Introduction
Dental implants are placed in the human body to replicate the function and appearance of natural teeth, offering long-term solutions for missing teeth. Prosthodontic components, such as abutments and crowns, are connected to these implants to restore both functionality and aesthetics [
1]. Due to their high predictability, dental implants have become the most recommended and widely practiced treatment option for restoring lost teeth in totally or partially edentulous patients. The discovery of osseointegration—the process by which bone heals around titanium—has been the foundation for the success of modern implant dentistry [
2].
There are two critical interfaces between living tissues and artificial components in implant dentistry. The first is the bone-implant interface, known as osseointegration, which is well-established and extensively studied [
3]. The second is the peri-implant soft tissue interface, often referred to as the “soft tissue seal” or “mucointegration.” Unlike osseointegration, our understanding of this soft tissue interface is still evolving. The term “soft tissue seal” reflects the fact that its characteristics are not yet fully defined or understood [
4,
5].
When osseointegration lacks adequate protection from a functional soft tissue barrier, external threats—particularly bacterial infiltration—can compromise implant stability and long-term success. This soft tissue barrier plays a pivotal role in preventing peri-implant diseases such as peri-implantitis, which can lead to bone loss and, if untreated, implant failure. Enhancing our understanding of the peri-implant soft tissue interface is essential for improving implant outcomes and developing preventive strategies against peri-implant complications [
6].
Despite its importance, there is no unified consensus on the anatomy, histology, physiology, or clinical guidelines for optimizing the soft tissue seal around implants. While interest in this area has grown in recent years, many questions remain unresolved [
7,
8,
9,
10].
Similar to periodontal diseases—gingivitis and periodontitis—peri-implant diseases include peri-implant mucositis and peri-implantitis. Peri-implant mucositis is limited to the soft tissue, while peri-implantitis involves progressive bone loss [
11,
12,
13]. These conditions typically begin with disruption of the soft tissue seal, which, if left unchecked, can eventually compromise the osseointegration achieved during the early phase of treatment.
Like periodontal bone loss, peri-implant bone loss can result from bacterial invasion. However, bone loss around implants may also occur due to inadequate soft tissue width, independent of bacterial factors. Linkevicius et al. reported that when vertical soft tissue thickness (STT) at the alveolar crest is 2 mm or less, bone resorption is likely to occur, as the body attempts to establish an adequate biologic width [
14]. This adaptive resorption highlights the importance of ensuring a critical soft tissue thickness to maintain peri-implant health and minimize crestal bone loss.
To address the inherent weakness of peri-implant soft tissue in forming a biological seal, various strategies have been explored:
- -
Optimizing abutment surface characteristics to enhance tissue attachment [
15,
16].
- -
Modifying prosthodontic components, such as the emergence profile and platform-switching, to improve soft tissue stability [
17,
18].
- -
Adjusting the implant placement depth to strengthen the soft tissue’s protective barrier [
19].
Among these approaches, subcrestal implant placement (SPI) has gained attention as a method for improving peri-implant soft tissue adaptation. Vervaeke et al. described SPI as a technique that promotes bone remodeling and soft tissue stability, particularly in cases with thin peri-implant mucosa [
22,
23].
Several studies have demonstrated that subcrestally placed implants provide stable peri-implant conditions and minimize crestal bone loss over time. (
Figure 1) Bicon implants, widely used during the 2000s and 2010s, were often placed subcrestally, leading to lasting biological stability [
24]. SPIs are commonly used in cases with narrow or sloped ridges, where deeper placement ensures that the implant’s rough surface is fully embedded in bone. This approach is also beneficial for achieving a natural emergence profile, especially in molar regions. Despite their clinical success, SPI outcomes in the literature have been inconsistent, possibly due to:
- Lack of standardized clinical guidelines.
- Variability in surgical and prosthodontic techniques.
- Limited data on peri-implant soft tissue adaptation [
23,
24,
25].
Most analyses of peri-implant soft tissue have focused on horizontal extensions between the implant restoration and the underlying soft tissue, while vertical soft tissue dimensions have been largely overlooked. The Crest to Restoration Distance (CRD) represents the vertical space between the crestal bone and the implant restoration. This space is occupied by peri-implant soft tissue and is critical for biologic stability.
When CRD is within an optimal range:
The biologic seal is reinforced, reducing bacterial infiltration.
Void spaces or pockets are eliminated, lowering the risk of mucositis.
Soft tissue adaptation is improved, preventing excessive sulcular epithelial downgrowth.
When CRD is too large
Soft tissue stability is compromised, leading to pocket formation.
Bacterial accumulation increases, raising the risk of peri-implant disease.
This case study examines how CRD optimization affects peri-implant soft tissue stability by comparing two implants placed in the same patient:
- -
Control Implant: Maintained peri-implant health with stable CRD.
- -
Test Implant: Developed peri-implant mucositis due to excessive CRD.
By modifying CRD through prosthodontic adjustments, this study explores its role in disease prevention and peri-implant soft tissue integrity.
Crest to Restoration Distance (CRD) plays a fundamental role in ensuring peri-implant soft tissue stability, particularly in subcrestally placed implants. This study underscores the importance of CRD optimization as a modifiable factor in implant success and suggests that future research should focus on establishing standardized CRD guidelines to improve clinical protocols and long-term outcomes.
Case Presentation
A 57-year-old woman underwent extraction of her lower right first molar on June 3, 2020, due to a severe periodontal abscess. Later, on September 22, 2020, her upper left first molar was extracted due to a fracture involving both the crown and root. (
Figure 2)
On October 29, 2020, an implant (Oneplant, Warrentec, manufactured in Seoul, South Korea) was placed at the upper left first molar site. The procedure included a sinus lift using the transcrestal approach along with bone grafting surgery. (
Figure 3)
A second implant (Oneplant, Warrentec) was placed at the lower right first molar site on December 21, 2020.
The second operation to connect the healing abutments on both sides was performed on March 3, 2021. Healing abutments with a diameter of 6.0 mm and a height of 5.0 mm were placed at each site.
The final implant restoration for the lower right molar was completed on March 18, 2021, using a 4.5 mm diameter, 3 mm gingival cuff abutment.
For the upper left molar, the restoration was completed on March 25, 2021, with a 4.5 mm diameter, 4 mm gingival cuff abutment.
Zirconia crowns were cemented onto the prefabricated abutments (manufactured by Warrentec) using the extraoral cementation technique. (
Figure 4)
She was provided with instructions on implant care, including thorough cleaning using proximal brushes and water irrigation devices.
Until October 17, 2022, the patient had not exhibited any signs or symptoms of implant-associated diseases, such as peri-implant mucositis or peri-implantitis, with the implant functioning well during routine check-ups for approximately 19 months. However, starting from that date, she reported discomfort in the upper left first molar implant area, including gingival bleeding, swelling, and food impaction.
On clinical examination, the peri-implant soft tissue around the upper left first molar implant exhibited redness and slight swelling; however, radiographic evaluation showed no signs of bone loss. Consequently, the patient was diagnosed with peri-implant mucositis in the upper left first molar implant, while the lower right first molar implant remained healthy.
For the treatment of peri-implant mucositis, the following measures were implemented: reinforcing the patient’s oral hygiene around the implant, scheduling more frequent recall appointments for check-ups and saline irrigation around the sulcus, and applying topical antibiotics to the sulcus. However, there was no improvement, and the patient’s discomfort due to food impaction persisted. Clinical evaluation revealed that the interproximal contact point was adequately tight, preventing food from becoming lodged between the teeth. The lateral profile of the crown appeared natural, with sufficient space in the embrasure area for cleaning. Despite this, food seemed to accumulate in the sulcus rather than in other areas, such as the interproximal or contact zones. In contrast, the lower right first molar implant remained stable, with no signs or symptoms of peri-implant disease.
For the purpose of analyzing and comparing the peri-implant soft tissue structure and peri-implant bone topography, a CBCT (Cone-Beam Computed Tomography) scan was performed. The analysis specifically focused on the Crest to Restoration Distance (CRD), which represents the vertical distance between the implant restoration and the underlying crestal bone. The results of this analysis are summarized in
Table 1. (
Table 1)
Discussions
Peri-implant mucositis and peri-implantitis are biological complications with an infectious etiology, primarily caused by the disruption of the soft tissue seal around implants [
24]. The peri-implant soft tissue serves a crucial function as a biological barrier, protecting the underlying crestal bone from bacterial infiltration. Linkevicius et al. highlighted the importance of vertical soft tissue thickness in forming a strong biologic seal around implants but did not fully address the three-dimensional architecture of peri-implant soft tissue. This omission raises concerns about the potential for pocket formation, which can lead to bacterial colonization and food accumulation, particularly in subcrestally placed implants (SPI), where deeper positioning can create greater challenges for maintaining a stable soft tissue seal (25, 13).
Bosshardt et al. described the pathogenesis of periodontal pocket formation, emphasizing that the breakdown of the junctional epithelium serves as the initiating event in disease progression. Their study highlighted the critical function of the junctional epithelium as the first line of defense and reinforced the importance of probing as a diagnostic tool [
26].
Although acquiring sufficient Supracrestal Tissue Height (STH) is essential for maintaining biologic width and preventing esthetic complications, excessive STH has been linked to pocket formation and an increased risk of peri-implant disease. A balanced STH within the range of 3 mm < STH < 5 mm has been suggested to optimize tissue health [
27]. While numerous studies emphasize the vertical component of peri-implant soft tissue as a key determinant of tissue stability, there is a lack of literature addressing the horizontal component of peri-implant soft tissue, which is inevitably generated in SPI cases due to the extended transitional interface between the submucosal portion of the implant restoration and the underlying peri-implant tissue.
This case report suggests that maintaining an optimal CRD plays a pivotal role in stabilizing peri-implant soft tissue architecture within this transitional interface. A properly maintained CRD may limit epithelial downgrowth and prevent deep pocket formation, thus reinforcing the biological seal.
The observed clinical improvement in peri-implant mucositis following CRD optimization suggests a structural transformation in peri-implant soft tissue.
Figure 11 presents a schematic representation of this proposed histologic adaptation. Prior to modification, the peri-implant interface exhibited void spaces (pockets) alongside sulcular epithelium and connective tissue layers. Following restoration revision, it is hypothesized that the void was eliminated, leading to the reorganization of peri-implant soft tissue into a more structured zone composed of sulcular epithelium, junctional epithelium, and connective tissue.
This transformation may contribute to a more effective biologic seal, preventing bacterial infiltration and promoting peri-implant health. However, while these observations are inferred from clinical and radiographic data, further histologic research is required to validate the proposed structural adaptation.
- 2.
Clinical Indicators and Diagnosis of Peri-Implant Disease
Clinical signs of peri-implant mucositis include redness, swelling, bleeding on probing (BOP), suppuration on probing (SOP), and increased probing pocket depth (PPD). In contrast, peri-implantitis is confirmed through radiographic evidence of bone loss. Periodic probing is emphasized as a critical diagnostic tool for monitoring peri-implant conditions, as increased PPD, accompanied by profuse BOP and SOP, is strongly associated with peri-implantitis [
28].
The diagnosis of peri-implant mucositis relies on clinical criteria without requiring histological confirmation. Unlike peri-implantitis, peri-implant mucositis is reversible. Destruction of the junctional epithelium attachment is a hallmark of gingivitis, underscoring the role of hemidesmosomes in periodontal gingiva [
26]. However, peri-implant tissues exhibit significant morphological differences from periodontal tissues, particularly weaker hemidesmosomal attachment in the junctional epithelium around implants [
29]. This weaker attachment may render peri-implant tissues more susceptible to bacterial infiltration and disease progression, including peri-implantitis.
Despite skepticism regarding the role of hemidesmosomes around implants [
10], the resistance observed during probing suggests that peri-implant soft tissue retains some sealing ability. This may be attributed to the functional role of hemidesmosomes, similar to that observed in natural teeth [
9,
30]. This sealing function highlights the importance of peri-implant soft tissue integrity in maintaining peri-implant health and preventing disease progression.
Numerous reports underscore the pivotal role of probing in maintaining peri-implant soft tissue health. Probing serves not only as an essential diagnostic tool for early detection of peri-implant diseases but also as a preventive measure to mitigate progression toward peri-implant bone destruction. When combined with clinical signs such as erythema, BOP, SOP, and swelling, probing provides a comprehensive assessment of peri-implant tissue health, facilitating timely intervention [
28].
- 3.
Probing implants’ sulcus with IPPP (Implant Paper Point Probing)
For natural teeth, periodontal sulcus depth is measured using periodontal probes with a limited probing force. Sulcus depth is defined as the distance from the gingival margin to the most apically penetrated portion of the junctional epithelium. In a healthy state, the probe tip remains within the junctional epithelium. However, in the presence of inflammation, the probe penetrates beyond the junctional epithelium into the connective tissue, reflecting the compromised barrier function of the epithelial attachment.
Similarly, in peri-implant tissues, inflammation causes the probe tip to penetrate deeper into the connective tissue, reaching closer to the alveolar bone crest than in natural teeth. The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions recommended that peri-implant pocket probing should be performed with a light force (~0.25 N), as healthy peri-implant pocket depths generally remain <5 mm [
24].
Controlled probing with a force of 0.2 N has demonstrated similar probe penetration depths in healthy implants and natural teeth, aligning with the dimensions of the epithelial barrier, as observed in studies by Abrahamsson and Soldini [
31].
The challenge in clinical examinations lies in applying a standardized probing force. While automated periodontal probes have been proposed to ensure consistent force, their utility for probing implants remains limited due to variations in the curvature and design of implant prosthetic restorations [
32].
To overcome these challenges, the author used the Implant Paper Point Probe (IPPP), developed by Suredent in South Korea. The IPPP has a yield strength of 0.25–0.35 N, allowing it to flexibly penetrate the sulcus within this range. If excessive force is applied, the paper points bend rather than damage the tissue, ensuring controlled probing. This approach provides reliable sulcus depth measurements while minimizing the risk of over-penetration, offering an effective alternative to metal probes for implants.
Additionally, the Implant Paper Point Probe (IPPP) offers dual functionality, providing valuable insights into sulcus health by detecting sulcus fluid and bleeding on probing (BOP). These conditions can be directly observed through wetting and discoloration of the paper probe, enhancing diagnostic accuracy.
Some authors have raised concerns about potential tissue damage caused by probing, particularly when the probe penetrates deeper than intended. However, the IPPP method alleviates this concern, as its supple design allows it to bend upon reaching a preset force, preventing damage to the underlying peri-implant soft tissue beyond the appropriate depth.
- 4.
Possible explanation for the results from this study
This case study primarily focused on the impact of Crest to Restoration Distance (CRD) on peri-implant soft tissue health. The key finding was that maintaining CRD within an optimal range was associated with peri-implant soft tissue stability, preventing the onset of peri-implant mucositis—a precursor to peri-implantitis.
To explain this observation, three possible mechanisms are proposed:
-
Preservation of Hemidesmosomal Attachment
o By eliminating pocket formation at the peri-implant interface, the hemidesmosomal attachment of the junctional epithelium remains functional at the entrance.
o This prevents sulcular epithelial downgrowth and maintains the integrity of the peri-implant mucosa, reinforcing the soft tissue barrier against bacterial infiltration.
-
Hydraulic Pressure from the Connective Tissue
o With a stable junctional epithelium at the entrance, the connective tissue beneath the implant restoration is able to exert hydraulic pressure against the prosthesis.
o This pressure generates a protective sealing force, further contributing to the biologic defense mechanism.
-
Sustained Structural and Immunologic Integrity
-
o Maintaining structural stability ensures that each component of the peri-implant soft tissue performs its specialized role:
▪ Sulcular epithelium: High turnover rate providing rapid barrier renewal.
▪ Junctional epithelium: Adhesion to the implant surface with immunologic function.
▪ Connective tissue: Mechanical support, vascular supply, and immune defense.
o These roles are well-established in basic histology and can be inferred without direct histological investigation. The structural integrity of peri-implant soft tissue can be assessed through dimensional analysis of each layer, as demonstrated in this case study.
From this case, two key clinical findings support this hypothesis:
Peri-implant mucositis resolved following the optimization of CRD.
Peri-implant soft tissue returned to a healthy state, confirmed through Implant Paper Point Probing (IPPP).
These findings suggest that maintaining CRD within an optimal range may help prevent downward migration of the sulcular epithelium, allowing the underlying connective tissue to remain stable without requiring sulcular epithelial coverage. This self-sustained tissue maintains its structural and functional integrity, preventing inflammatory pocket formation and minimizing the risk of disease progression.
Conversely, when CRD exceeds the critical range, peri-implant soft tissue stability is compromised. Without sufficient support, the connective tissue becomes unsustainable, requiring compensatory epithelial coverage. This leads to apical migration of the junctional epithelium, forming deep pockets that facilitate bacterial colonization and inflammation. The resulting inflammatory response, aimed at managing the foreign material, often manifests as soft tissue swelling, further deepening the pocket and increasing susceptibility to peri-implant mucositis and peri-implantitis.
This mechanism closely parallels the pathogenesis of periodontitis in natural teeth, where the junctional epithelium converts into pocket epithelium, marking disease progression [
26]. Thus, this study reinforces the importance of CRD as a modifiable factor in maintaining peri-implant health and preventing disease progression.
These results challenge the traditional assumption that subcrestally placed implants (SPIs) are inherently predisposed to deep pocket formation and peri-implant complications. Instead, this study demonstrates that when CRD is maintained within a critical range, SPIs can achieve stable peri-implant soft tissue adaptation—even when a greater peri-implant soft tissue dimension develops beneath the restoration. Conversely, this broader and more voluminous connective tissue may contribute to peri-implant soft tissue health through its intrinsic biological properties, including enhanced vascularization, mechanical support, and immunologic function. This underscores the necessity of meticulous soft tissue management and precision in prosthetic design to ensure long-term implant success.
Furthermore, all cases in this study employed an extraoral cementation technique, effectively eliminating excess cement—a well-documented factor contributing to peri-implant disease. These findings highlight the importance of integrating both soft tissue and prosthetic management strategies to optimize peri-implant health and prevent complications.
- 5.
Histologic Features of the Junctional Epithelium and Connective Tissue
The peri-implant soft tissue consists of two primary components:
Junctional epithelium
Connective tissue
These structures work synergistically to form a biologic seal around implants, functionally mimicking the protective roles observed in natural teeth.
Junctional Epithelium
The junctional epithelium serves as a critical defense barrier, forming the first line of protection against microbial colonization and infection. It plays a vital role in adapting gingival tissues to implants while also contributing to immunologic defense mechanisms [
9].
Key characteristics of the junctional epithelium:
High cell turnover: Comprising 15–30 cell layers, with a maximum average thickness of about 0.5 mm.
Hemidesmosomal adhesion: Hemidesmosomes are present on both the implant-facing and connective tissue-facing surfaces of the epithelium, facilitating adhesion.
Larger intercellular spaces: Compared to oral epithelium, these spaces allow immune cells to traverse the epithelium, enhancing its defensive function.
Endocytosis and decomposition: The epithelium has the ability to process and neutralize exogenous factors, contributing to its protective role [
34].
Clinically, the junctional epithelium is indistinguishable from connective tissue, but its presence can often be inferred by the absence of bleeding on probing (BOP). Its high cell turnover rate and ability to facilitate immune cell migration further enhance its role as a protective barrier. Therefore, in self-sustained peri-implant soft tissue, the upper portion may include junctional epithelium, even if composed of only a single cell layer, distinguishing it from pathologic sulcular epithelial downgrowth observed in disease progression.
Connective Tissue
Located apical to the junctional epithelium, the peri-implant connective tissue is primarily composed of collagen fibers. In natural teeth, this connective tissue is anchored to cementum via dentogingival fibers, which provide a mechanical barrier against bacterial invasion. However, dental implants lack this intrinsic fibrous attachment.
Instead, the peri-implant connective tissue compensates by forming a functional seal, which is maintained through:
These biomechanical interactions ensure stability and integration of the peri-implant soft tissue. (
Figure 12) The connective tissue effectively supports the overlying junctional epithelium, playing a critical role in maintaining peri-implant soft tissue stability and protecting the underlying crestal bone.
Figure 12 illustrates a temporary submucosal swelling, representing the resilience and hydraulic positive pressure generated within the connective tissue. This phenomenon occurs during the removal of the prosthesis for temporary repair, highlighting the structural integrity and adaptability of peri-implant connective tissue.
- 6.
New model for biologic width for implants
To structurally understand peri-implant soft tissue, the traditional concept of biologic width, originally developed for natural teeth, has been adapted for implants. This concept was first introduced by Gargiulo et al. [
35] and later expanded by Vacek et al. [
36], defining biologic width as comprising:
These studies reported consistent connective tissue measurements of approximately 1.07 mm and 0.77 mm, respectively. Further investigations by Saso Ivanovski explored the supra-alveolar transmucosal architecture, highlighting both similarities and distinct features between teeth and implants [
37].
Limitations of Traditional Biologic Width Models in Implant Dentistry
Traditional biologic width models emphasize vertical soft tissue dimensions but largely overlook horizontal and oblique components of peri-implant connective tissue. This is particularly relevant for subcrestally placed implants (SPIs), where the peri-implant soft tissue adapts due to the coronal flaring of implant restorations.
One key adaptation is the formation of an extended soft tissue interface between the crestal bone and the implant restoration, referred to in this study as Crest to Restoration Distance (CRD). Without an adequate understanding of this peri-implant soft tissue, the overall transition zone from the fixture-abutment connection to the coronal gingival margin may be misinterpreted as pathological or mistaken for a socket-like condition.
Previous efforts to standardize peri-implant soft tissue evaluation have largely focused on:
However, these approaches are limited by their two-dimensional perspective, failing to capture the complex three-dimensional architecture of peri-implant tissues [
39]. This oversight underscores the need for a new model tailored to subcrestally placed implants (SPIs) with internal platform-switching connections, where the three-dimensional interplay of soft tissue components plays a critical role in long-term stability and function.
Proposed New Model for Biologic Width in Implants
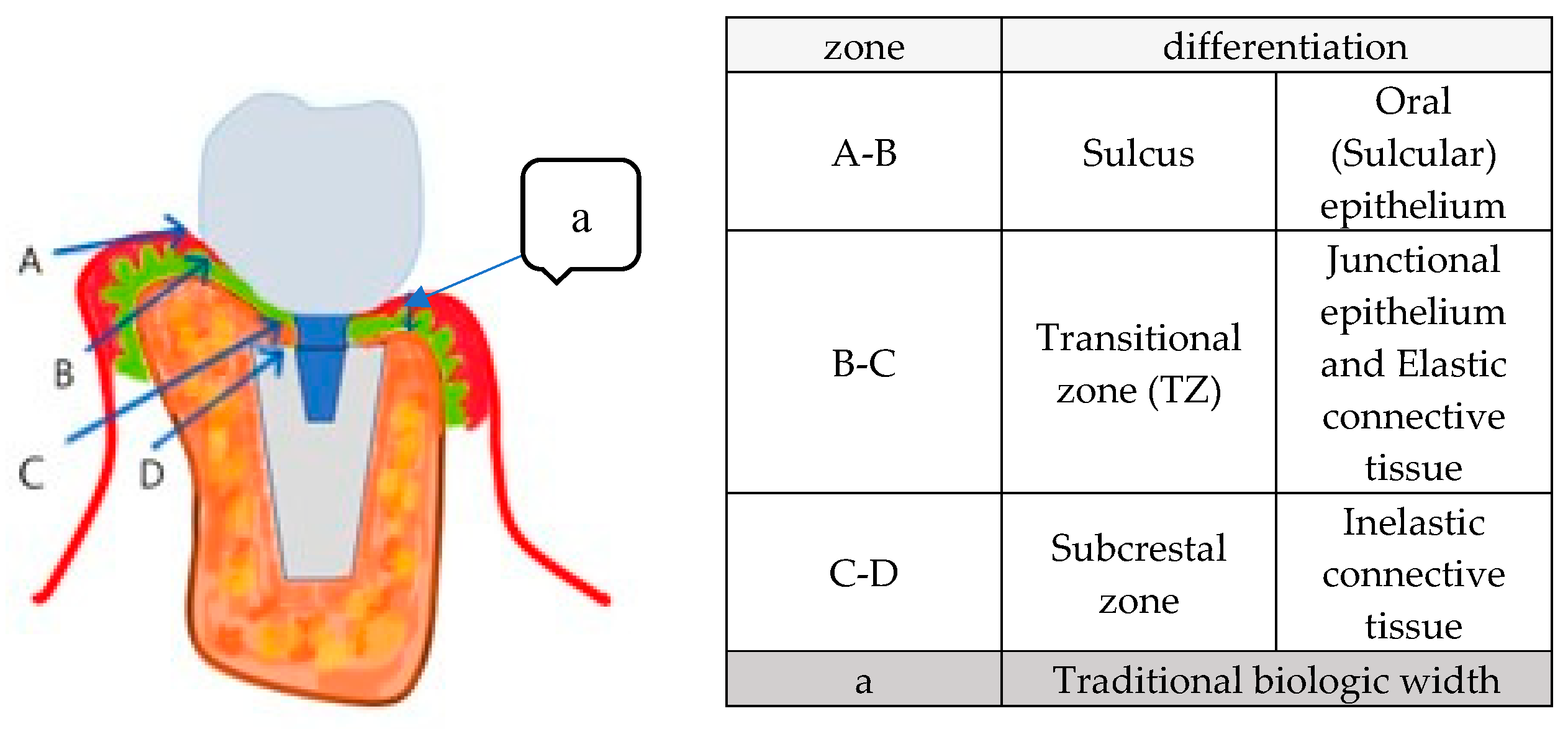
This study introduces a new biologic width model for implants, categorizing submucosal peri-implant soft tissue into three distinct zones: (
Figure 13)
This schematic model integrates real X-ray images and illustrations, enhancing visualization of peri-implant soft tissue structure. The Transitional Zone (B-C) and Subcrestal Zone (C-D) are emphasized, highlighting their distinct configurations and clinical significance in implant success.
Additionally, this model visually incorporates traditional biologic width (A-E) for comparison.
Differences Between Epi-Crestal Placement and SPI: Considerations for CRD Optimization
The adaptation of peri-implant soft tissue varies depending on implant positioning, prosthetic design, and recipient site characteristics. Understanding these differences underscores the necessity of a comprehensive biologic width model that accounts for both vertical and horizontal peri-implant soft tissue dimensions.
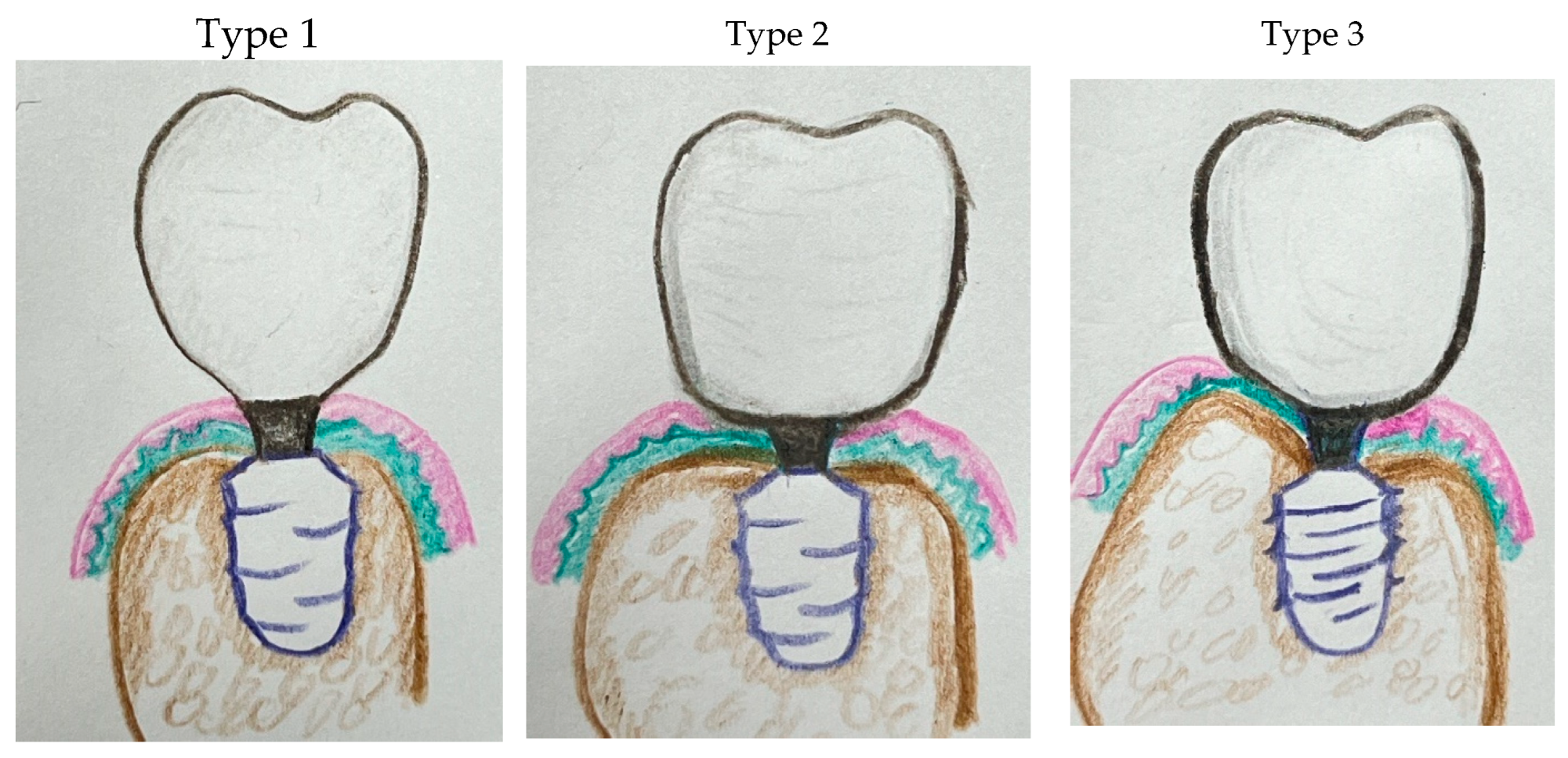
Classification of Implant Placement Approaches (Figure 14)
Type 1: Epi-Crestal Placement with Vertical Biologic Width
When the implant platform is positioned at the crestal bone without horizontal extension of the implant restoration in contact with the underlying mucosa, the peri-implant soft tissue aligns vertically, forming what is traditionally referred to as biologic width. The soft tissue primarily consists of vertically oriented connective tissue and epithelial layers. Because no coronal flaring occurs at the submucosal level, the resulting emergence profile is less natural in appearance.
A slender prosthetic design is typically required to minimize soft tissue compression, and a large CRD is created to facilitate easier hygiene maintenance.
Type 2: Epi-Crestal Placement with Horizontal Extension Generating a Transitional Zone (TZ)
When the implant platform is placed at the crestal bone level with horizontal extension of the implant restoration in contact with the underlying mucosa, the coronal flaring extends the soft tissue attachment laterally, generating a horizontal component within the biologic width. This adaptation stabilizes the peri-implant soft tissue and results in the formation of a Transitional Zone (TZ).
Compared to Type 1, this configuration enhances the natural emergence profile while maintaining biologic function. However, it requires sufficient crestal bone width to support the extended interface between the implant restoration and the soft tissue.
Biological Implications of SPI with CRD Optimization
Enhances vascularization and mechanical support.
Reduces the risk of epithelial downgrowth and peri-implant mucositis.
Provides long-term stability, particularly in cases with thin crestal bone, where additional soft tissue volume serves as a protective barrier.
This figure illustrates three distinct types of peri-implant soft tissue adaptation, categorized by implant placement depth and the morphology of the implant restoration:
Type 1: Epi-Crestal Placement with Vertical Biologic Width – The implant is positioned at the crestal bone level without horizontal extension of the restoration in contact with the underlying mucosa. This results in a vertically aligned peri-implant soft tissue seal, forming a classical biologic width composed of connective tissue and epithelial layers. A slender restoration design is typically required to reduce soft tissue compression and facilitate hygiene maintenance.
Type 2: Epi-Crestal Placement with Horizontal Extension Forming a Transitional Zone (TZ) – The implant is placed at the crestal bone level with horizontal extension of the implant restoration in contact with the underlying mucosa. This coronal flaring extends the soft tissue attachment laterally, forming a Transitional Zone (TZ) that enhances soft tissue stabilization. This configuration provides a more natural emergence profile than Type 1 while maintaining biologic function, but it requires adequate crestal bone width for optimal support.
Type 3: Subcrestal Placement (SPI) with Subcrestal Zone (SZ) Formation – The implant is positioned below the crestal bone level, promoting peri-implant soft tissue adaptation in both vertical and horizontal dimensions. This leads to the formation of both a Transitional Zone (TZ) and a Subcrestal Zone (SZ), contributing to enhanced soft tissue sealing and biologic stability. Proper CRD management and optimized Soft Tissue Thickness (STT) are critical for maintaining peri-implant health and preventing complications such as peri-implant mucositis.
These models highlight the structural differences in peri-implant soft tissue adaptation and emphasize the importance of CRD optimization in achieving long-term implant stability.
- 7.
Summary of Key Findings
In the presented case of a subcrestally placed implant (SPI) in the upper left first molar region, the peri-implant soft tissue interposed between the crestal bone and the implant restoration emerged as a critical factor in maintaining peri-implant health. Initially, probing depths exceeding 3 mm indicated compromised peri-implant conditions. Following interventions to optimize soft tissue thickness and adjust the CRD within an optimal range, the probing depth improved to less than 1 mm, and this stability was consistently maintained over time.
These findings underscore the vital importance of maintaining CRD within a critical range. When CRG exceeds a certain threshold, the soft tissue overlying the crestal bone may transform into oral epithelium, leading to the formation of a pocket prone to bacterial colonization and inflammation. Conversely, by optimizing CRD, epithelial downgrowth was prevented, and the peri-implant soft tissue remained stable.
This case highlights the proposed roles of the TZ and SC as structural components essential for peri-implant health.
Figure 12 illustrates a temporary submucosal swelling observed during the removal of the prosthesis for repair (
Figure 12). This swelling reflects the hydraulic positive pressure and resilience of the connective tissue within the TZ, suggesting a self-sustaining nature that maintains soft tissue integrity and prevents external irritation.
Interestingly, the palatal soft tissue remained unaffected by the implant restoration (
Figure 15). This may be attributed to the unique characteristics of palatal tissue, including its abundant blood supply and keratinized nature, which contribute to its resilience and stability despite external modifications [
40].
Ultimately, this case highlights the importance of precisely modulating peri-implant soft tissue dimensions. By maintaining an optimal CRD and promoting the formation of TZ and SZ, clinicians can enhance peri-implant health, reduce complications such as inflammation and mucositis, and ensure both functional and esthetic stability. These findings offer valuable insights for integrating advanced peri-implant soft tissue management strategies into clinical practice.
Future research should aim to further elucidate the structural and functional roles of the peri-implant soft tissue and establish standardized guidelines for its management.
A comparison of palatal mucosal thickness at three vertical levels (from apical to crestal) between 2021 and 2024. The data demonstrates minimal changes in palatal mucosal thickness over time, suggesting that the palatal soft tissue remains stable despite external influences. This thickness stability may contribute to its strong sealing capability, providing an optimal peri-implant environment in the maxilla.
- 8.
Future Directions
- 1.
Histologic Analysis of Peri-Implant Soft Tissues in SPIFurther histologic studies are required to provide detailed insights into the junctional epithelium and connective tissue adaptations in subcrestally placed implants (SPIs). These studies should analyze how soft tissue interacts with implant surfaces and its role in maintaining peri-implant health. Given the challenges associated with direct histologic validation in human subjects, alternative methodologies, such as advanced imaging techniques and computational modeling, may also be explored to enhance our understanding.
- 2.
Standardization of CRD Measurements for SPILarge-scale clinical studies are necessary to establish standardized CRD measurement protocols for SPIs. A systematic approach to defining optimal CRD parameters for Transitional Zone (TZ) and Subcrestal Zone (SZ) formation would improve clinical assessments and long-term peri-implant health. By analyzing a broader patient population, future research can refine these parameters and develop evidence-based guidelines for implant placement and prosthetic design.
- 3.
Advancing Prosthodontic Design Based on Bony ArchitectureFuture prosthodontic designs should transition from empirical approaches to data-driven methodologies tailored to individual bony architecture. The integration of Cone Beam Computed Tomography (CBCT) and STL file technology could allow for customized restorations that optimize both esthetic and functional outcomes. Computational simulations and machine learning applications may further enhance precision in predicting peri-implant soft tissue behavior.
- 4.
Establishing a Logical Framework for TZ and SZWhile this case report introduces a novel perspective on peri-implant soft tissue adaptation, its findings are derived from a single case and require validation through larger-scale studies. The proposed concepts of the Transitional Zone (TZ) and Subcrestal Zone (SZ) must be tested across various clinical scenarios to determine their reproducibility and clinical relevance. Logical reasoning serves as a fundamental tool in shaping these research directions, forming hypotheses, and refining clinical applications. By systematically integrating empirical data with structured theoretical frameworks, future investigations can strengthen the scientific foundation for peri-implant soft tissue management, ensuring long-term implant success.
A comprehensive research approach that combines histologic, clinical, and theoretical validation will refine peri-implant soft tissue management, ultimately advancing implant dentistry and improving patient outcomes.
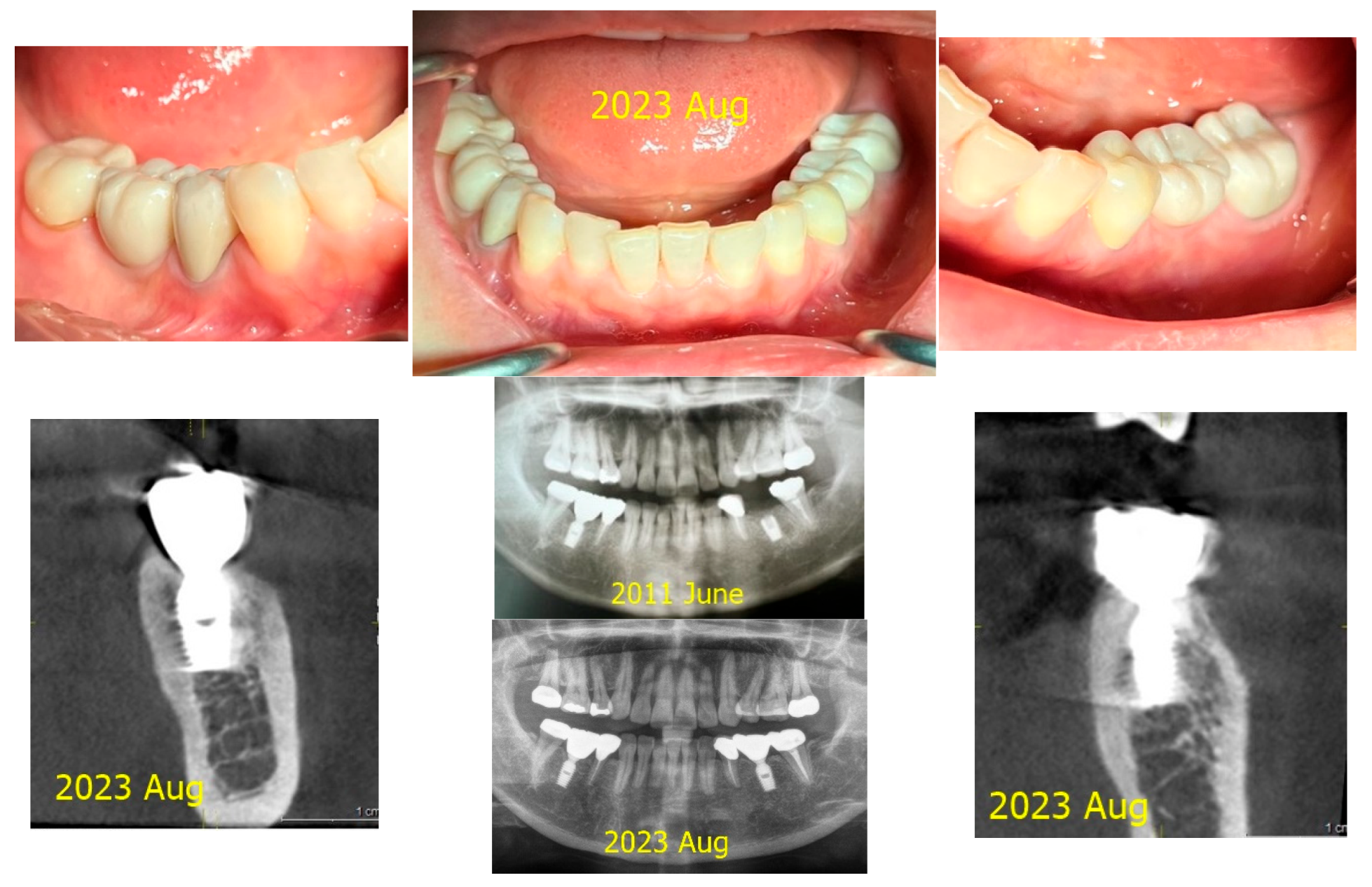
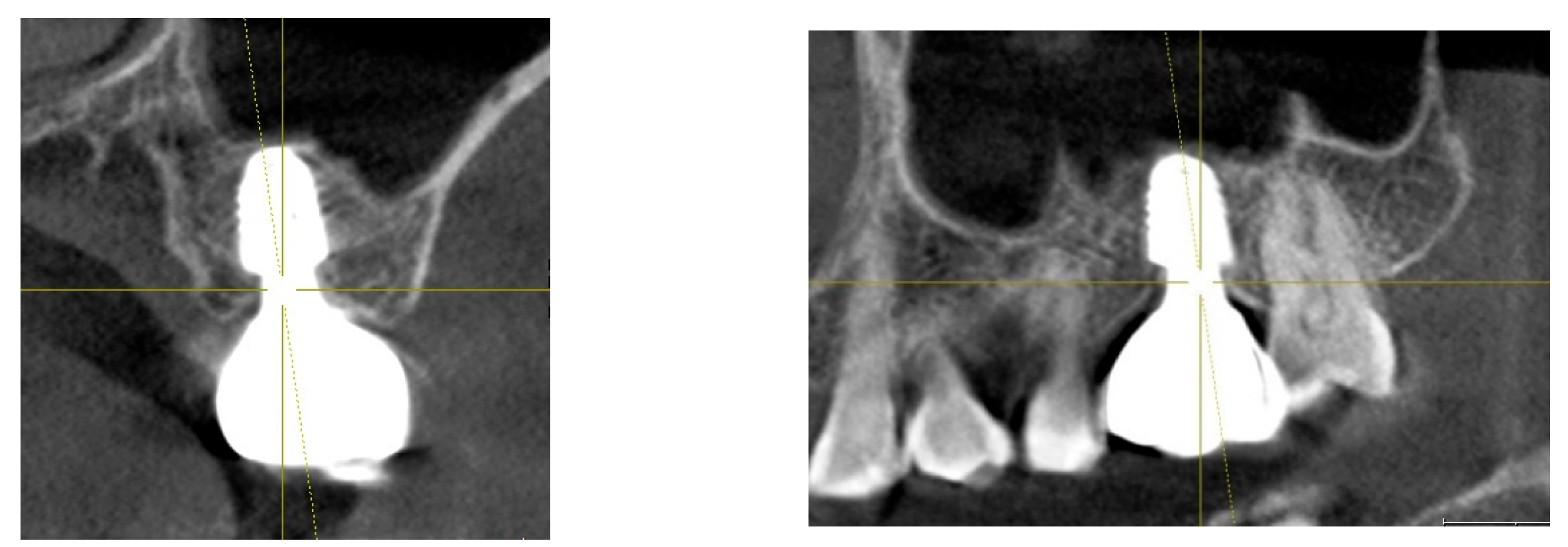
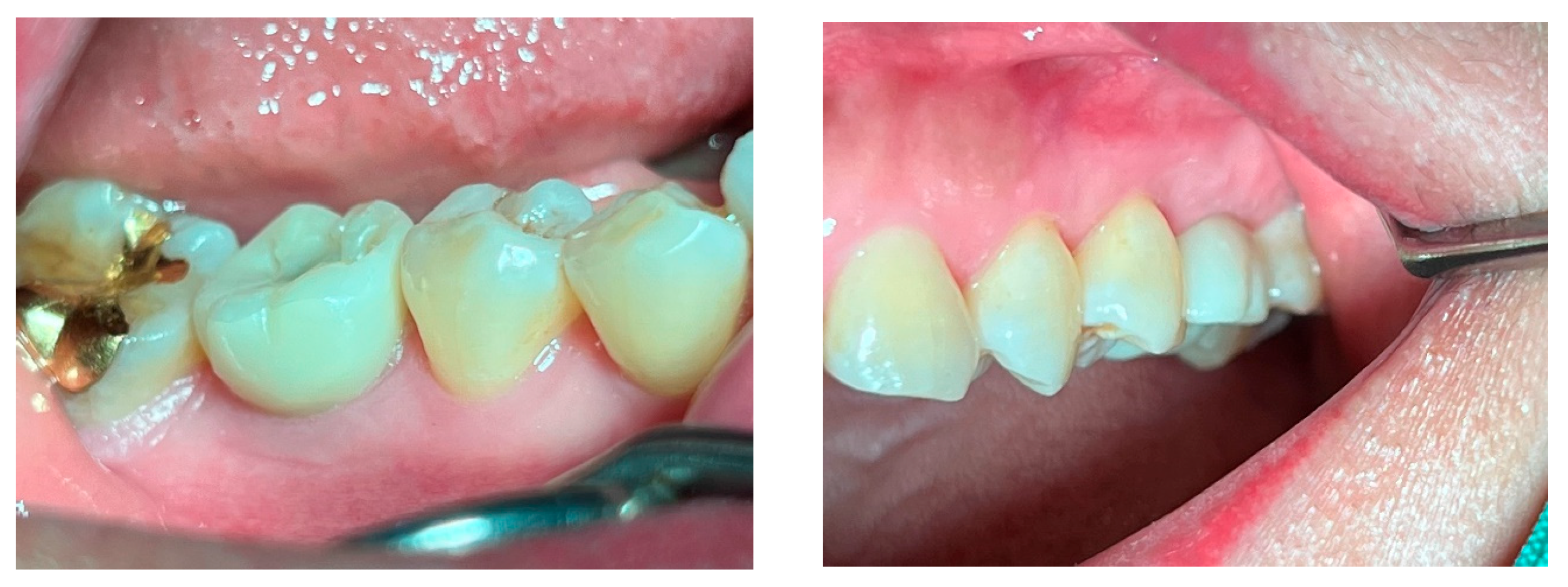
Figure 1.
Results after 10 years of subcrestally placed implants (SPI) – Fixtures were placed subcrestally, with X-rays showing no crestal bone loss and clinical photos demonstrating healthy peri-implant soft tissue and a natural emergence profile. This approach reflects a trend seen with Bicon implants during the 2000s to 2010s, where implant fixtures were often placed slightly deeper than those of other systems to support lasting stability.
Figure 1.
Results after 10 years of subcrestally placed implants (SPI) – Fixtures were placed subcrestally, with X-rays showing no crestal bone loss and clinical photos demonstrating healthy peri-implant soft tissue and a natural emergence profile. This approach reflects a trend seen with Bicon implants during the 2000s to 2010s, where implant fixtures were often placed slightly deeper than those of other systems to support lasting stability.
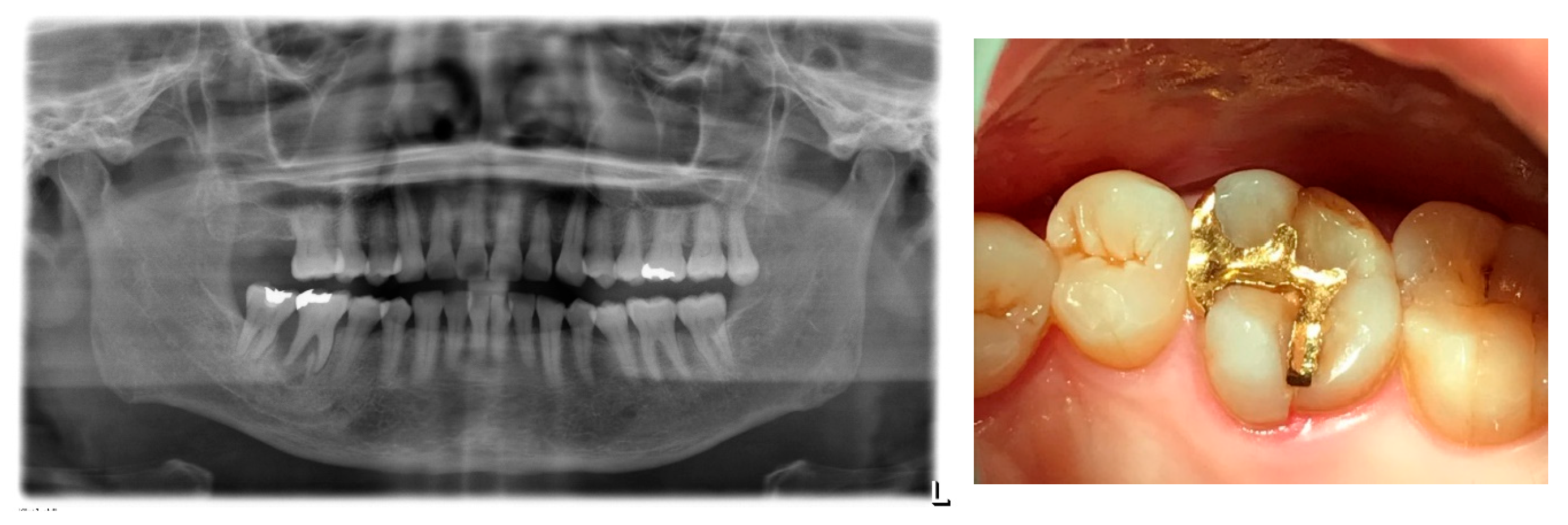
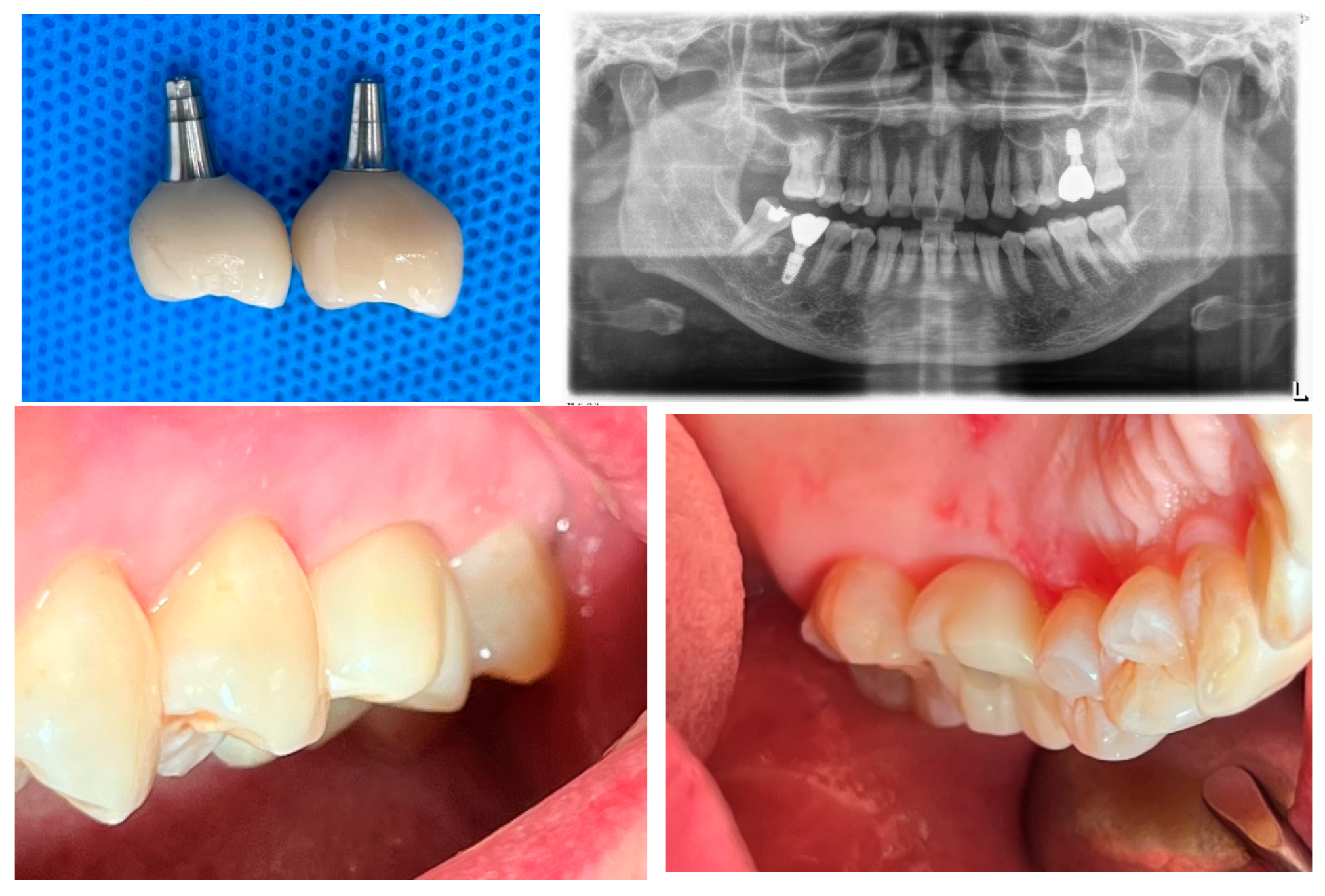
Figure 2.
Initial radiograph and clinical image of the upper left first molar on the patient’s first visit (September 22, 2020). The radiograph illustrates the condition of the tooth and surrounding structures. The clinical image provides a clear view of the crown and root fracture prior to extraction.
Figure 2.
Initial radiograph and clinical image of the upper left first molar on the patient’s first visit (September 22, 2020). The radiograph illustrates the condition of the tooth and surrounding structures. The clinical image provides a clear view of the crown and root fracture prior to extraction.
Figure 3.
October 29, 2020 – Implant placement at the upper left first molar site with sinus lift and bone grafting surgery.
Figure 3.
October 29, 2020 – Implant placement at the upper left first molar site with sinus lift and bone grafting surgery.
Figure 4.
March 25, 2021 – Restoration completed using a 4 mm gingival cuff abutment.
Figure 4.
March 25, 2021 – Restoration completed using a 4 mm gingival cuff abutment.
Figure 5.
October 31, 2022 The restoration was remade with gingival cuff 3 mm abutment.
Figure 5.
October 31, 2022 The restoration was remade with gingival cuff 3 mm abutment.
Figure 6.
The upper left image presents a comparison of the two restorations before and after the revision, while the upper right image shows a panoramic X-ray taken post-revision. The lower two images display the clinical appearance of the upper left first molar implant restoration following the revision procedure conducted on October 31, 2022.
Figure 6.
The upper left image presents a comparison of the two restorations before and after the revision, while the upper right image shows a panoramic X-ray taken post-revision. The lower two images display the clinical appearance of the upper left first molar implant restoration following the revision procedure conducted on October 31, 2022.
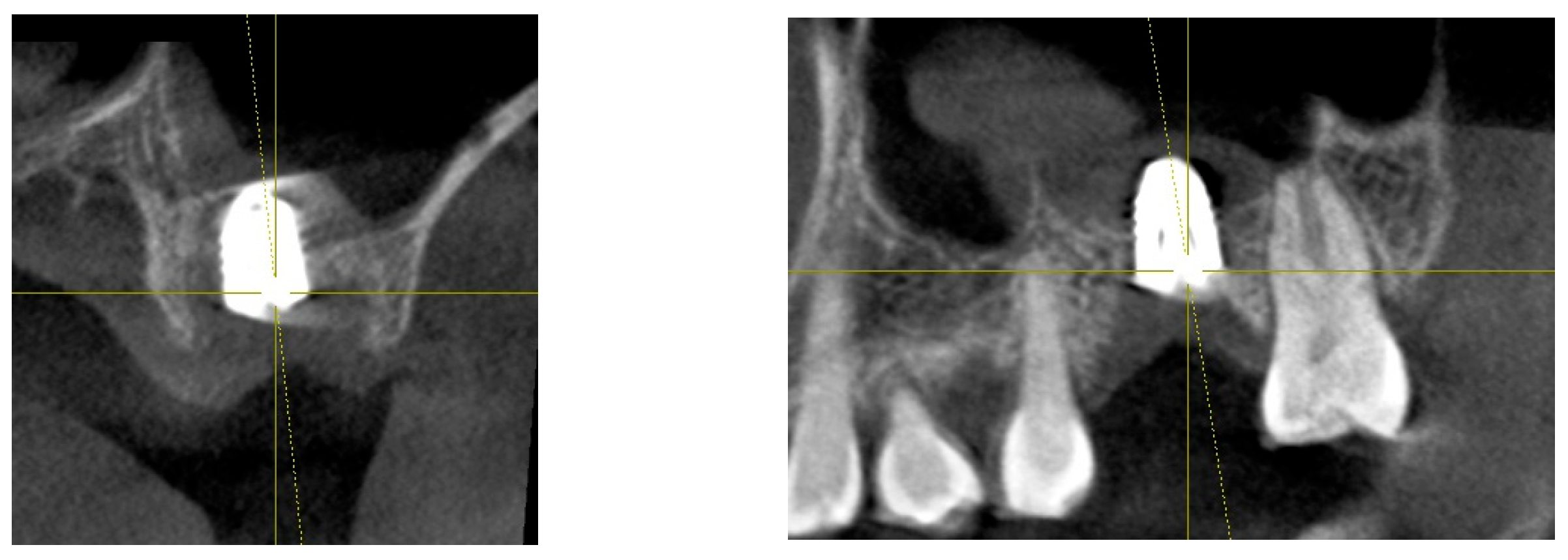
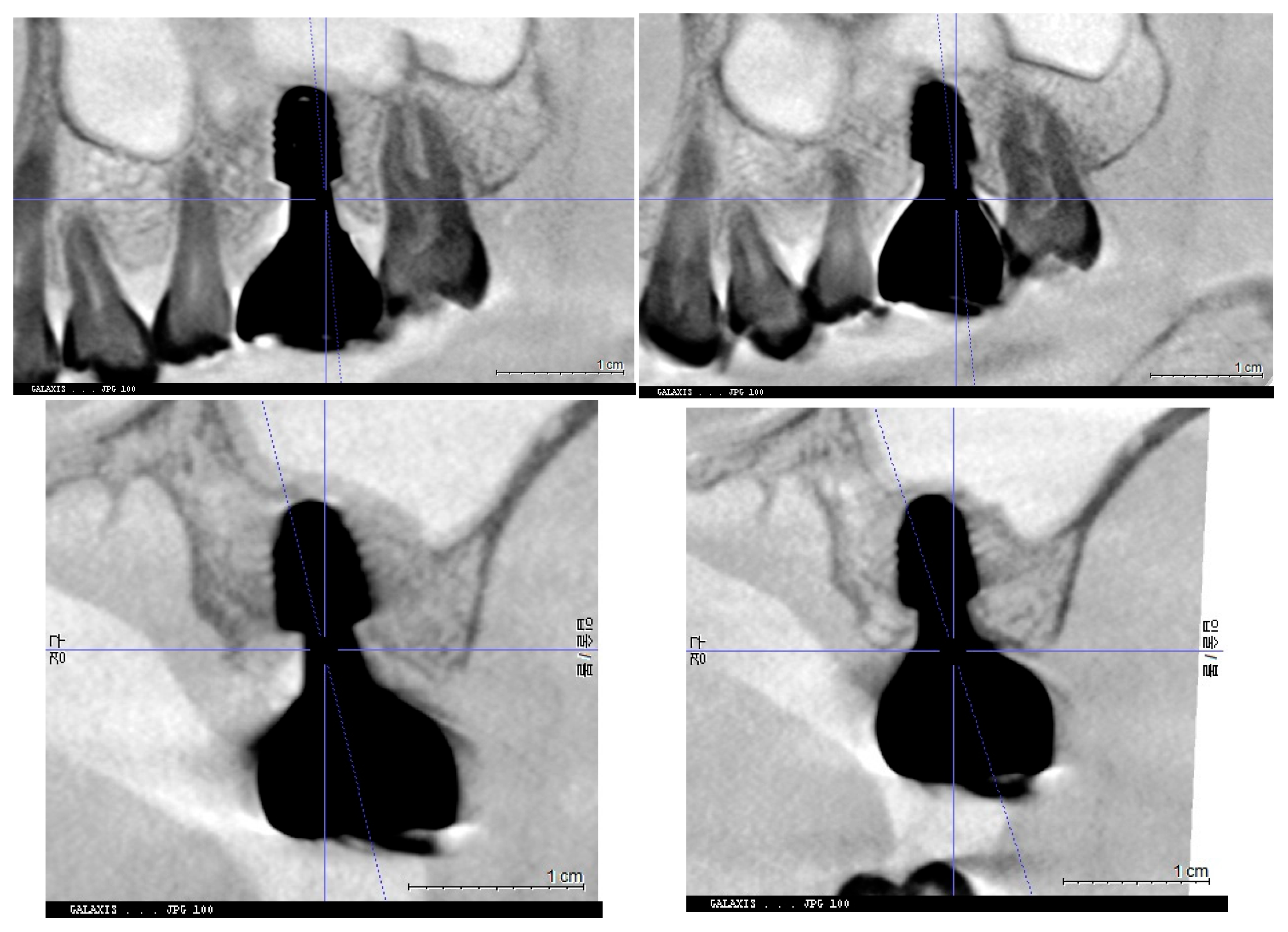
Figure 7.
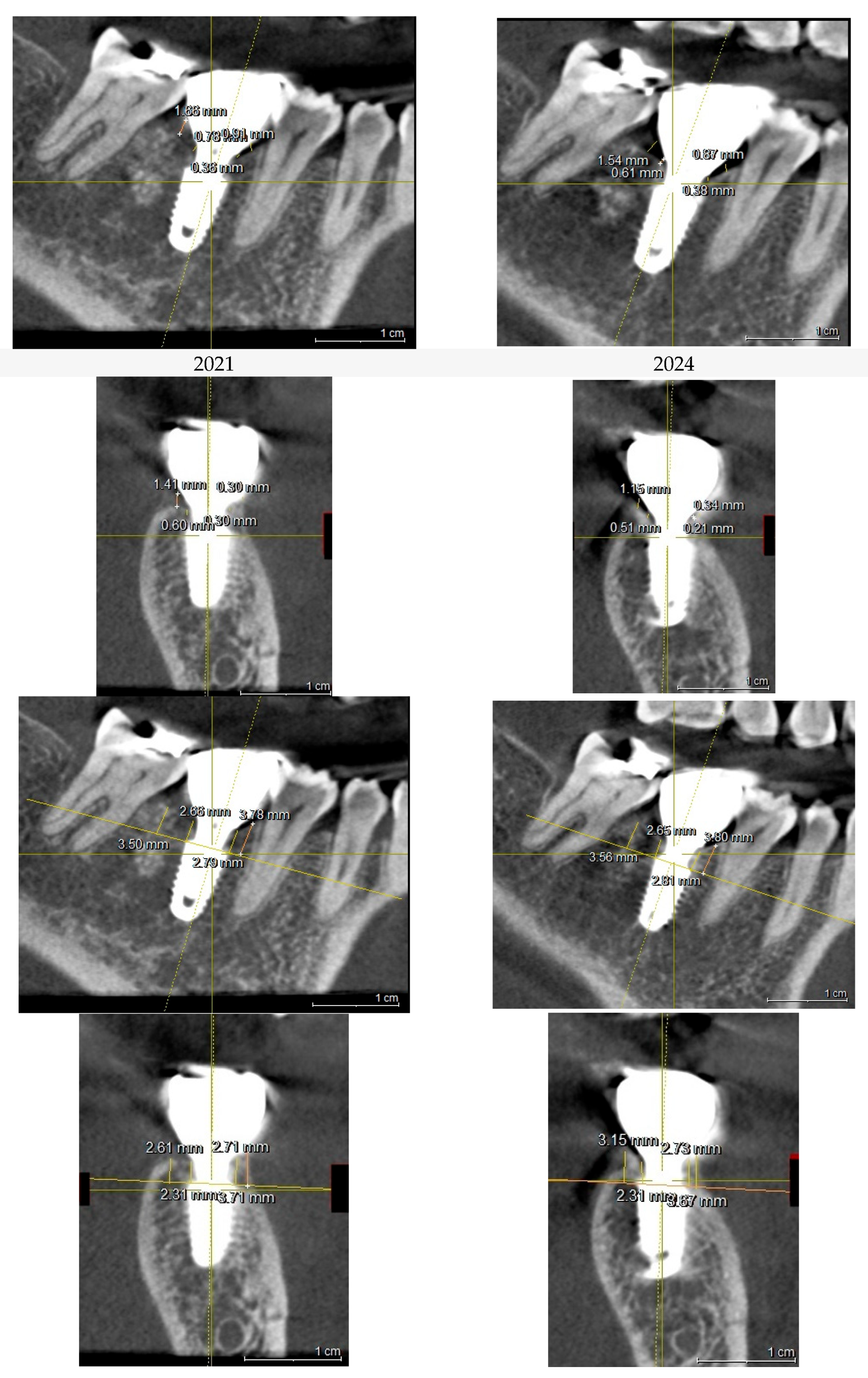
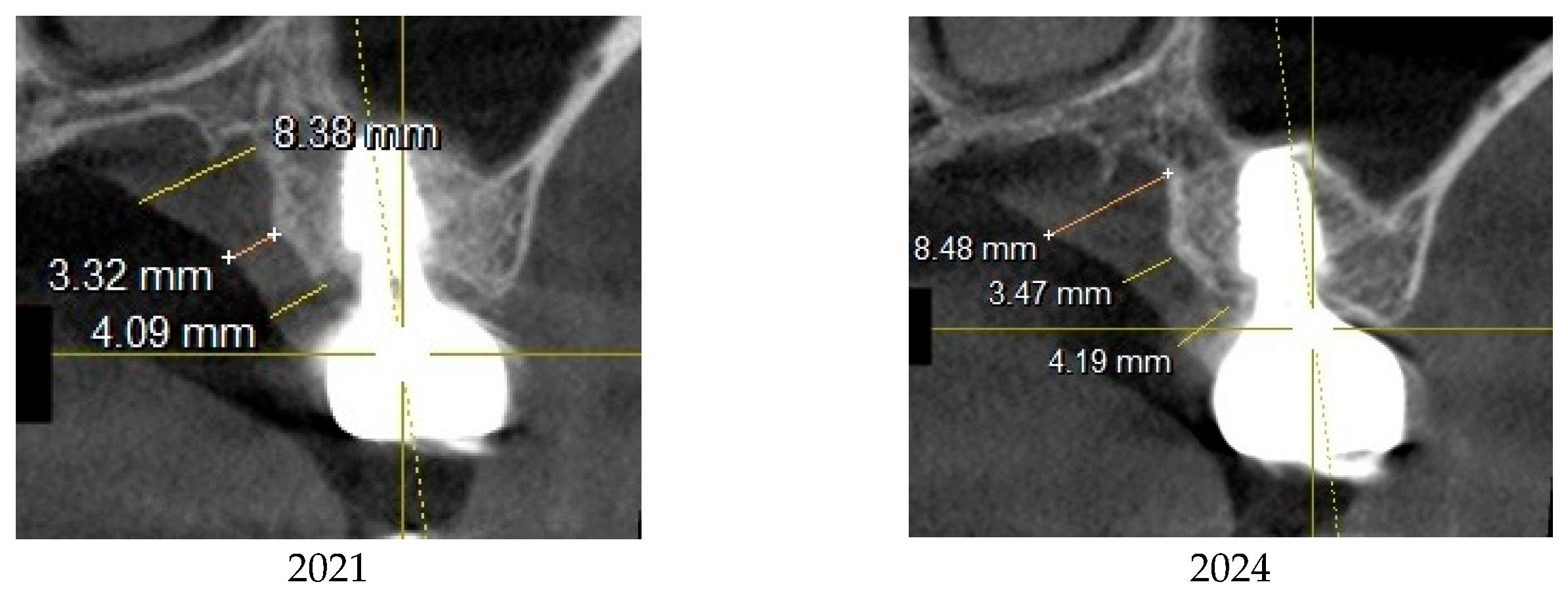
This image illustrates the X-ray measurements conducted on the lower right first molar implant, comparing data from 2021 and 2024.
Figure 7.
This image illustrates the X-ray measurements conducted on the lower right first molar implant, comparing data from 2021 and 2024.
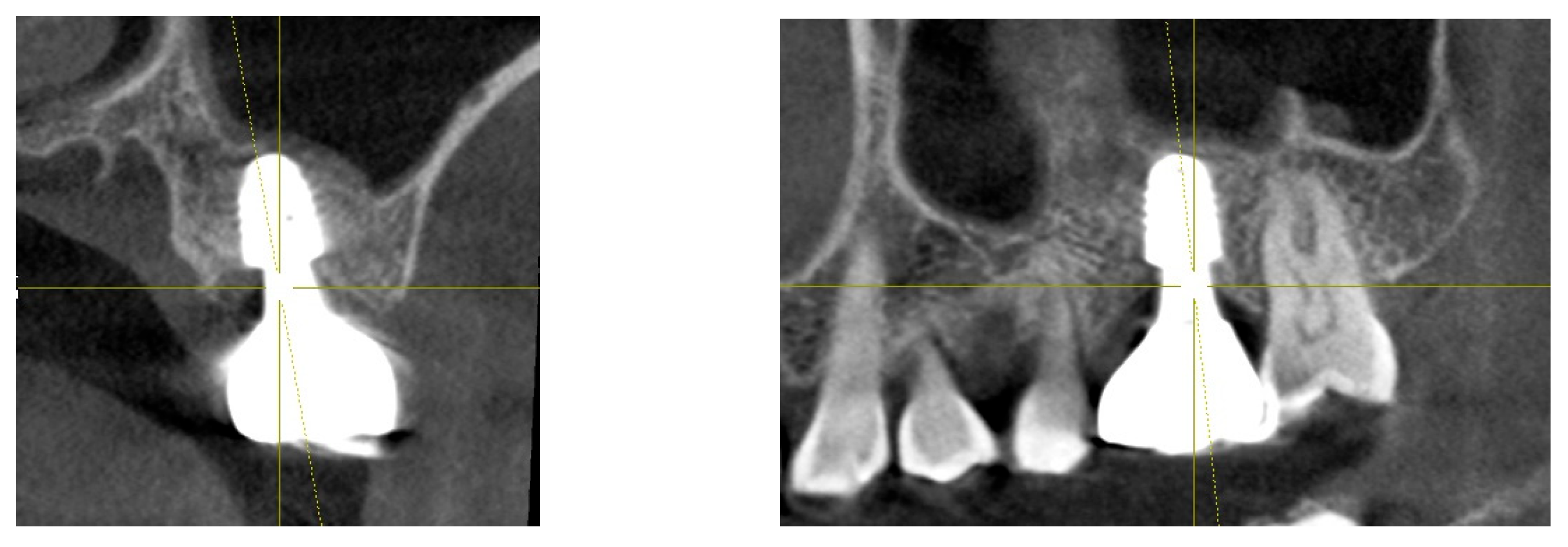
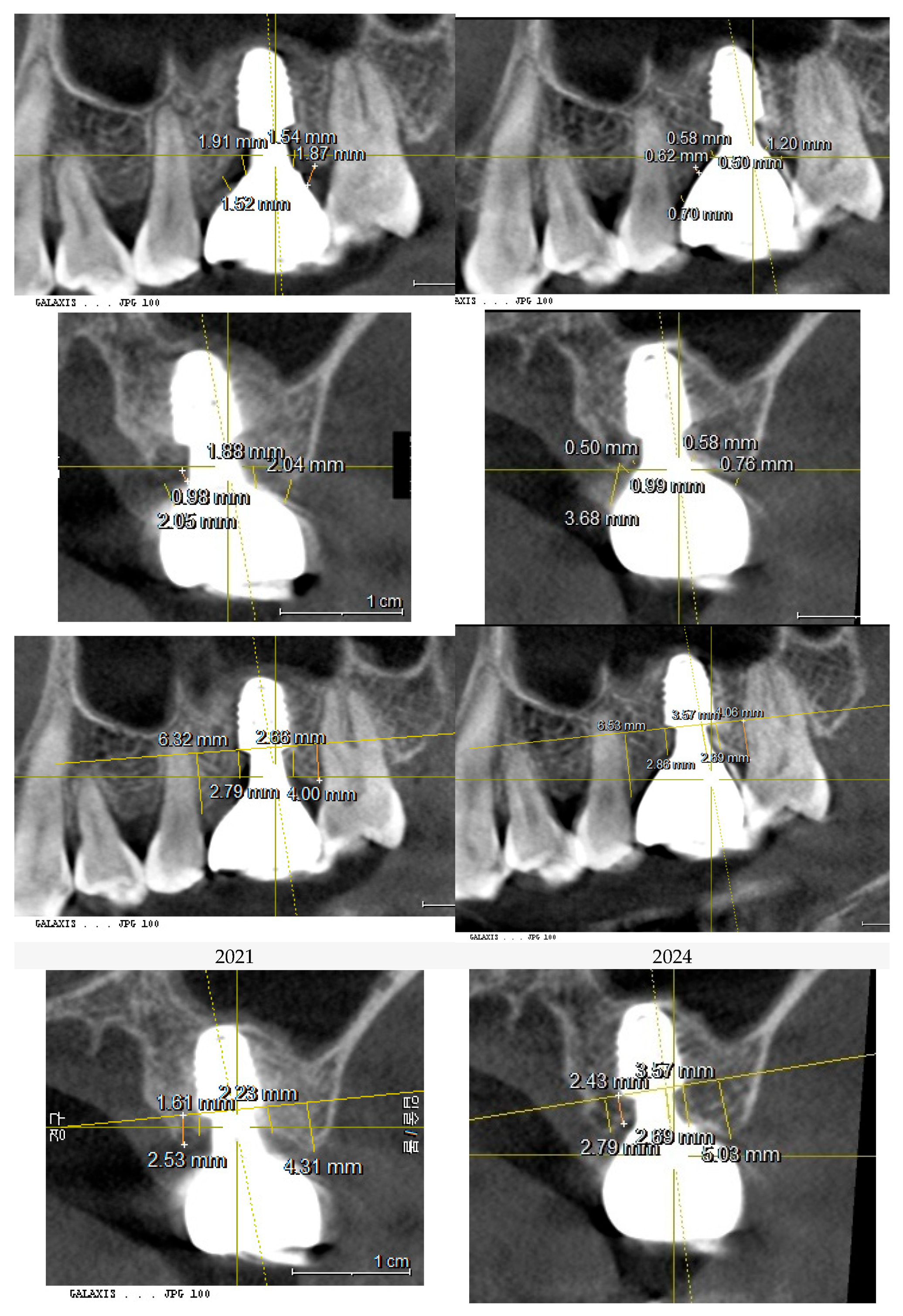
Figure 8.
This image illustrates the X-ray measurements conducted on the upper left first molar implant, comparing data from 2021 and 2024..
Figure 8.
This image illustrates the X-ray measurements conducted on the upper left first molar implant, comparing data from 2021 and 2024..
Figure 9.
Clinical images of control and experimental sites post-revision.
Figure 9.
Clinical images of control and experimental sites post-revision.
Figure 10.
Comparison of X-rays taken in October 2021 and July 2024 after the restoration revision, demonstrating a decrease in the CRD. When the CRD is maintained within the critical range, it can be inferred that the gap space is occupied exclusively by peri-implant soft tissue, without voids, and the GRD can be represented as Soft Tissue Thickness (STT).
Figure 10.
Comparison of X-rays taken in October 2021 and July 2024 after the restoration revision, demonstrating a decrease in the CRD. When the CRD is maintained within the critical range, it can be inferred that the gap space is occupied exclusively by peri-implant soft tissue, without voids, and the GRD can be represented as Soft Tissue Thickness (STT).
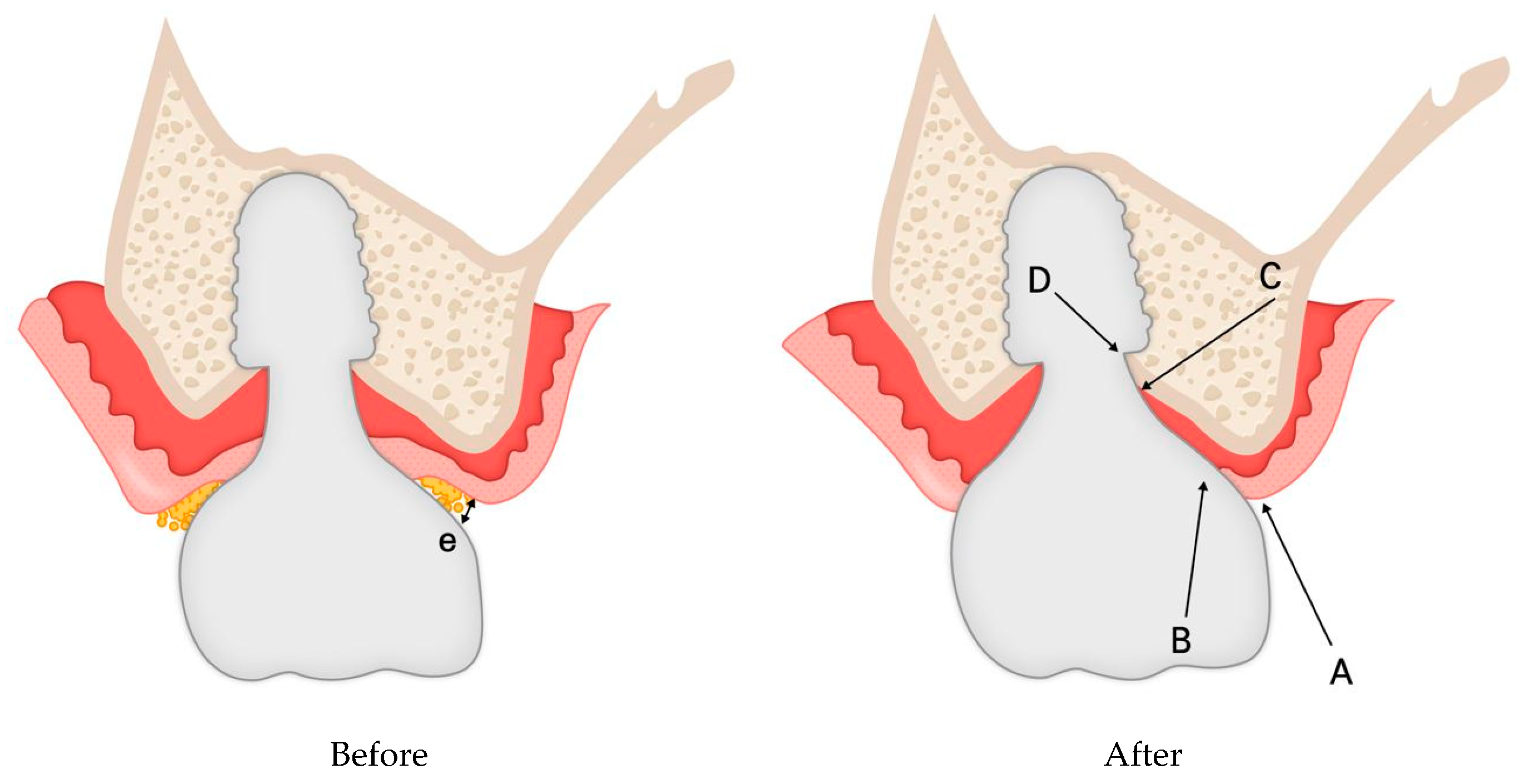
Figure 11.
Hypothetical schematic representation of histologic changes in peri-implant soft tissue following restoration modification. Before modification, the Crest to Restoration Distance (CRD) contained voids (pockets), sulcular epithelium, and connective tissue. After modification, it is hypothesized that the CRD was restructured into a stable peri-implant soft tissue barrier, consisting of sulcular epithelium, junctional epithelium, and connective tissue, reinforcing the biological seal and reducing the risk of peri-implant inflammation.
Figure 11.
Hypothetical schematic representation of histologic changes in peri-implant soft tissue following restoration modification. Before modification, the Crest to Restoration Distance (CRD) contained voids (pockets), sulcular epithelium, and connective tissue. After modification, it is hypothesized that the CRD was restructured into a stable peri-implant soft tissue barrier, consisting of sulcular epithelium, junctional epithelium, and connective tissue, reinforcing the biological seal and reducing the risk of peri-implant inflammation.
Figure 12.
Submucosal Swelling and Hydraulic Pressure in the Connective Tissue.
Figure 12.
Submucosal Swelling and Hydraulic Pressure in the Connective Tissue.
Figure 13.
provides a schematic drawing that differentiates submucosal peri-implant soft tissue into the three distinct zones:.
Figure 13.
provides a schematic drawing that differentiates submucosal peri-implant soft tissue into the three distinct zones:.
Figure 14.
Schematic Models of Peri-Implant Soft Tissue Adaptation Based on Implant Placement Depth and Restoration Design.
Figure 14.
Schematic Models of Peri-Implant Soft Tissue Adaptation Based on Implant Placement Depth and Restoration Design.
Figure 15.
Palatal Mucosal Thickness Over Time.
Figure 15.
Palatal Mucosal Thickness Over Time.
Table 1.
presents the measurements of the Crest to Restoration Distance (CRD), which quantifies the vertical distance between the implant restoration and the crestal bone. The values are divided into central CRD (cCRD) and peripheral CRD (pCRD) to distinguish the dimensional variations along the implant interface. These measurements are taken at both central and peripheral locations, evaluating the mesial (M), distal (D), buccal (B), and lingual (L) aspects around the implants of the upper left first molar and lower first molar. The data were collected from the CBCT scan conducted on October 26, 2021.
Table 1.
presents the measurements of the Crest to Restoration Distance (CRD), which quantifies the vertical distance between the implant restoration and the crestal bone. The values are divided into central CRD (cCRD) and peripheral CRD (pCRD) to distinguish the dimensional variations along the implant interface. These measurements are taken at both central and peripheral locations, evaluating the mesial (M), distal (D), buccal (B), and lingual (L) aspects around the implants of the upper left first molar and lower first molar. The data were collected from the CBCT scan conducted on October 26, 2021.
| |
Upper left 1st molar |
Lower right 1st molar |
| cCRD |
pCRD |
cCRD |
pCRD |
| M |
1.52 |
1.91 |
0.36 |
0.91 |
| D |
1.54 |
1.87 |
0.78 |
1.66 |
| B |
1.88 |
2.04 |
0.3 |
0.3 |
| L |
0.98 |
2.05 |
0.6 |
1.41 |
| Average |
1.48 |
1.97 |
0.51 |
1.07 |
Table 2.
Summary of CRD measurements in 20 cases of subcrestally placed implants, as analyzed by Won. The average pCRD was 0.6 mm, and the average cCRD was 0.3 mm.
Table 2.
Summary of CRD measurements in 20 cases of subcrestally placed implants, as analyzed by Won. The average pCRD was 0.6 mm, and the average cCRD was 0.3 mm.
| |
cCRD |
pCRD |
| |
cCRD/M |
cCRD/D |
cCRD/B |
cCRD/L |
pCRD/M |
pCRD/D |
pCRD/B |
pCRD/L |
| average |
0.4 |
0.2 |
0.2 |
0.2 |
0.8 |
0.6 |
0.4 |
0.5 |
| |
0.3 |
0.6 |
Table 3.
Changes in CRD from 2021 to 2024 at the peripheral and central areas for the control site (the lower right first molar implant).
Table 3.
Changes in CRD from 2021 to 2024 at the peripheral and central areas for the control site (the lower right first molar implant).
| pCRD |
2021 |
2024 |
changes |
| M |
0.91 |
0.87 |
-0.04 |
| D |
1.66 |
1.54 |
-0.12 |
| B |
0.3 |
0.34 |
0.04 |
| L |
1.41 |
1.15 |
-0.26 |
| average |
1.07 |
0.98 |
-0.10 |
| cCRD |
2021 |
2024 |
changes |
| M |
0.36 |
0.38 |
0.02 |
| D |
0.78 |
0.61 |
-0.17 |
| B |
0.3 |
0.21 |
-0.09 |
| L |
0.6 |
0.51 |
-0.09 |
| average |
0.51 |
0.43 |
-0.08 |
Table 4.
Changes in Depth of Placement (DP) from 2021 to 2024 at the peripheral and central areas for the control Site (the lower right first molar implant) The DP changes over time indicate variations in crestal bone level, signifying potential bone loss or gain around the implant.
Table 4.
Changes in Depth of Placement (DP) from 2021 to 2024 at the peripheral and central areas for the control Site (the lower right first molar implant) The DP changes over time indicate variations in crestal bone level, signifying potential bone loss or gain around the implant.
| pDP |
2021 |
2024 |
changes |
| M |
3.78 |
3.8 |
0.02 |
| D |
3.5 |
3.56 |
0.06 |
| B |
3.71 |
3.67 |
-0.04 |
| L |
2.61 |
3.15 |
0.54 |
| average |
3.4 |
3.55 |
0.15 |
| cDP |
2021 |
2024 |
changes |
| M |
2.79 |
2.81 |
0.02 |
| D |
2.65 |
2.65 |
0 |
| B |
2.71 |
2.73 |
0.02 |
| L |
2.31 |
2.31 |
0 |
| average |
2.62 |
2.63 |
0.01 |
Table 5.
Clinical parameters for soft tissue condition at the lower right first molar implant. This table presents the clinical assessment of soft tissue health around the lower right first molar implant, including Implant Paper Point Probing (IPPP), Bleeding on Probing (BOP), redness, and swelling, demonstrating a healthy soft tissue state.
Table 5.
Clinical parameters for soft tissue condition at the lower right first molar implant. This table presents the clinical assessment of soft tissue health around the lower right first molar implant, including Implant Paper Point Probing (IPPP), Bleeding on Probing (BOP), redness, and swelling, demonstrating a healthy soft tissue state.
| Depth of Probing |
BOP |
redness |
swelling |
| Less than 1 mm |
no |
no |
no |
Table 6.
Table 6. Changes in CRD from 2021 to 2024 at the peripheral and central areas for the treatment site (the upper left first molar implant).
Table 6.
Table 6. Changes in CRD from 2021 to 2024 at the peripheral and central areas for the treatment site (the upper left first molar implant).
| pCRD |
2021 |
2024 |
changes |
| M |
1.91 |
0.58 |
-1.33 |
| D |
1.87 |
1.2 |
-0.67 |
| B |
2.04 |
0.76 |
-1.28 |
| L |
2.05 |
0.99 |
-1.06 |
| average |
1.97 |
0.88 |
-1.09 |
| cCRD |
2021 |
2024 |
changes |
| M |
1.52 |
0.62 |
-0.90 |
| D |
1.54 |
0.5 |
-1.04 |
| B |
1.88 |
0.58 |
-1.3 |
| L |
0.98 |
0.5 |
-0.48 |
| average |
1.48 |
0.55 |
-0.93 |
Table 7.
Changes in Depth of Placement (DP) from 2021 to 2024 at the peripheral and central areas for the treatment site (the upper left first molar implant) The DP changes over time indicate variations in crestal bone level, signifying potential bone loss or gain around the implant.
Table 7.
Changes in Depth of Placement (DP) from 2021 to 2024 at the peripheral and central areas for the treatment site (the upper left first molar implant) The DP changes over time indicate variations in crestal bone level, signifying potential bone loss or gain around the implant.
| pDP |
2021 |
2024 |
changes |
| M |
6.32 |
6.53 |
0.21 |
| D |
4.00 |
4.06 |
0.06 |
| B |
4.31 |
5.03 |
0.72 |
| L |
1.61 |
2.79 |
1.18 |
| average |
4.06 |
4.60 |
0.54 |
| cDP |
2021 |
2024 |
changes |
| M |
2.79 |
2.86 |
0.07 |
| D |
2.66 |
2.69 |
0.03 |
| B |
2.23 |
3.57 |
1.34 |
| L |
2.53 |
2.43 |
-0.1 |
| average |
2.55 |
2.89 |
0.34 |
Table 8.
Clinical parameters for soft tissue condition at the upper left first molar implant. This table presents the clinical assessment of soft tissue health around the upper left first molar implant, including Implant Paper Point Probing (IPPP), Bleeding on Probing (BOP), redness, and swelling, demonstrating a healthy soft tissue state after revision.
Table 8.
Clinical parameters for soft tissue condition at the upper left first molar implant. This table presents the clinical assessment of soft tissue health around the upper left first molar implant, including Implant Paper Point Probing (IPPP), Bleeding on Probing (BOP), redness, and swelling, demonstrating a healthy soft tissue state after revision.
| Depth of Probing |
BOP |
redness |
swelling |
| Less than 1 mm |
no |
no |
no |
Table 9.
Summary of the comparison for the average changes between the control site (lower right molar) and the treatment site (upper left molar) for CRD.
Table 9.
Summary of the comparison for the average changes between the control site (lower right molar) and the treatment site (upper left molar) for CRD.
| |
Control site |
Treated site |
| The average change in pCRD |
-0.10 |
-1.09 |
| The average change in cCRD |
-0.08 |
-0.93 |