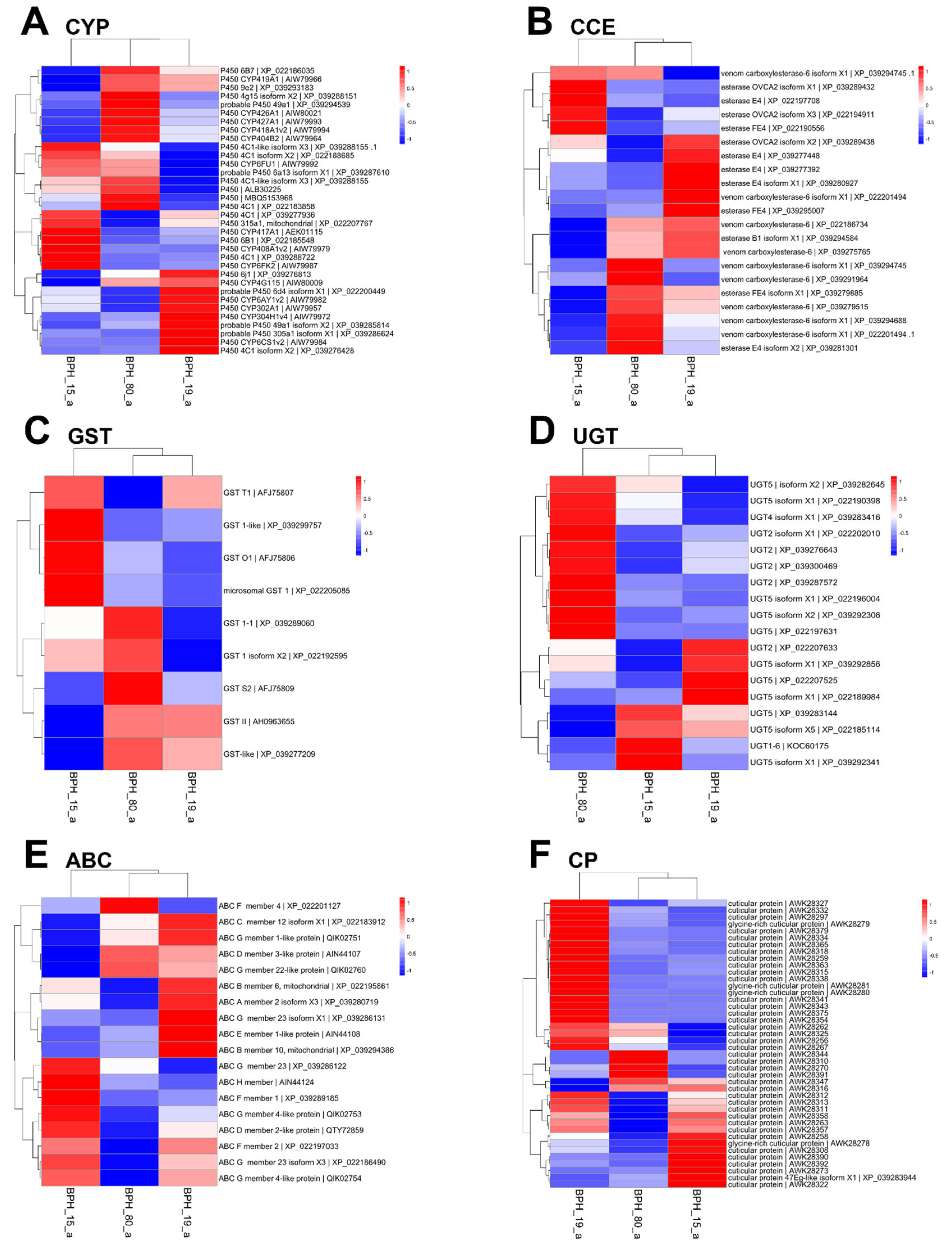
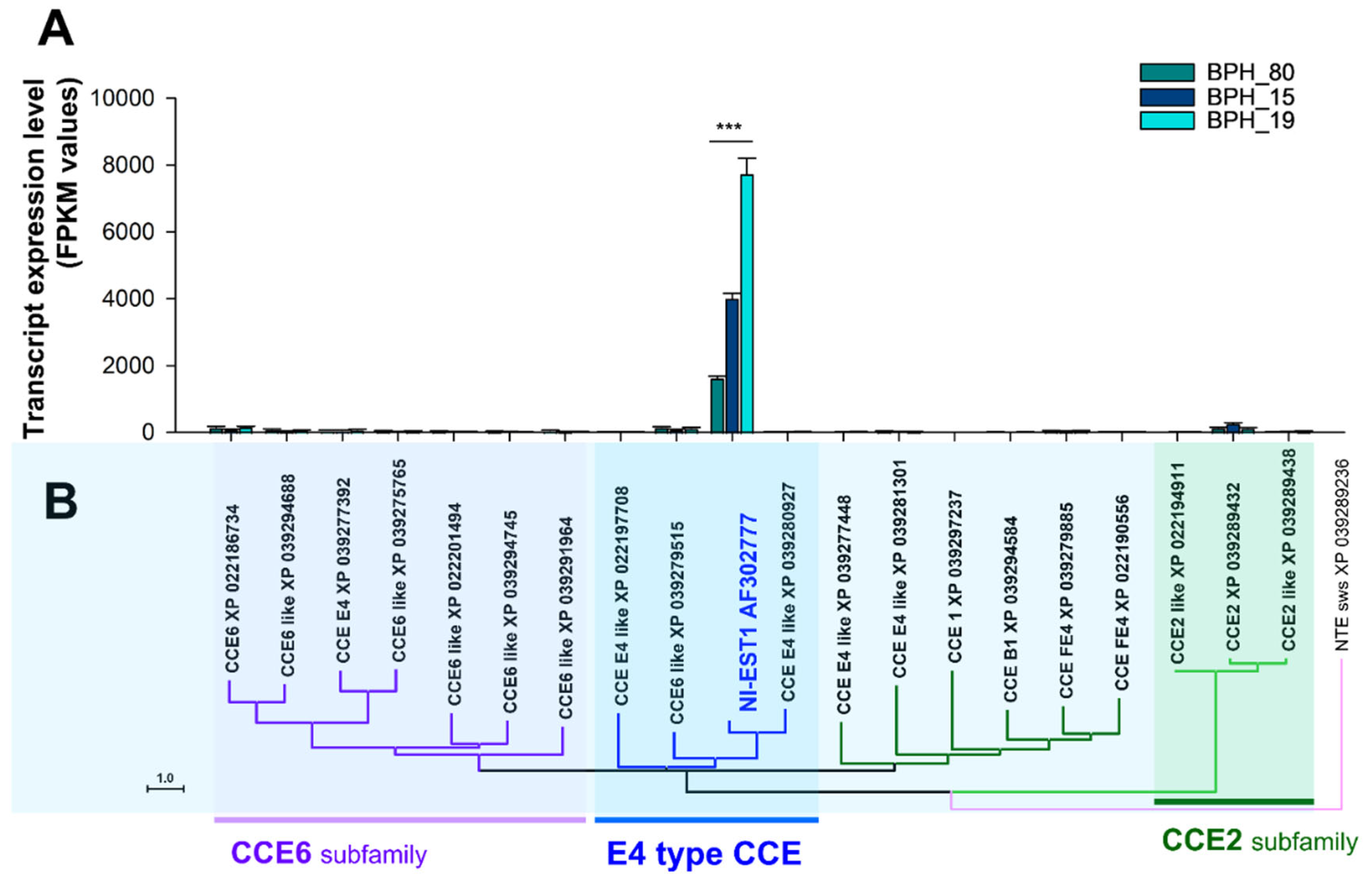
3.8. DEG Analysis of Detoxification Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.-G.; Lee, S.-U.; Yun, B.-W. Evaluation of Iraqi rice cultivars for their tolerance to drought stress. Agronomy 2020, 10, 1782. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends in Food Science & Technology 2020, 97, 265–285. [Google Scholar]
- Min, S.; Lee, S.W.; Choi, B.-R.; Lee, S.H.; Kwon, D.H. Insecticide resistance monitoring and correlation analysis to select appropriate insecticides against Nilaparvata lugens (Stål), a migratory pest in Korea. Journal of Asia-Pacific Entomology 2014, 17, 711–716. [Google Scholar] [CrossRef]
- Phatthalung, T.N.; Tangkananond, W. Rice grassy stunt virus-free and pathogenic rice plants affect the brown planthopper (Nilaparvata lugens Stål) life cycle. Agriculture and Natural Resources 2021, 55, 331–340. [Google Scholar]
- Phatthalung, T.N.; Tangkananond, W. The Infectivity Survival and Transmissibility of Rice ragged stunt virus from the Frozen-Infected Rice Leaves by the Brown Planthopper, Nilaparvata lugens Stål. Trends in Sciences 2022, 19, 5097–5097. [Google Scholar] [CrossRef]
- Yoo, J.-K.; Lee, S.-W.; Ahn, Y.-J.; Nagata, T.; Shono, T. Altered acetylcholinesterase as a resistance mechanism in the brown planthopper (Homoptera: Delphacidae), Nilaparvata lugens Stål. Applied Entomology and Zoology 2002, 37, 37–41. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, D.J.; Kim, Y.H.; Lee, S.W.; Lee, S.H. Cloning of the acetylcholinesterase 1 gene and identification of point mutations putatively associated with carbofuran resistance in Nilaparvata lugens. Pesticide biochemistry and physiology 2012, 103, 94–100. [Google Scholar] [CrossRef]
- Khan, S.; Uddin, M.N.; Rizwan, M.; Khan, W.; Farooq, M.; Shah, A.S.; Subhan, F.; Aziz, F.; Rahman, K.U.; Khan, A. Mechanism of Insecticide Resistance in Insects/Pests. Polish Journal of Environmental Studies 2020, 29. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Luttrell, R. Altered gene regulation and potential association with metabolic resistance development to imidacloprid in the tarnished plant bug, Lygus lineolaris. Pest management science 2015, 71, 40–57. [Google Scholar] [CrossRef]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLOS genetics 2010, 6, e1000999. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Liang, Q.-M.; Xu, Y.; Gurr, G.M.; Bao, Y.-Y.; Zhou, X.-P.; Zhang, C.-X.; Cheng, J.; Zhu, Z.-R. Genomic insights into the glutathione S-transferase gene family of two rice planthoppers, Nilaparvata lugens (Stål) and Sogatella furcifera (Horváth)(Hemiptera: Delphacidae). PLoS One 2013, 8, e56604. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Andrews, M.; Cutler, P.; Daniels, M.; Elias, J.; Paul, V.L.; Crossthwaite, A.J.; Denholm, I.; Field, L.M. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. Bmc Neuroscience 2011, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Puggioni, V.; Chiesa, O.; Panini, M.; Mazzoni, E. Qualitative Sybr Green real-time detection of single nucleotide polymorphisms responsible for target-site resistance in insect pests: the example of Myzus persicae and Musca domestica. Bulletin of entomological research 2017, 107, 96–105. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Gao, X. Characterisation of spinosad resistance in the housefly Musca domestica (Diptera: Muscidae). Pest Management Science 2011, 67, 335–340. [Google Scholar] [CrossRef]
- Holderman, C.J.; Swale, D.R.; Bloomquist, J.R.; Kaufman, P.E. Resistance to permethrin, β-cyfluthrin, and diazinon in Florida horn fly populations. Insects 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Ren, Z.; Li, W.; Cai, T.; Qin, X.; Wan, H.; Jin, B.R.; He, S.; Li, J. Carboxylesterase genes in nitenpyram-resistant brown planthoppers, Nilaparvata lugens. Insect science 2021, 28, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. Journal of hazardous materials 2021, 411, 125026. [Google Scholar] [CrossRef]
- Lu, K.; Li, Y.; Xiao, T.; Sun, Z. The metabolic resistance of Nilaparvata lugens to chlorpyrifos is mainly driven by the carboxylesterase CarE17. Ecotoxicology and Environmental Safety 2022, 241, 113738. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect biochemistry and molecular biology 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Lan, W.-s.; Cong, J.; Jiang, H.; Jiang, S.-r.; Qiao, C.-l. Expression and characterization of carboxylesterase E4 gene from peach–potato aphid (Myzus persicae) for degradation of carbaryl and malathion. Biotechnology letters 2005, 27, 1141–1146. [Google Scholar] [CrossRef]
- Han, C.; Rahman, M.-M.; Shin, J.; Kim, J.H.; Lee, S.H.; Kwon, M.; Timm, A.E.; Ramasamy, S.; Lee, Y.; Kang, S. Exaptation of I4760M mutation in ryanodine receptor of Spodoptera exigua (Lepidoptera: Noctuidae): Lessons from museum and field samples. Pesticide Biochemistry and Physiology 2023, 195, 105579. [Google Scholar] [CrossRef]
- Adelman, Z.N.; Kilcullen, K.A.; Koganemaru, R.; Anderson, M.A.; Anderson, T.D.; Miller, D.M. Deep sequencing of pyrethroid-resistant bed bugs reveals multiple mechanisms of resistance within a single population. PLoS One 2011, 6, e26228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Gujar, H.; Gordon, J.R.; Haynes, K.F.; Potter, M.F.; Palli, S.R. Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Scientific reports 2013, 3, 1456. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature biotechnology 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: protein domains identifier. Nucleic acids research 2005, 33, W116–W120. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic acids research 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nature methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: an integrated platform for exploring and visualizing orthologous data across genomes. Nucleic acids research 2023, 51, W397–W403. [Google Scholar] [CrossRef]
- Muduli, L.; Pradhan, S.K.; Mishra, A.; Bastia, D.N.; Samal, K.C.; Agrawal, P.K.; Dash, M. Understanding brown planthopper resistance in rice: Genetics, biochemical and molecular breeding approaches. Rice Science 2021, 28, 532–546. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. Journal of Plant Protection Research 2015, 55. [Google Scholar] [CrossRef]
- Guedes, R.; Roditakis, E.; Campos, M.; Haddi, K.; Bielza, P.; Siqueira, H.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: patterns, spread, mechanisms, management and outlook. Journal of Pest Science 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Kwon, D.H.; Min, S.; Lee, S.W.; Park, J.H.; Lee, S.H. Monitoring of carbamate and organophosphate resistance levels in Nilaparvata lugens based on bioassay and quantitative sequencing. Journal of Asia-Pacific Entomology 2012, 15, 635–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Li, J.; Liu, M.; Liu, Z. Point mutations in acetylcholinesterase 1 associated with chlorpyrifos resistance in the brown planthopper, Nilaparvata lugens Stål. Insect molecular biology 2017, 26, 453–460. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, Y.; Li, Y.; Li, W.; Ma, Y.; Zhou, Q.; Lu, K. Adipokinetic hormone regulates cytochrome P450-mediated imidacloprid resistance in the brown planthopper, Nilaparvata lugens. Chemosphere 2020, 259, 127490. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Sun, H.; Wang, J.; Wu, M.; Wang, K.; Denholm, I.; Han, Z. Multiple cis-acting elements involved in up-regulation of a cytochrome P450 gene conferring resistance to deltamethrin in smal brown planthopper, Laodelphax striatellus (Fallén). Insect biochemistry and molecular biology 2016, 78, 20–28. [Google Scholar] [CrossRef]
- Malathi, V.M.; Jalali, S.K.; Gowda, D.K.S.; Mohan, M.; Venkatesan, T. Establishing the role of detoxifying enzymes in field-evolved resistance to various insecticides in the brown planthopper (Nilaparvata lugens) in South India. Insect science 2017, 24, 35–46. [Google Scholar] [CrossRef]
- Wang, H.L.; Rao, Q.; Chen, Z.Z. Identifying potential insecticide resistance markers through genomic-level comparison of Bemisia tabaci (Gennadius) lines. Archives of Insect Biochemistry and Physiology 2023, 114, e22034. [Google Scholar] [CrossRef]
- Field, M.L.; Devonshire, L.A. Evidence that the E4 and FE4 esterase genes responsible for insecticide resistance in the aphid Myzus persicae (Sulzer) are part of a gene family. Biochemical Journal 1998, 330, 169–173. [Google Scholar] [CrossRef] [PubMed]
| Purpose | Primers | Sequence |
| For mutation survey of Nl-EST1 | Nl-EST1_5UTR-F1 | TGCCGAGCCGTAGTTGATGAT |
| Nl-EST1_5UTR-F2 | TCGAGCATCTATCCTGCCTCTT | |
| Nl-EST1_ORF-R1 | GGCCATAGTTTCCAGCAAAGTC | |
| Nl-EST1_ORF-R2 | GTCAGGGTCATCGAGGAAATCT | |
| Nl-EST1_ORF-R3 | GCTCCTGGGAAGTTCTTCTTCA | |
| Nl-EST1_3UTR-R1 | GCCTACCTACCGTACTCAATTTTAATG | |
| For mutation survey of ace1, G119A | Nl-ace1_G119A-F | CATGACTCGCACATCCTCAACA |
| Nl-ace1_G119A-R | CTGCATGCTGACAAGTATGACG | |
| For mutation survey of ace1, F331H | Nl-ace1_F331H-F | GGTCGTTGGCGACGAAAAACTT |
| Nl-ace1_F331H-R | TGTAGAAACTCGTCCCGGTTGA |
| Assembly | No of genes | No of transcripts | GC (%) | N50 | Avg. contig length (bp) |
Total assembled bases (bp) |
| merge | 119,664 | 119,664 | 41 | 896 | 611 | 73,153,339 |
| BPH80 | 75,069 | 75,069 | 40 | 816 | 579 | 43,504,047 |
| BPH15 | 69,319 | 69,319 | 38 | 765 | 560 | 38,844,156 |
| BPH19 | 93,427 | 93,427 | 41 | 860 | 600 | 56,127,492 |
| Assembly | Total Unigene | ORF predicted Unigene |
Single ORF predicted Unigene |
Multiple ORF predicted Unigene |
| merge | 119,664 | 30,788 (25.73%) | 30,064 (97.65%) | 724 (2.35%) |
| BPH80 | 75,069 | 18,593 (24.77%) | 18,349 (98.69%) | 244 (1.31%) |
| BPH15 | 69,319 | 15,880 (22.91%) | 15,721 (99.0%) | 159 (1.0%) |
| BPH19 | 93,427 | 26,147 (27.99%) | 25,721 (98.37%) | 426 (1.63%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





