Submitted:
09 January 2025
Posted:
09 January 2025
Read the latest preprint version here
Abstract
Keywords:
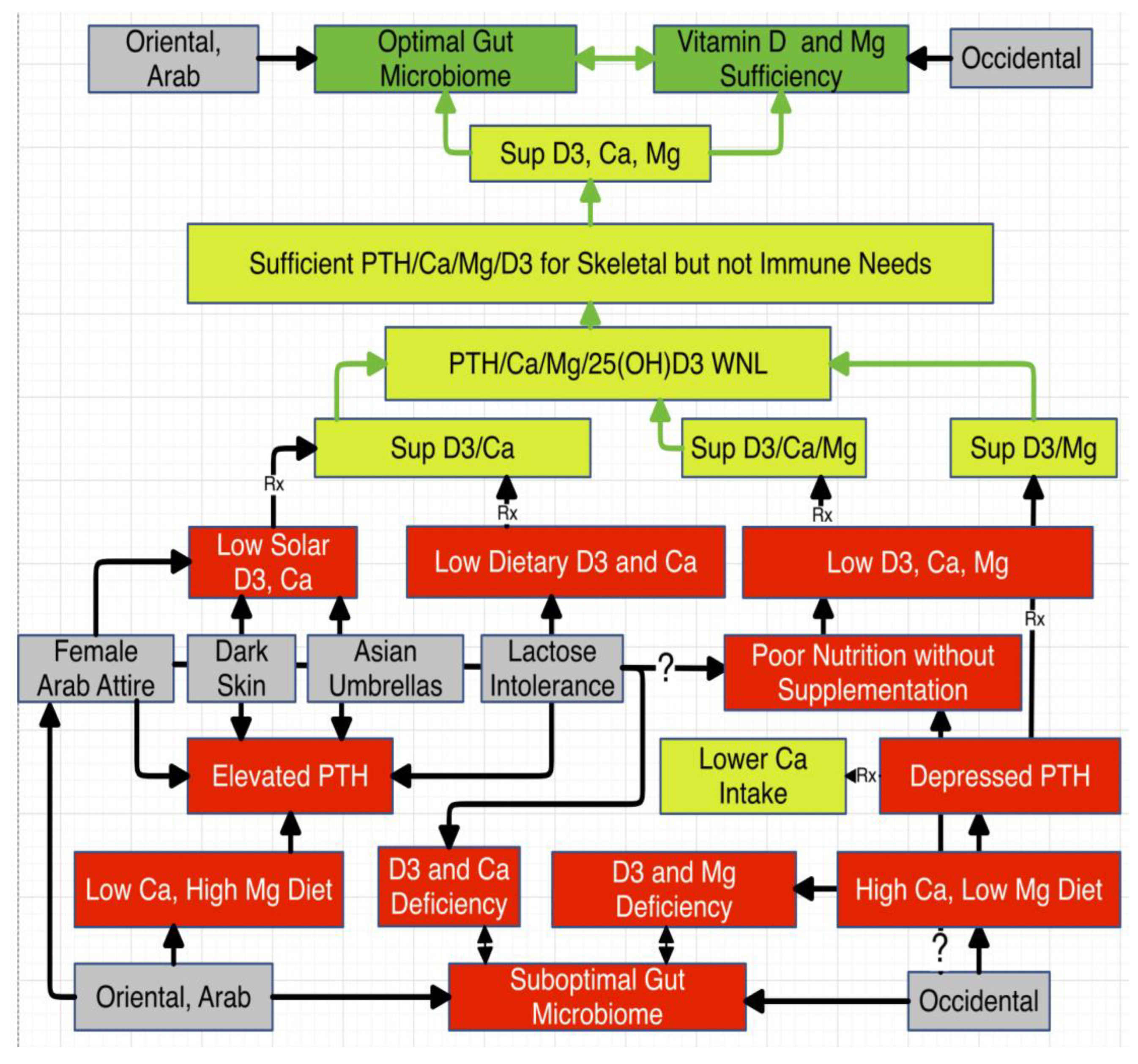
Introduction
Discussion
- I.
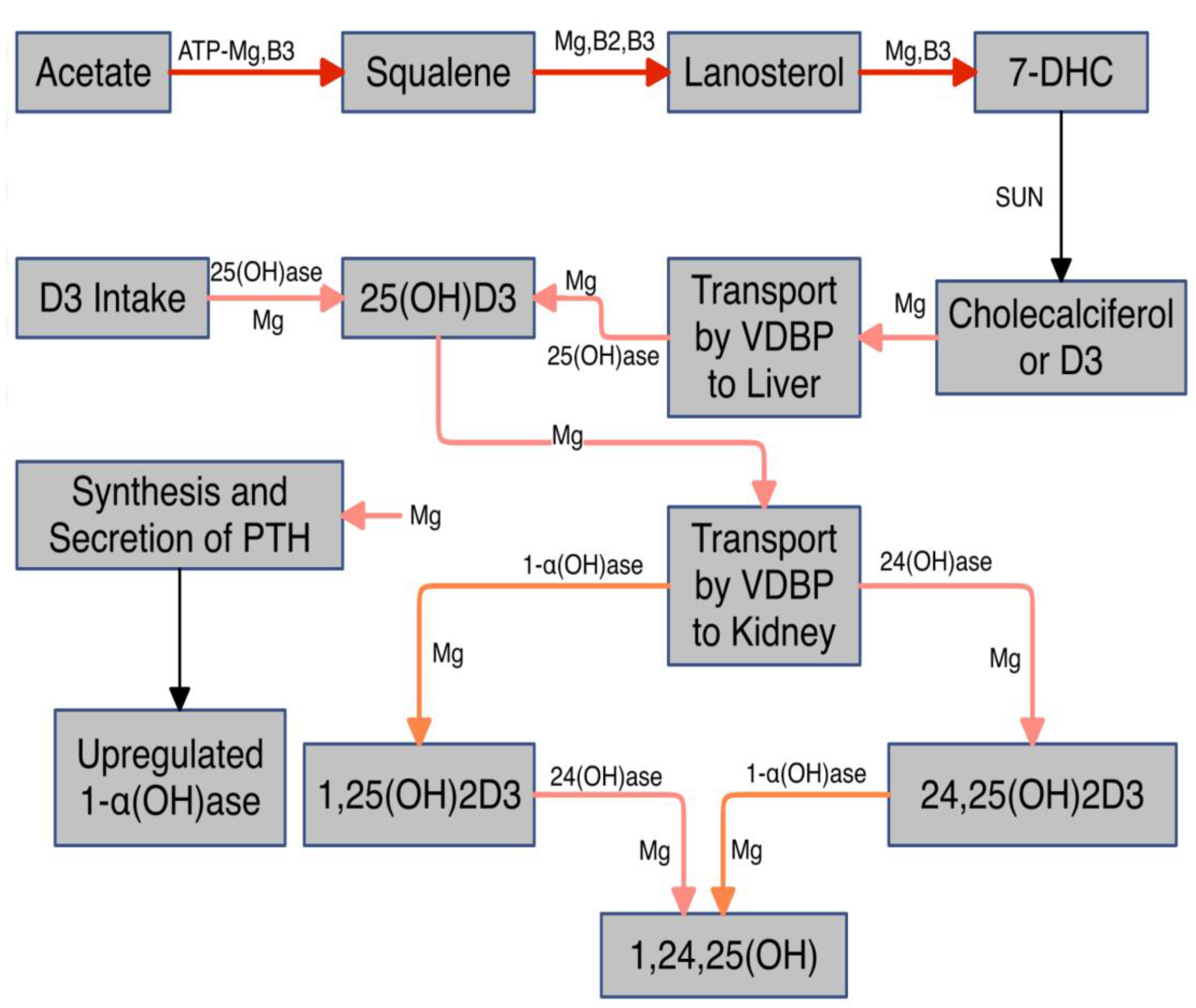
- Vitamin D and Magnesium
- II.
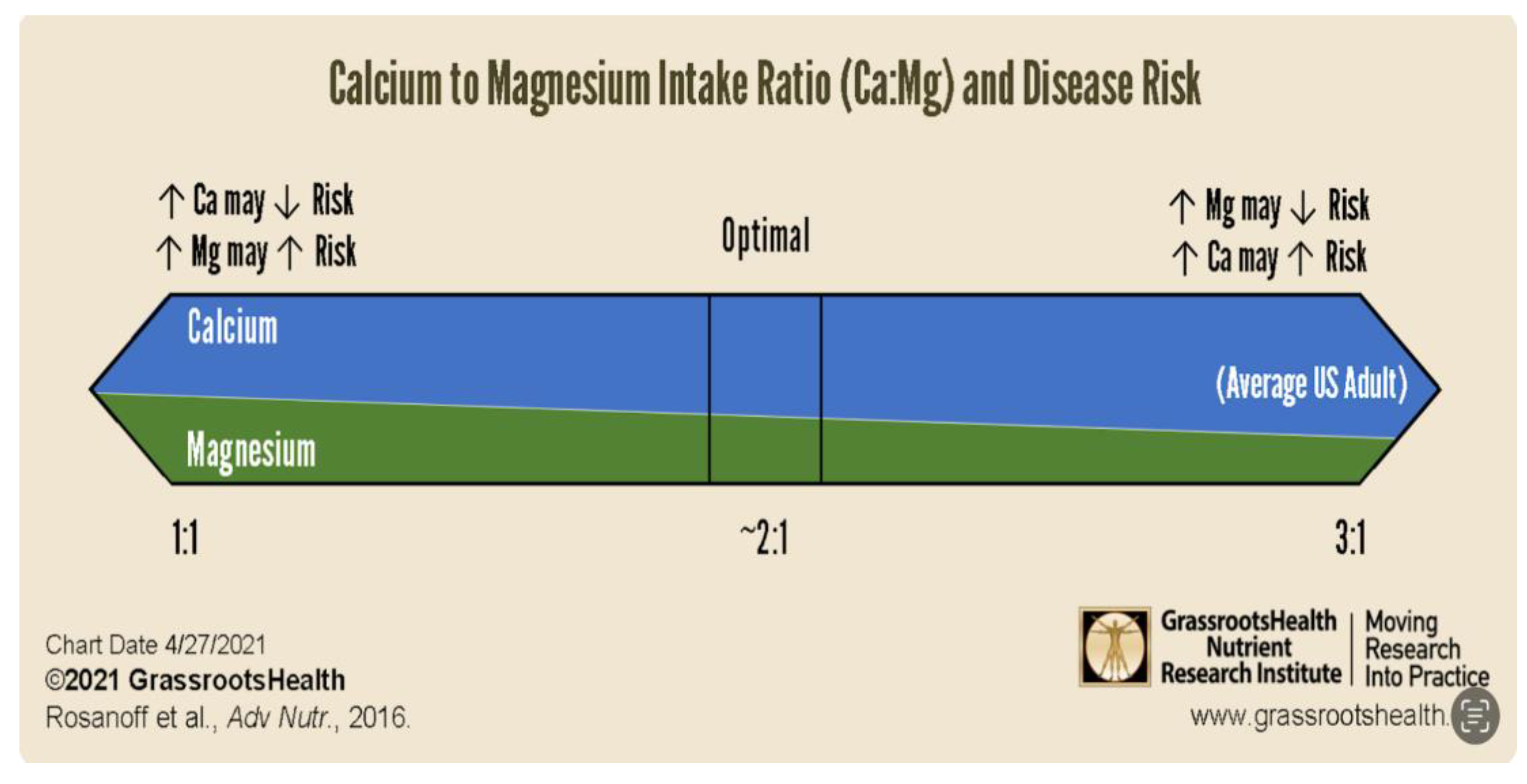
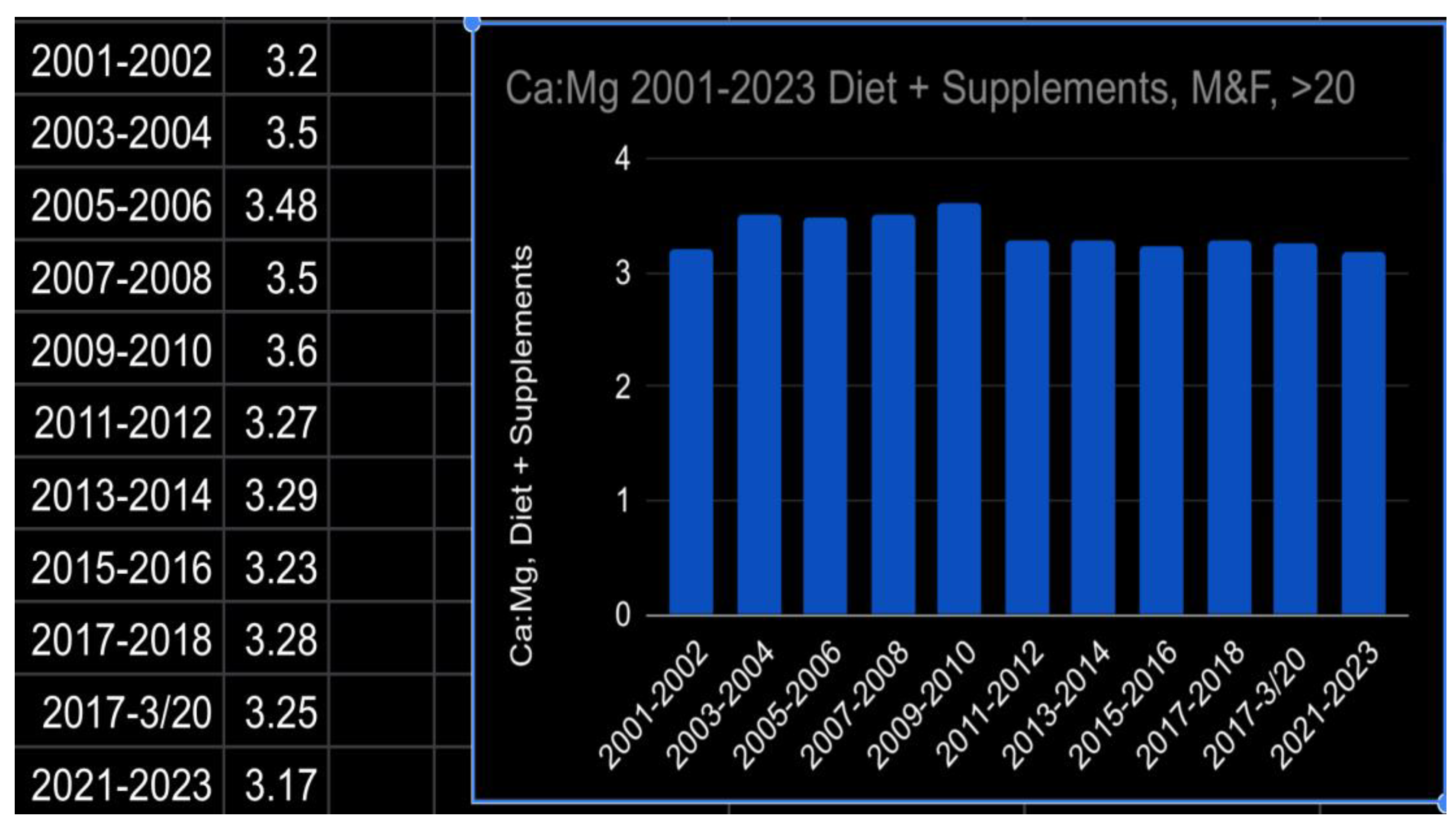
- Calcium to Magnesium Ratio
- Increasing Mg intake when Ca:Mg is less than 1.7 increases risk for some cancers [24].
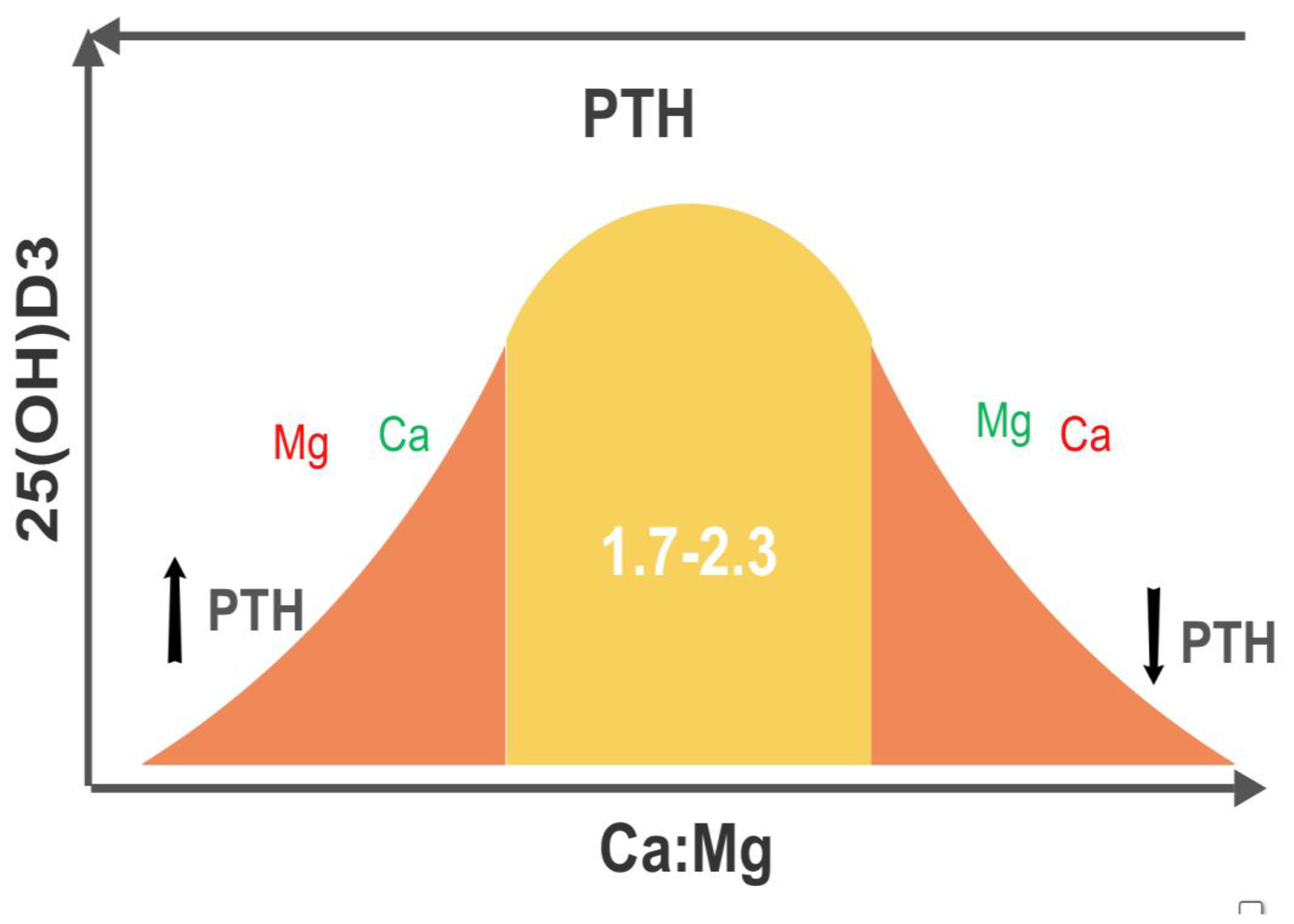
- Low Mg in the setting of elevated Ca:Mg translates to low VD [29]. This can be explained physiologically, as Mg is required for the synthesis and secretion of PTH and upregulation of VD. Elevated Ca also displaces Mg from CaSRs.
- Low Ca in the setting of depressed Ca:Mg is physiologically contradictory to a concomitant low VD. However, this may be explained based on discrepant but mutually reinforcing genetic, cultural, socioeconomic, and dietary considerations. These may complicate and compromise clinical correlations.
- Skin pigmentation is directly linked to VD deficiency [30].
- Cultural customs can drive VD deficiency. Most in the Middle East dress modestly [33] and many Asians are averse to solar exposure.
- III.
- Vitamin D and the Gut Microbiome
- Lack of baseline data indicating insufficiency/deficiency
- Failure to property separate placebo and target groups by baseline
- Less than 2-3 months between start of D3 supplementation and measurement of results
- Insufficient D3 dosage
- Failure to normalize for Ca:Mg as a confounding factor
- Target group too small
- IV.
- Magnesium and the Gut Microbiome
- V.
- Therapeutic Interventions
- 1.
- Probiotics, e.g., yogurt, alone are insufficient, if diet is suboptimal. The “good” bacteria must be fed and require fiber or indigestible carbohydrates, i.e., prebiotic, e.g., d-mannose. Butyrate is a commercially available postbiotic
- 2.
- Target a Ca:Mg of 2.0, either by weight of intake or by serum cation mM values. If elevated, lower dietary Ca, e.g., dairy products, sardines, first and then increase dietary Mg, e.g., nuts, seeds, leafy greens, avocados. The damage due to an elevated ratio is greatly underappreciated [38]
- 3.
- 4.
- Take supplemental Mg with pyridoxal phosphate and perhaps D3, the active form of B6. Mg is required for the hydroxylation of D3 in the liver (storage form). Taking pyridoxal phosphate concomitantly with magnesium can enhance absorption and availability of magnesium [95,96]. Not only does pyridoxal phosphate enhance cellular uptake of magnesium but magnesium enhances that of pyridoxal phosphate [97]. Several studies have challenged this [98,99]. But both employed the inactive form - pyridoxine.
- 5.
- Avoid simultaneous Ca and Mg intake. Although CaSRs are primarily found in the parathyroid gland and the kidney, they are also present in many other organs, including the alimentary canal [100].
- 6.
- Avoid simultaneous processed food/soft drinks and Mg intake. The former contain phosphates, which bind magnesium, limiting absorption.
- 7.
- Exercise induced elevation of lactate may enhance serum butyrate. Lactate may permeate intestinal endothelial and epithelial cells into the alimentary canal, where it can crossfeed butyrogenic bacteria [91].
- 8.
- Pay close attention to proper hydration. Dehydration triggers release of aldosterone, which increases renal reabsorption of Na+ and urinary excretion of Mg++ and K+. Cortisol possesses similar aldosterone properties and can to a lesser degree trigger this same cationic exchange. Stress induced cortisol can lead to Mg deficiency, while magnesium deficiency in turn enhances the body’s susceptibility to stress [101].
- 9.
- Increase VD intake with age and increasing morbidity.
- 10.
- Replenish water soluble B vitamins that require Mg for activation and are required for synthesis of 7-dehydrocholesterol from acetate, enabling solar conversion to D3, (see Figure 1).
Conclusions
References
- Fantini C, Corinaldesi C, Lenzi A, Migliaccio S, Crescioli C. Vitamin D as a Shield against Aging. International Journal of Molecular Sciences. 2023; 24(5):4546. [CrossRef]
- Chambers, P. (2024). Magnesium and Longevity. Qeios. [CrossRef]
- Badal VD, Vaccariello ED, Murray ER, Yu KE, Knight R, Jeste DV, Nguyen TT. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients. 2020; 12(12):3759. [CrossRef]
- Bidell MR, Hobbs ALV, Lodise TP. Gut microbiome health and dysbiosis: A clinical primer. Pharmacotherapy. 2022 Nov;42(11):849-857. [CrossRef]
- Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, Lagishetty V, Jacobs JP, Sinha-Hikim AP, Friedman TC. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front Endocrinol (Lausanne). 2021 Jun 8;12:667066. [CrossRef]
- Norman DA, Fordtran JS, Brinkley LJ, Zerwekh JE, Nicar MJ, Strowig SM; et al. Jejunal and ileal adaptation to alterations in dietary calcium: Changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1,25-dihydroxyvitamin D. J Clin Invest. 1981 Jun;67(6):1599-603. [CrossRef]
- Mahaffee DD, Cooper CW, Ramp WK, Ontjes DA. Magnesium promotes both parathyroid hormone secretion and adenosine 3’,5’-monophosphate production in rat parathyroid tissues and reverses the inhibitory effects of calcium on adenylate cyclase. Endocrinology. 1982 Feb;110(2):487-95. [CrossRef]
- Brown EM, Hurwitz S, Aurbach GD. Beta-adrenergic stimulation of cyclic AMP content and parathyroid hormone release from isolated bovine parathyroid cells. Endocrinology. 1977 Jun;100(6):1696-702. [CrossRef]
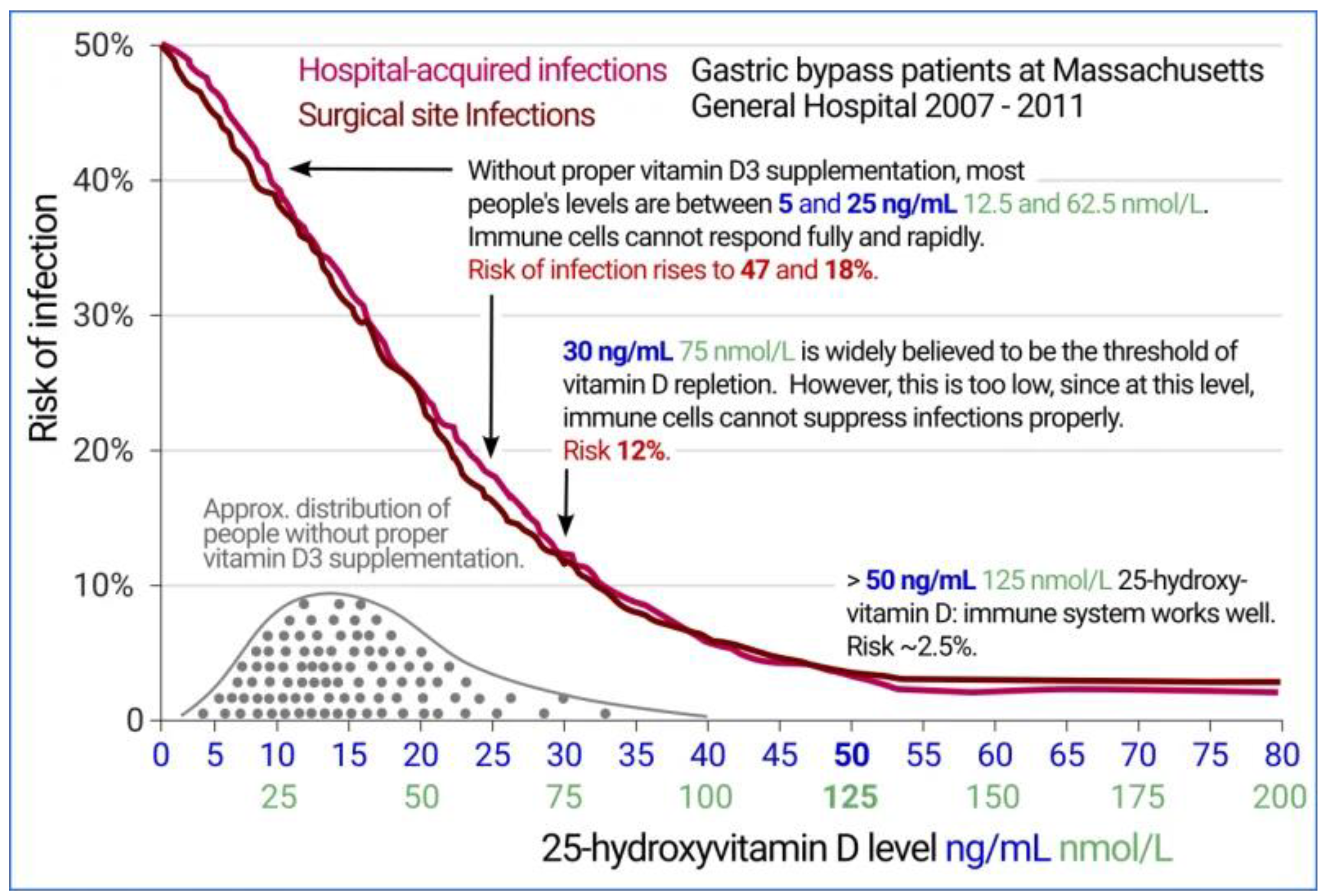
- Figure 2 graph of infection versus 25(OH)D3 level may be viewed at https://vitamindstopscovid.info/02-intracrine/.
- Quraishi SA, Bittner EA, Blum L, Hutter MM, Camargo CA. Association Between Preoperative 25-Hydroxyvitamin D Level and Hospital-Acquired Infections Following Roux-en-Y Gastric Bypass Surgery. JAMA Surg.2014;149(2):112–118. [CrossRef]
- Israel A, Cicurel A, Feldhamer I; et al. The link between vitamin D deficiency and Covid-19 in a large population. medRxiv; 2020. [CrossRef]
- Veugelers, P.J.; Ekwaru, J.P. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients 2014, 6, 4472–4475. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P. Comment on Huțanu et al. Low Serum Vitamin D in COVID-19 Patients Is Not Related to Inflammatory Markers and Patients’ Outcomes—A Single-Center Experience and a Brief Review of the Literature. Nutrients 2022, 14, 1998. [Google Scholar] [CrossRef]
- Sempos CT, Looker AC, Durazo-Arvizu RA, Yetley EA, Chaudhary-Webb M, Maw KL; et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016 Aug;104(2):454-61. [CrossRef]
- Subramanian A, Burrowes HB, Rumph JT, Wilkerson J, Jackson CL, Jukic AMZ. Vitamin D Levels in the United States: Temporal Trends (2011-2018) and Contemporary Associations with Sociodemographic Characteristics (2017-2018). Nutrients. 2024 Oct 9;16(19):3414. [CrossRef]
- Durlach, J. Recommended dietary amounts of magnesium: Mg RDA. Magnes Res. 1989 Sep;2(3):195-203. https://pubmed.ncbi.nlm.nih.gov/2701269/.
- 18 Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv Nutr. 2016;7:977–993. [CrossRef]
- Razzaque, MS. Magnesium: Are We Consuming Enough? Nutrients. 2018;10(12):1863. [CrossRef]
- Elin, RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010 Dec;23(4):S194-8. [CrossRef]
- Micke O, Vormann J, Kraus A, Kisters K. Serum Magnesium: Time for a Standardized and Evidence-Based Reference Range. Magnetic Resonance. 2021;34:84-89. https://www.magnesium-ges.de/Micke_et_al._2021.pdf.
- Rosanoff A, West C, Elin RJ, Micke O, Baniasadi S, Barbagallo M; et al. MaGNet Global Magnesium Project (MaGNet). Recommendation on an updated standardization of serum magnesium reference ranges. Eur J Nutr. 2022 Oct;61(7):3697-3706. [CrossRef]
- Weiss D, Brunk DK, Goodman DA. “Scottsdale Magnesium Study: Absorption, Cellular Uptake, and Clinical Effectiveness of a Timed-Release Magnesium Supplement in a Standard Adult Clinical Population”. J Am Coll Nutr. 2018;37(4):316-327. [CrossRef]
- Quinn SJ, Thomsen AR, Egbuna O, Pang J, Baxi K, Goltzman D, Pollak M, Brown EM. CaSR-mediated interactions between calcium and magnesium homeostasis in mice. Am J Physiol Endocrinol Metab. 2013 Apr 1;304(7):E724-33. [CrossRef]
- Shah SC, Dai Q, Zhu X, Peek RM Jr, Roumie C, Shrubsole MJ. Associations between calcium and magnesium intake and the risk of incident oesophageal cancer: An analysis of the NIH-AARP Diet and Health Study prospective cohort. Br J Cancer. 2020 Jun;122(12):1857-1864. [CrossRef]
- Han, C. , Shin, A., Lee, J. et al. Dietary calcium intake and the risk of colorectal cancer: A case control study. BMC Cancer 15, 966 (2015). [CrossRef]
- Takata Y, Yang JJ, Yu D, Smith-Warner SA, Blot WJ, White E; et al. Calcium Intake and Lung Cancer Risk: A Pooled Analysis of 12 Prospective Cohort Studies. J Nutr. 2023 Jul;153(7):2051-2060. [CrossRef]
- Huang, JH. , Tsai, LC., Chang, YC. et al. High or low calcium intake increases cardiovascular disease risks in older patients with type 2 diabetes. Cardiovasc Diabetol 13, 120 (2014). [CrossRef]
- Hyung-Suk Yoon, Xiao Ou Shu, Hui Cai, Wei Zheng, William J. Blot, Qiuyin Cai; Abstract 851: Associations of dietary calcium and magnesium intakes with lung cancer risk among low-income Americans: Results from the Southern Community Cohort Study. Cancer Res ; 81 (13_Supplement): 851. 1 July. [CrossRef]
- Deng, X. , Song, Y., Manson, J.E. et al. Magnesium, vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med 11, 187 (2013). [CrossRef]
- Ken Batai, Zuxi Cui, Amit Arora, Ebony Shah-Williams, Wenndy Hernandez, Maria Ruden; et al. Genetic loci associated with skin pigmentation in African Americans and their effects on vitamin D deficiency. e: PLOS Genetics, 2021; 17 (2), 2021. [CrossRef]
- Scully H, Laird E, Healy M, Crowley V, Walsh JB, McCarroll K. Socioeconomic status predicts vitamin D status in a large cohort of Irish children. Proceedings of the Nutrition Society. 2022;81(OCE4):E87. [CrossRef]
- Tønnesen, R. , Hovind, P.H., Jensen, L.T. et al. Determinants of vitamin D status in young adults: Influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health 16, 385 (2016). [CrossRef]
- Hussein, Dahat A. et al. “Pattern of vitamin D deficiency in a Middle Eastern population: A cross-sectional study. 2022. [CrossRef]
- Darling, A.L.; et al. (2020) Very High Prevalence of 25-hydroxyvitamin D Deficiency in 6433 UK South Asian adults: Analysis of the UK Biobank Cohort. British Journal of Nutrition. doi.org/10.1017/S0007114520002779.
- Chan, Chin Yi et al. “Attitude of Asians to Calcium and Vitamin D Rich Foods and Supplements: A Systematic Review.” Sains Malaysiana (2018):. [CrossRef]
- Rosanoff A, Dai Q, Shapses SA. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv Nutr. 2016 Jan 15;7(1):25-43. [CrossRef]
- Dai Q, Sandler R, Barry E, Summers R, Grau M, Baron J. Calcium, magnesium, and colorectal cancer. Epidemiology. 2012 May;23(3):504-5. [CrossRef]
- Dai, Q. , Zhu, X., Manson, J.E., Song, Y., Li, X., Franke, A.; et al. (2018) Magnesium Status and Supplementation Influence Vitamin D Status and Metabolism: Results from a Randomized Trial. The American Journal of Clinical Nutrition, 108, 1249-1258. [Google Scholar] [CrossRef]
- NHANES data for nutrient intake by gender and age from 2001 through Aug 2023 https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/.
- Guerrero-Romero F, Mercado M, Rodriguez-Moran M, Ramírez-Renteria C, Martínez-Aguilar G, Marrero-Rodríguez D, Ferreira-Hermosillo A, Simental-Mendía LE, Remba-Shapiro I, Gamboa-Gómez CI; et al. Magnesium-to-Calcium Ratio and Mortality from COVID-19. Nutrients. 2022; 14(9):1686. [CrossRef]
- Díez JJ, Iglesias P, García A, Martín-Casasempere I, Bernabéu-Andréu FA. Serum Calcium, Magnesium, and Phosphorus Levels in Patients with COVID-19: Relationships with Poor Outcome and Mortality. Horm Metab Res. 2023 Jan;55(1):31-39. [CrossRef]
- Kherad Z, Yazdanpanah S, Saadat F, Pakshir K, Zomorodian K. Vitamin D3: A promising antifungal and antibiofilm agent against Candida species. Curr Med Mycol. 2023 Jun;9(2):17-22 https://pmc.ncbi.nlm.nih.gov/articles/PMC10874479/.
- Lei J, Xiao W, Zhang J, Liu F, Xin C, Zhou B, Chen W, Song Z. Antifungal activity of vitamin D3 against Candida albicans in vitro and in vivo. Microbiol Res. 2022 Dec;265:127200. [CrossRef]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms. 2022; 10(5):1014. [CrossRef]
- Thomas, R.L. , Jiang, L., Adams, J.S. et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun 11, 5997 (2020). [CrossRef]
- Tangestani H, Boroujeni HK, Djafarian K, Emamat H, Shab-Bidar S. Vitamin D and The Gut Microbiota: A Narrative Literature Review. Clin Nutr Res. 2021 Jul;10(3):181-191. [CrossRef]
- Singh, P. , Rawat, A., Alwakeel, M. et al. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep 10, 21641 (2020). [CrossRef]
- Evangelos, Giampazolias; et al. Vitamin D regulates microbiome-dependent cancer immunity.Science384,428-437(2024). [CrossRef]
- Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol. 2020 Jan 21;10:3141. [CrossRef]
- Tabatabaeizadeh, SE, Tafazoli, N, Ferns, GA, Avan, A, Ghayour-Mobarhan M. Vitamin D, the gut microbiome and inflammatory bowel disease. Journal of Research in Medical Sciences 23(1):p 75. [CrossRef]
- Kanhere, M, He, J, Chassaing, B, Ziegler, TR, Alvarez, JA, Ivie, EA; et al. Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults With Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 103, Issue 2, 18, Pages 564–574. 20 February. [CrossRef]
- Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, Venkatesan A, Fraser CM, Mowry EM. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J Investig Med. 2015 Jun;63(5):729-34. [CrossRef]
- Velizarova, M. , Yanachkova, V., Boneva, T., Giragosyan, S., Mihaleva, I., Andreeva-Gateva, P.; et al. (2023). Relationship between Vitamin D status and microbiome changes in Bulgarian patients with type 2 diabetes mellitus. Biotechnology & Biotechnological Equipment, 37(1). [CrossRef]
- Daley DK, Myrie SB. Diabetes and vitamin D: The effect of insulin sensitivity and gut microbial health. Adv Food Nutr Res. 2024;109:160-184. [CrossRef]
- Breuling M, Tomeva E, Ivanovic N, Haslberger A. Butyrate- and Beta-Hydroxybutyrate-Mediated Effects of Interventions with Pro- and Prebiotics, Fasting, and Caloric Restrictions on Depression: A Systematic Review and Meta-Analysis. Life. 2024; 14(7):787. [CrossRef]
- Wang, C. , Zheng, D., Weng, F. et al. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes.Psychopharmacology 239, 215–227 (2022). [CrossRef]
- Chen J, Zhao K-N, Vitetta L. Effects of Intestinal Microbial–Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients. 2019; 11(5):1026. [CrossRef]
- Zhang Y, Tao Y, Gu Y, Ma Q. Butyrate facilitates immune clearance of colorectal cancer cells by suppressing STAT1-mediated PD-L1 expression. Clinics (Sao Paulo). 2023 Nov 4;78:100303. [CrossRef]
- Coppola S, Avagliano C, Calignano A, Berni Canani R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules. 2021; 26(3):682. [CrossRef]
- Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G; et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes. 2020 Sep 1;11(5):411-455. https://doiorg/10.3920/BM2020.0057.
- Facchin S, Bertin L, Bonazzi E, Lorenzon G, De Barba C, Barberio B, Zingone F, Maniero D, Scarpa M, Ruffolo C; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life. 2024; 14(5):559. [CrossRef]
- Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020 Jan;40(1):551-556. [CrossRef]
- Cooney OD, Nagareddy PR, Murphy AJ, Lee MKS. Healthy Gut, Healthy Bones: Targeting the Gut Microbiome to Promote Bone Health. Front Endocrinol (Lausanne). 2021 Feb 19;11:620466. [CrossRef]
- Roberto Pacifici, Role of Gut Microbiota in the Skeletal Response to PTH, The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 3, 21, pp 636–645. 20 March. [CrossRef]
- Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L; et al. Vitamin D Signaling through Induction of Paneth Cell Defensins Maintains Gut Microbiota and Improves Metabolic Disorders and Hepatic Steatosis in Animal Models. Front Physiol. 2016 Nov 15;7:498. [CrossRef]
- Hansdah, K. , Lui, JC. Emerging Insights into the Endocrine Regulation of Bone Homeostasis by Gut Microbiome, Journal of the Endocrine Society, Volume 8, Issue 8, 24, bvae117. 20 August. [CrossRef]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019 Jan 10;7(1):14. [CrossRef]
- Bellerba F, Muzio V, Gnagnarella P, Facciotti F, Chiocca S, Bossi P, Cortinovis D, Chiaradonna F, Serrano D, Raimondi S; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients. 2021; 13(10):3378. [CrossRef]
- Gominak, SC. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med Hypotheses. 2016 Sep;94:103-7. [CrossRef]
- Wibowo, S. , & Pramadhani, A. (2024). Vitamin B, Role of Gut Microbiota and Gut Health. IntechOpen. [CrossRef]
- Nysten, J. , & Van Dijck, P. (2023). Can we microbe-manage our vitamin acquisition for better health? PLoS Pathogens 19(5). [CrossRef]
- Mansmann, H.C. (1994). Consider magnesium homeostasis: III: Cytochrome P450 enzymes and drug toxicity. Applied Immunohistochemistry & Molecular Morphology, 8, 7-28. https://www.liebertpub.com/doi/abs/10.1089/pai.1994.8.7.
- Collins, S.L. , Stine, J.G., Bisanz, J.E. et al. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat Rev Microbiol 21, 236–247 (2023). [CrossRef]
- Ji S, Pan Y, Zhu L, Tan J, Tang S, Yang Q, Zhang Z, Lou D, Wang B. A novel 7α-hydroxysteroid dehydrogenase: Magnesium ion significantly enhances its activity and thermostability. Int J Biol Macromol. 2021 Apr 30;177:111-118. [CrossRef]
- Kim, D.M. , Liu, J., Whitmore, M.A. et al. Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis. J Animal Sci Biotechnol 15, 29 (2024). [CrossRef]
- Ojo ES, Tischkau SA. The Role of AhR in the Hallmarks of Brain Aging: Friend and Foe. Cells. 2021 Oct 13;10(10):2729. https//doi.org/10.3390/cells10102729.
- Wang Z, Snyder M, Kenison JE, Yang K, Lara B, Lydell E; et al. How the AhR Became Important in Cancer: The Role of Chronically Active AhR in Cancer Aggression. International Journal of Molecular Sciences. 2020 Dec 31;22(1):387. [CrossRef]
- Zhu K, Meng Q, Zhang Z, Yi T, He Y, Zheng J, Lei W. Aryl hydrocarbon receptor pathway: Role, regulation and intervention in atherosclerosis therapy (Review). Molecular Medicine Reports. 2019 Dec;20(6):4763-4773. https://doi.org10.3892/mmr.2019.10748.
- Koper JEB, Kortekaas M, Loonen LMP, Huang Z, Wells JM, Gill CIR ; et al. Aryl hydrocarbon Receptor activation during in vitro and in vivo digestion of raw and cooked broccoli (brassica oleracea var. Italica). Food Funct. 2020 ;11(5):4026-4037. 1 May. [CrossRef]
- Marinelli, L. , Martin-Gallausiaux, C., Bourhis, JM. et al. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep 9, 643 (2019). [CrossRef]
- Li X, Zhang B, Hu Y, Zhao Y. New Insights Into Gut-Bacteria-Derived Indole and Its Derivatives in Intestinal and Liver Diseases. Front Pharmacol. 2021 Dec 13;12:769501. [CrossRef]
- Gribble FM, Reimann F. Metabolic Messengers: Glucagon-like peptide 1. Nat Metab. 2021;3:142–148. [CrossRef]
- Chaudhary P, Kathuria D, Suri S, Bahndral A, Kanthi Naveen A. Probiotics- its functions and influence on the ageing process: A comprehensive review. Food Bioscience. 2023;52:102389. https://doi.ofrg/10.1016/j.fbio.2023.102389.
- Mahboobi S, Ghasvarian M, Ghaem H, Alipour H, Alipour S, Eftekhari MH. Effects of probiotic and magnesium co-supplementation on mood, cognition, intestinal barrier function and inflammation in individuals with obesity and depressed mood: A randomized, double-blind placebo-controlled clinical trial. Front Nutr. 2022 Sep 28;9:1018357. [CrossRef]
- Seefeldt JM, Homilius C, Hansen J, Lassen TR, Jespersen NR, Jensen RV; et al. Short-Chain Fatty Acid Butyrate Is an Inotropic Agent With Vasorelaxant and Cardioprotective Properties. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease. 2024;13. [CrossRef]
- Yu Z, Han J, Chen H, Wang Y, Zhou L, Wang M; et al. Oral Supplementation With Butyrate Improves Myocardial Ischemia/Reperfusion Injury via a Gut-Brain Neural Circuit. Front Cardiovasc Med. 2021 Sep 23;8:718674. [CrossRef]
- Sasaki H, Hayashi K, Imamura M, Hirota Y, Hosoki H, Nitta L; et al. Combined resistant dextrin and low-dose Mg oxide administration increases short-chain fatty acid and lactic acid production by gut microbiota. J Nutr Biochem. 2023 Oct;120:109420. [CrossRef]
- Hou Y, Li J, Ying S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites. 2023; 13(11):1166.), an essential amino acid, also associated with longevity. [CrossRef]
- Kherad Z, Yazdanpanah S, Saadat F, Pakshir K, Zomorodian K. Vitamin D3: A promising antifungal and antibiofilm agent against Candida species. Curr Med Mycol. 2023 Jun;9(2):17-22. https://pubmed.ncbi.nlm.nih.gov/38375518/.
- Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front Neurosci. 2014 Feb 6;8:12. [CrossRef]
- Louis P, Duncan SH, Sheridan PO, Walker AW, Flint HJ (2022). “Microbial lactate utilisation and the stability of the gut microbiome.” Gut Microbiome. 3: e3. [CrossRef]
- Li, J. , Yan, Y., Fu, Y. et al. ACE2 mediates tryptophan alleviation on diarrhea by repairing intestine barrier involved mTOR pathway. Cell Mol Biol Lett 29, 90 (2024). [CrossRef]
- Wimalawansa, SJ. Physiology of Vitamin D—Focusing on Disease Prevention. Nutrients. 2024; 16(11):1666. [CrossRef]
- AlHewishel M A, Bahgat M, Al Huwaiyshil A; et al. (, 2020) 25(OH)D Serum Level in Non-Diabetic and Type II Diabetic Patients: A Cross-Sectional Study. Cureus 12(6): e8910. 29 June. [CrossRef]
- Abraham GE, Schwartz UD, Lubran MM (1981) Effect of vitamin B-6 on plasma and red blood cell magnesium levels in premenopausal women. Ann Clin Lab Sci 11(4):333-336 https://pubmed.ncbi.nlm.nih.gov/7271227.
- Boylan LM, Spallholz JE (1990) In vitro evidence for a relationship between magnesium and vitamin B-6. Magnes Res 3:79-85 https://pubmed.ncbi.nlm.nih.gov/2133627.
- Planells E, Lerma A, Sánchez-Morito N, Aranda P, LLopis J (1997) Effect of magnesium deficiency on vitamin B2 and B6 status in the rat. 3: J Am Coll Nutr 16(4). [CrossRef]
- Noah L, Pickering G, Dubray C, Mazur A, Hitier S; et al. (2020) Effect of vitamin B6 supplementation, in combination with magnesium, on severe stress and magnesium status: Secondary analysis from an RCT. [CrossRef]
- Pouteau E, Kabir-Ahmadi M, Noah L, Mazur A, Dye L; et al. (2018) Superiority of magnesium and vitamin B6 over magnesium alone on severe stress in healthy adults with low magnesemia: A randomized, single-blind clinical trial. PLoS ONE, 0208. [CrossRef]
- Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T; et al. (10). Involvement of the calcium-sensing receptor in human taste perception. The Journal of Biological Chemistry. 285 (2): 1016–1022. 20 January. [CrossRef]
- Pickering G, Mazur A, Trousselard M, Bienkowski P, Yaltsewa N, Amessou M, Noah L, Pouteau E. Magnesium Status and Stress: The Vicious Circle Concept Revisited. Nutrients. 2020 Nov 28;12(12):3672. [CrossRef]
- Akimbekov NS, Digel I, Sherelkhan DK, Lutfor AB, Razzaque MS. Vitamin D and the Host-Gut Microbiome: A Brief Overview. Acta Histochem Cytochem. 2020 Jun 26;53(3):33-42. [CrossRef]
- Rosanoff A, Dai Q, Shapses SA. Essential Nutrient Interactions: Does Low or Suboptimal Magnesium Status Interact with Vitamin D and/or Calcium Status? Adv Nutr. 2016 Jan 15;7(1):25-43. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).