1. Introduction
The giant honey bee (
Apis dorsata F.) is an economically important species as a crop pollinator in Pakistan, South Asia, and South-East Asia [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. This honey bee lives in the Asian tropics; the colony produces a large quantity of honey and beeswax in a single comb that is several square meters in area, suspended from a strong lateral support in the open, and the honey and beeswax are harvested by local communities [
1].
In Pakistan, Apis dorsata contributed 3-35% of the total honey production [
14]. In recent years, the number of colonies of this honey bee has markedly declined [
7]. The main reason for such a decline is the erosion of nesting sources and traditional destructive methods of honey hunting. The latter practice involves a method of colony smoking/burning and destruction of combs that leads to large-scale bee mortality. It is opined that obtaining good quality honey from
A. dorsata using traditional methods of honey harvesting was not a desirable technique [
15]. This honey-hunting practice leads to the mass destruction of colonies, thus adversely affecting the indigenous pollination service. For the sustainability of pollination services and the safe honey harvest from this honey bee, proper domestication, handling and conservation of this honey bee has become very important. That prompted me to undertake these investigations.
The giant honey bee has a migratory habit. Many colonies of this honey bee migrate seasonally, living in two or more rich forage areas in the course of each year [
16,
17,
18,
19,
20,
21]. In the semi-arid environments of Northwest India, the migratory swarms of this honey bee arrive in October/November; stay there, reproduce and produce reproductive swarms, and then emigrate in mid-May [
7]. In the southernmost provinces in Vietnam, Minh Hai, Kien Giang and Hau Giang, which are west of the Mekong River,
A. dorsata migrates between mangrove forests on the coast and the swamp forests of
Melaleuca leucadendron farther inland, which seasonally produce much pollen and nectar [
1].
Recent reports have revealed that the colonies of this honey bee in the semi-arid environments of Northwest India make nests on the cliffs/projections of multi-storey buildings and the branches of tall trees. This honeybee has some fixed preferences for many nesting alternatives, including a preference for the nesting place/site, the height of the nesting site and the direction of the nest. These parameters seemed to play a decisive role in the selection of the source and the site for nesting [
22]. For example,
A. dorsata prefers smooth surfaces over the unevenly grooved surfaces for nesting. The majorities of the colonies nest at heights between 14 and 17 m, on supports with an inclination from 0° to 45°; construct their nests in an east/west direction, and choose a site that has relics of the abandoned nest. The majority of the “immigrated swarm colonies” have 100–120 cm nest length and 30–50 cm nest height. The basal thickness of the comb in the non-honey region is 2.04 ± 0.6 cm whilst in the honey region; it is 5.7 ± 1.2 cm [
22].
The giant honey bee (
A dorsata) has been bearing predatory pressure from human beings for ages, as they have been resorting to destructive methods of honey hunting against this species. The latter practice leads to the destruction of a whole colony, resulting in the death of large numbers of live bees and a large amount of brood. Therefore, there is a need to develop some non-destructive method of honey hunting for this species.
Not many reports are, however, available on the domestication and conservation of this honey bee. Rafter beekeeping with
Apis dorsata is known in some southeastern countries [
23,
24,
25,
26,
27,
28,
29,
30]. However, this practice has been done in areas where natural nesting sources for this honey bee are abundant. No such efforts have been made in areas where nesting sources of this honey bee have been depleted due to man-engineered activities. Some efforts have also been made towards the hiving of this honey bee [
15,
31], but with limited success. It was reported that annual migrations of
Apis dorsata severely affected its honey production activity
in Pakistan. Therefore, some trials to control migration in this honey bee were tested [
14]. These included re-queening of colonies with superior queens and feeding them in times of dearth. However, these trials proved futile. Some ‘attraction planks’ to capture migrating
Apis dorsata swarms were also developed and tested in Southwest India [
15]. The researcher claimed that his device had improved the method of honey harvest from this honey bee; however, the method was never in use.
The foregoing information clearly reveals that this honey bee is an important honey, beeswax and pollination service provider in the regions of its natural abode. For the sustainability of these services, domestication and conservation of this honey bee has become very important. That is why I conducted this study with three objectives; viz., i) examining the role of the giant honey bee as a crop pollination service provider, ii) testing the efficacy of some nesting devices for alluring and domestication of migratory swarms of this honey bee, iii) devising some suitable method for the safe handling of this honey bee, and iv) evaluating the potential of this honey bee for honey and beeswax production.
2. Material and Methods
This study was carried out at the College of Basic Sciences and Humanities, Haryana Agricultural University, Hisar, India (
Figure 1). The following observations were recorded:
2.1. Giant Honey Bee as a Pollination Service Provider for Crop Plants
Year-round surveys were conducted for the last 40 years (from 1984 to 2024) to record the crop plants visited by the foragers of the giant honey bee (
Apis dorsata) at Hisar (India). The foraging behavior of the visiting bees was recorded following methods suggested earlier [
32,
33]. It was determined whether the foragers of this honey bee acted as pollinators or nectar thieves on the visited flowers. Accordingly, their value as pollination service providers in this region was ascertained.
2.2. Trials for Domestication and Conservation of Giant Honey Bee
The major nesting sources of this honey bee in this region were identified. Based on my earlier study on the nesting preferences of this honey bee [
22], I designed and fabricated the nesting planks (made up of
Acacia nilotika wood) of 1m length, 15 cm breadth, and 1/2m height (
Figure 2). Molten beeswax was applied on the undersurface of the treated planks. The untreated planks were kept without beeswax (
Figure 2).
These planks were hung from the eves of the building of the College of Basic Sciences and Humanities, Haryana Agricultural University (Hisar, India), with the help of thin iron wires (
Figure 3). The latter were subjected to the following four treatments before their testing:
- i)
Planks were treated with beeswax and tied loosely with the building eaves.
- ii)
Planks were treated with beeswax and tied tightly with the building eaves.
- iii)
Planks were not treated with beeswax and tied loosely with the building eaves.
- iv)
Planks were not treated with beeswax and were tied tightly with the building eaves.
The planks were hung in the preferred direction and height in October, the time of immigration of the swarm colonies of
Apis dorsata in this region [
22]. Each treatment was replicated 4 times (4 planks were used for each treatment), and the experiment ran for 10 years (1984 to 1993). Therefore, each treatment had 40 replications during those 10 years. The acceptance of wooden planks was adjudged /confirmed on the basis of their occupancy by the migratory swarm colonies of the giant honey bee.
2.3. Occupation and Re-Occupation Indices
Six nesting sites were selected for testing the preferred location of the migratory swarms of
Apis dorsata. On each site, one beeswax-treated plank was hung and tied tightly with the projection of the building in 1994 and the experiment continued till 2012 (for 19 years). The occupation and re-occupation indices were determined by the following equations:
Where
OI = Occupation index.
n = Number of years when the plank was available for the first occupation.
N = Number of years when the plank was actually occupied.
ROI = Re-occupation index.
m = Number of times when the plank was utilized for reoccupation.
M = Number of times when the plank was actually available for reoccupation.
2.4. Trials for Handling and Taming of Colonies
These trials were performed during a bright day on the colonies of this honey bee already settled on the artificial nesting planks. Two kinds of trials were performed and the aggressive response of the colonies was tested in the following two ways:
2.4.1. Pre-Handling Disturbance Trials
This experiment started on colonies after a month of their settlement on the nesting planks. Under this experiment, colonies were divided into two groups, viz. i) periodically disturbed colonies; and ii) undisturbed (control) colonies. Periodical disturbance to the colonies was caused five times, each at a 10 day interval i.e. on the 40th, 50th, 60
th, 70
th, and 80th day after settlement. Likewise, the disturbance was caused to the control colonies. However, there was a difference between the disturbed and undisturbed (control) colonies. The repeated disturbance treatment was given to the same five colonies selected for this purpose. However, in undisturbed colonies (control), every time fresh colonies were selected to cause disturbance. Therefore, the disturbed colonies received disturbance for five times, but the undisturbed colonies received only once. The method adopted for the undisturbed colonies was: to the five colonies, the disturbance was caused after the 40
th day of their settlement; to another five colonies disturbance was caused after the 50
th day of their settlement; to another five colonies disturbance was caused after the 60
th day of their settlement; to another five colonies disturbance was caused after the 70
th day of their settlement; and to another five colonies disturbance was caused after the 80
th day of their settlement. The latter trial, therefore, could not be completed in the same year due to the limited number of colonies and the experiment spread over the years. The disturbance to the colonies was caused by loosening the wire of the planks carrying the colonies, waving them gently up-down and to and fro in all directions, giving them minor jerks, and going near these colonies. The aggressive response of the two types of colonies was recorded as shown in
Table 1, and the intensity of aggressiveness of the two types of experimental colonies was compared.
2.4.2. Pre-Handling Taming Trials
In these trials, the colonies were divided into four groups, viz.: i) smoke-treated colonies; ii) water-treated colonies; iii) sam-treated colonies, and iv) untreated (control) colonies. For the smoke treatment, 15-20 gentle puffs of smoke were applied to the experimental colonies with the help of a smoker. Jute cloth was used for burning in the smoker to produce smoke. In the water-treated colonies, normal clean water at room temperature was sprayed on the two faces of the colony with the help of a fine nozzle one-liter capacity hand spray pump. The sam-treatment was given by making 15-20 air puffs gently on the colonies. The control colonies did not receive any taming treatment. Two types of colonies were selected for this experiment; the undisturbed colonies and previously disturbed colonies. To perform these trials, the colonies were brought down to the chest height and precautions were taken to cause the least disturbance to the colonies other than the experimental disturbances. The aggressive response of the colonies subjected to four kinds of trials was recorded as shown in
Table 1.
2.5. Utilization of the Allured Giant Honey Bee for Honey and Beeswax Production, and for Live Studies
A method was developed to utilize the allured and tamed colonies of the giant honey bee for honey and beeswax production, and for live studies. For this purpose, the colonies were periodically disturbed on lines described in
Section 2.4.1. These colonies were brought down, and then suitably tamed on lines as described in Section-2.4.2. Then their honey part was excised manually with the help of a knife. The honey comb so excised was then squeezed by hands and thus honey was extracted. The beeswax so remained and from the deserted combs was also extracted by usual methods. In total, honey and beeswax production from 10 domesticated colonies were recorded and the potential of this honey bee in providing these commodities was determined. The allured colonies were also used for the live studies of this honey bee [
7,
22].
2.6. Statistical Analysis
For the domestication trials, there was no need to use any statistical test [
34]. However, for the handling and taming trials, an unpaired t-test [
35], and one-way ANOVA [
36] were used as per situation for finding differences between trials.
3. Results
3.1. Pollination Service Provided by the Giant Honey Bee (Apis dorsata) to Different Crop Plants
At Hisar, the foragers of the giant honey bee visited the flowers of more than 30 crop plants (
Figure 4 and
Figure 5;
Table 2).
The foraging behavior of the foragers of the giant honey bee revealed that they transferred pollen in each of their visits to the flowers of a plant species they visited and brought out pollination in each visited flower. Based on foraging behavior, this honey bee was found to be a faithful natural pollinator of these crops.
This way, this honey bee is an important and reliable pollination service provider to the crop plants of this region.
3.2. The Nesting Sources of the Giant Honey Bee (Apis dorsata)
At Hisar, Apis dorsata has very limited nesting sources. These include manmade structures like tall buildings and water towers (Figures 6 and 7) and some tall trees, e.g. Indian rose wood, eucalyptus (Figure 8), and sometimes the dwarf trees planted in undisturbed locations, like horticultural fields, too serve as nesting sources of this honey bee (Figure 9).
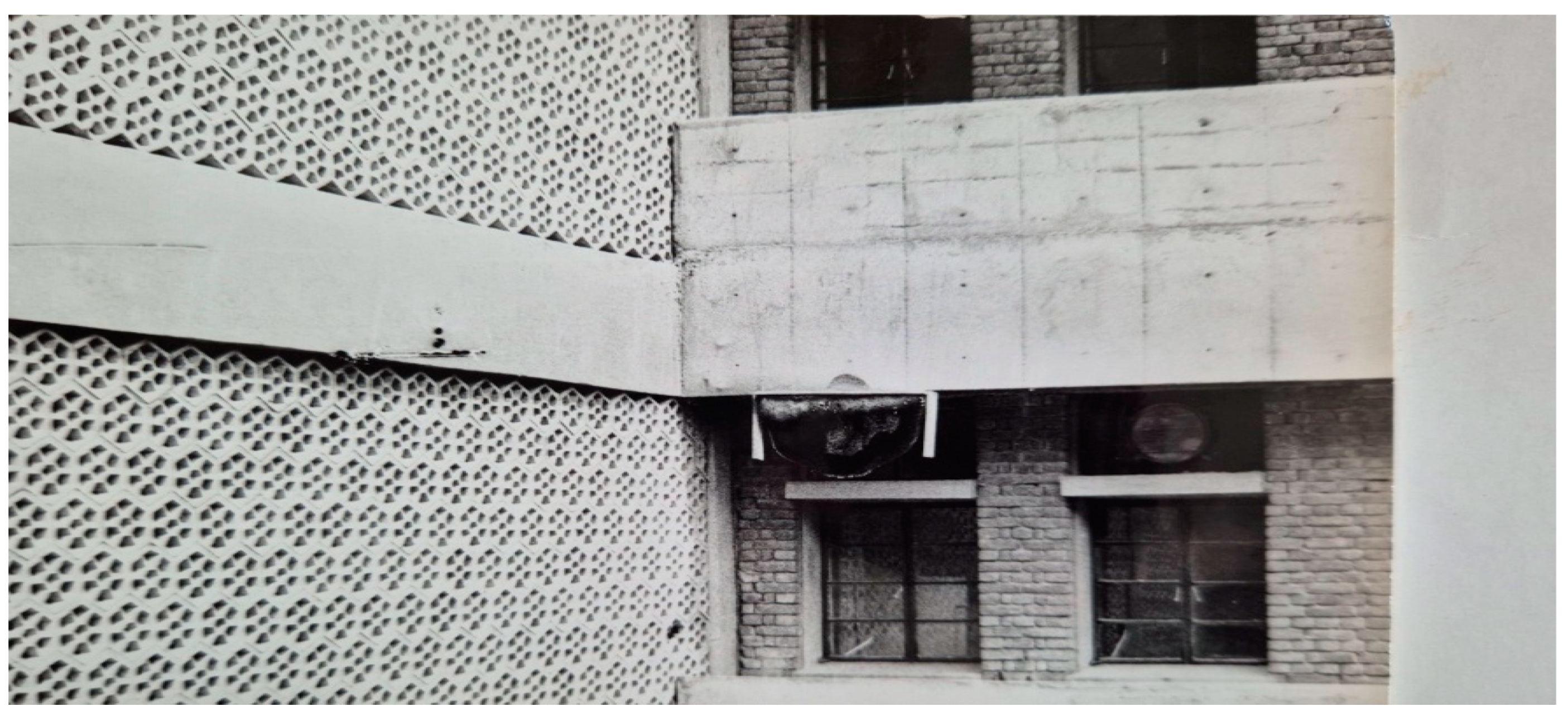
Figure 6.
A colony of Apis dorsata nesting on the projection of the college building (at a height of about 15m from the ground).
Figure 6.
A colony of Apis dorsata nesting on the projection of the college building (at a height of about 15m from the ground).
Figure 7.
Colonies of Apis dorsata nesting on the water tower building, (at a height of about 15m from the ground).
Figure 7.
Colonies of Apis dorsata nesting on the water tower building, (at a height of about 15m from the ground).
Figure 8.
A colony of Apis dorsata nesting on a eucalyptus tree (at a height of about 5m from the ground).
Figure 8.
A colony of Apis dorsata nesting on a eucalyptus tree (at a height of about 5m from the ground).
Figure 9.
A colony of Apis dorsata nesting on a jujube tree (at a height of about 2m from the ground).
Figure 9.
A colony of Apis dorsata nesting on a jujube tree (at a height of about 2m from the ground).
Due to the fast removal of host nesting trees of Apis
dorsata in this region [7], buildings
remain the ultimate and alternate sources for hanging the nesting planks to
allure the migratory swarms of this honey bee.
3.3. Trials for Domestication and Conservation
The results of the acceptable response of
immigrating swarm colonies of the giant honey bee to different nesting devices
and their hanging conditions revealed that this honey bee had a strong choice
in selecting the artificial nesting devices. The plank occupation gave very
interesting startling results (Table 3; Figures 10 and 11).
There were four treatment trials, each replicated 4 times each year, and repeated for 10 years. Thus, each treatment made 40 observations. Figures in the parenthesis are the percent occupation.
Of the four treatment trials, three treatment trials were completely ignored/ rejected by the migratory swarms of this honey bee. These included, i) planks treated with beeswax and tied loosely with the building eaves; ii) planks not treated with beeswax and tied loosely with the building eaves; and iii) planks not treated with beeswax and tied tightly with the building eaves. Interestingly, the migratory swarms of this honey bee accepted only the wooden planks of the fourth treatment trial, i.e. planks treated with beeswax and tied tightly with the building eves (
Figure 10 and
Figure 11). However, the occupation level of provided planks remained mediocre (58 percent), as out of 40 trials only 29 were occupied. The nested colonies stayed there from October through May and emigrated later on. In selecting the planks, a combination of two parameters seemed to play a role. This was: i) the presence of beeswax on the under surface of the hanging plank, and ii) the plank should be fully appositioned to the surface of the building projection.
3.4. Occupation and Reoccupation Indices
It is interesting to note that the two sites (Site-1 and Site-4) each had an occupation and re-occupation index equal to one. The planks hung on these sites were 100 percent occupied and re-occupied year after year by this honey bee for nesting purposes (
Table 4). Site 2 had an occupation index equal to one (as this was utilized in the same year as was provided), but its re-occupation index was 0.5 (out of 18 times availability, only 9 times were utilized). The planks hung at Site-3 had an occupation index equal to 0.5 (as this was utilized in the second year of its availability), but its re-occupation index was 0.29 (as out of 17 times of its availability, it was utilized only 5 times).
Likewise, the planks at Site-5 had 0.33 occupation index (it was occupied in the third year of its availability) but had a considerably higher reoccupation index, equal to 0.5 (out of 16 times of its availability, it was utilized 8 times), indicating that this site was moderately acceptable to this honey bee. The plank hung at Site-6 had 0.2 occupation index (it was utilized in the 5
th year after its availability), as well as a reoccupation index (utilized only 3 times despite its availability for 14 times), indicating that the nesting plank at this site had very low attraction for the swarms of the giant honey bee (
Table 4).
These results indicate that all the nesting sites were not equally attractive to this honey bee, and, given the availability of the nesting places, this honey bee preferred some sites over others.
3.5. Trials for Handling and Taming of Colonies
3.5.1. Pre-Handling Disturbance Trials
Table 5 shows the aggressive response of the test colonies under two kinds of trials. The colonies which were not previously disturbed showed very high aggressiveness in comparison to those which have not been frequently disturbed. The difference in aggressiveness was highly significant (p<0.0001, t-test value= 7.56; df=48,
Table 5). The frequently disturbed colonies seemed to start ignoring the disturbance in the vicinity and gradually became calm to the visitors. The difference in aggressiveness between days was highly significant (F
4,25=
∞, ANOVA,
Table 5), whereas the undisturbed colonies showed a very high degree of aggressiveness even after 80 days of their settlement if they were not frequently disturbed, as the aggressive response of these colonies remained exceptionally high (
Table 5).
3.5.2. Pre-Handling Taming Trials
Table 6 shows the aggressive response of the colonies of the giant honey bee subjected to four kinds of taming treatment. The effect of these taming trials was the same on the aggressiveness of previously disturbed and undisturbed colonies (p>0,05, t-test value=0,238, df=38,
Table 6).
However, the difference between taming trials was highly significant in previously disturbed as well as undisturbed colonies (F
3,16=
∞;ANOVA, Table 12). Undisturbed smoke-treated colonies showed little higher aggression over the water-treated colonies. However, previously disturbed water and smoke-treated colonies showed no difference in aggression. In fact, there was no aggression in these colonies. On the other hand, both undisturbed and disturbed sam-treated and control colonies showed a high degree of aggression; the aggression of previously disturbed colonies was certainly lower than the undisturbed colonies. This further supports the earlier findings that periodical disturbance of the colonies plays a role in subduing the aggression of the giant honey bee. Both disturbed and undisturbed colonies showed no aggression after they were sprayed with water (
Table 6).
3.6. Utilization of the Allured Giant Honey bee for Honey And beeswax Production, and for Live Studies
A non-destructive method of honey hunting from the giant honey bee (
Apis dorsata) was developed to utilize the allured and tamed colonies of this honey bee for honey and beeswax production [
Figure 12 and
Figure 13]; and for live studies also [7, 22;
Figure 14]. For this purpose, the colonies were brought down, and their honey part was excised. Then the honey was squeezed by hands and thus extracted. On average, this honey bee produced 6.58 ± 0.53 kg (mean ± s.d.; N=10) honey and 1.52 ± 0.18 kg (mean ± s.d.; N=10) beeswax per colony (Appendix 1). The colonies allured on the wooden planks also proved an easy resource for making live studies on otherwise very difficult to approach colonies of this honey bee [7, 22;
Figure 14].
4. Discussion
When there is some initiative for the conservation of a species, the first step in this direction is the conservation of its habitat. However, the latter practice may take several years in the restoration process. In such a situation, some alternatives have to be investigated for providing quick respite to the affected species for its survival. The present efforts have been directed towards conservation of the giant honey bee (Apis dorsata) in the semi-arid environments of Northwest India where its nesting sources have been destroyed by human-engineered activities, and its populations have declined markedly [
7].
Figure 12.
A deserted comb of the giant honey bee Apis dorsata; the whitish upper part shows the honey part; in the middle there is a sealed brood; at the lower end there are queen cells.
Figure 12.
A deserted comb of the giant honey bee Apis dorsata; the whitish upper part shows the honey part; in the middle there is a sealed brood; at the lower end there are queen cells.
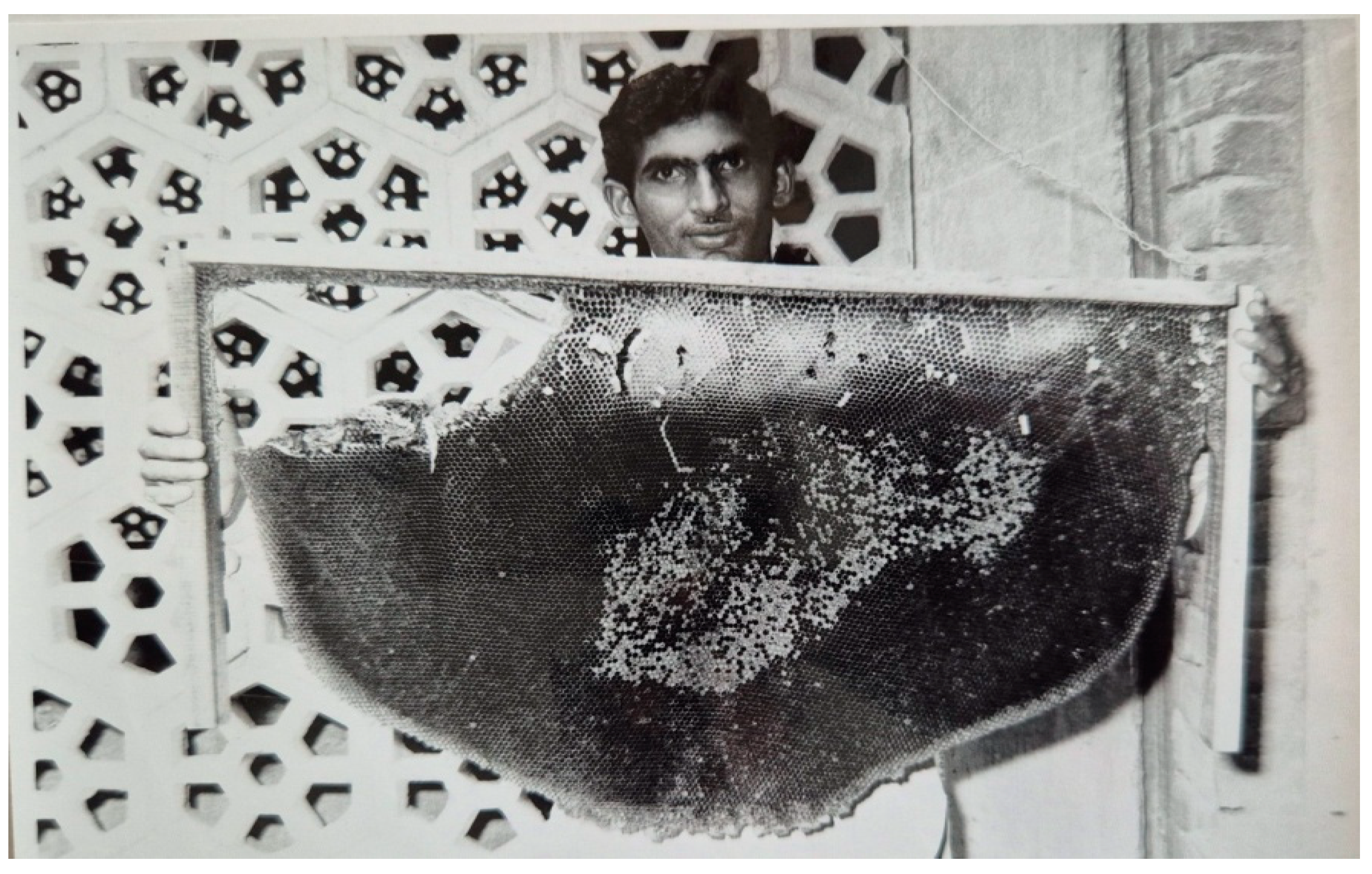
Figure 13.
A comb of the giant honey bee, Apis dorsata; from the upper left corner, the honey comb has been excised. The deserted comb makes an excellent source of beeswax, and can be used for study purposes.
Figure 13.
A comb of the giant honey bee, Apis dorsata; from the upper left corner, the honey comb has been excised. The deserted comb makes an excellent source of beeswax, and can be used for study purposes.
Figure 14.
A live colony of the giant honey bee, Apis dorsata. After the taming trials, the honey part has been excised, and the colony could easily be handled for live study purposes.
Figure 14.
A live colony of the giant honey bee, Apis dorsata. After the taming trials, the honey part has been excised, and the colony could easily be handled for live study purposes.
The giant honey bee (
Apis dorsata F.) immigrates in the Northwestern region of India during October/November, stays and reproduces there during winter and spring and emigrates in summer [
7]. During its stay in this region, this honey bee nests in tall trees and the eves of multi-storey buildings [
22]. In the past several years, this area has witnessed the loss of many host trees of this honey bee due to human-engineered activities [
7]. Viewing the potential of this honey bee in honey and beeswax production (Appendix 1) and providing the pollination service to the crops of this region (
Table 2), for its early conservation, restoration of its nesting resources has become very important. This study has made efforts in this direction.
Many efforts were made earlier in the form of rafter beekeeping with this honey bee [
23,
24,
25,
26,
27,
28,
29,
30]. However, the majority of earlier rafters were installed on the trees for the nesting sources of this honey bee. This effort, however, has been made in the region where the natural nesting resources (tall trees) of this honey bee have markedly declined due to human-engineered activities [
7]. The restoration of those nesting sources (tall trees) would take several years to return to their previous form. During this inordinate time lag of regeneration/restoration, and the availability of potential nesting sources on numerous tall buildings which have come up in recent years, the present effort would prove a great savior for this honey bee in its domestication, conservation and utilization for honey and beeswax production, and the sustainability of pollination services in the regions of its natural abode. This study indicated that all the nesting sites were not equally attractive to this honey bee, and, given the availability of the nesting places, this honey bee preferred some sites over others. Therefore, initially, efforts will have to be made to first select the preferred sites for alluring the migratory swarms of this honey bee before going for large-scale operation of beekeeping with this honey bee.
Rafter beekeeping with this honey bee notwithstanding, no efforts were earlier made to allure and tame the colonies; and very little efforts were made to domesticate this honey bee [
5,
31]. This study provides methods for i) how to allure the migratory swarms of this honey bee on the nesting planks hung on the projections of tall buildings; and ii) how to tame the colonies for subduing their aggressive behavior, for making close approaches to the colonies of this honey bee and for gently handling them for non-destructive harvesting honey and making scientific studies on the live colonies.
The information generated in this study is new to science. This will have great potential and would open a new chapter of beekeeping with the giant honey bee for honey and beeswax production, and the sustainability of pollination services in the regions of its natural abode.
Conclusion
The design and fabrication of wooden nesting devices for alluring and establishing the migratory swarms of the giant honey bee (Apis dorsata) for honey and wax production, and sustaining pollination services to the entomophilous crops in the region of its natural abode is novel to the science of beekeeping. This has led a step forward in the history of beekeeping, utilizing the giant honey bee, Apis dorsata for honey and beeswax production, and the sustainability of pollination services in the South and Southeast Asia. The methods used would be cheap, easy to employ and helpful in the alluring, efficient handling, safe and non-destructive honey gathering, and conservation of this honey bee for honey and beeswax production and the sustainability of pollination services in the regions where its natural nesting resources have vanished due to man-engineered activities.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, supervision, project administration, and funding acquisition were all done by R.C.Sihag.
Funding
This research was funded by the Government of Haryana.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
By request from the author.
Acknowledgements
I am thankful to the Head Department of Zoology for the necessary facilities. Thanks are due to the members of my field staff Dalbir Singh, Jhokhu Lal, Jagmal Singh and J.P. Narain for the field assistance in conducting this study. This study was completed under the scheme, B (IV) Non-Plan (Agri.) supported by the State Government of Haryana.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Annual Honey and Beeswax Production by the Giant Honey Bee (Apis dorsata)
| Colony. No. |
Honey production (kg) |
Wax production (kg) |
| 1 |
6.8 |
1.5 |
| 2 |
7.2 |
1.6 |
| 3 |
5.8 |
1.2 |
| 4 |
6.4 |
1.7 |
| 5 |
6.3 |
1.6 |
| 6 |
5.9 |
1.5 |
| 7 |
7.1 |
1.8 |
| 8 |
6.2 |
1.4 |
| 9 |
7.5 |
1.6 |
| 10 |
6.6 |
1.3 |
| Mean |
6.58 |
1.52 |
| Standard Deviation |
0.53 |
0.18 |
References
- Crane, E.; Luyen, V.V.; Mulder, V.; Ta, T.C. ran Cong Ta (1993). Traditional management system for Apis dorsata in submerged forests in southern Vietnam and central Kalimantan. Bee Wld, 1993, 74(1), 27‐40. [CrossRef]
- Arya, D.R.; Sihag, R. C.; Yadav, P. R. Diversity, abundance and foraging activity of insect pollinators of sunflower (Helianthus annuus L.) at Hisar (India). Indian Bee J. 1994, 56, 172‐78.
- Priti; Sihag, R. C. Diversity, visitation frequency, foraging behaviour and pollinating efficiency of insect pollinators visiting cauliflower (Brassica oleracea L. var. botrytis cv. Hazipur Local) blossoms. Indian Bee J, 1997, 59 (4), 230‐237.
- Chaudhary, N.; Sihag, R. C. Diversity, foraging behavior and foraging efficiency of different pollinators visiting onion (Allium cepa L.) blossoms. J. Apiculture, 2003, 18 (2), 103‐108.
- Priti, *!!! REPLACE !!!*; Sihag, R. C. Priti; Sihag, R. C. Diversity, visitation frequency, foraging behaviour and pollinating efficiency of different insect pollinators visiting coriander (Coriandrum sativum L.) blossoms. Asian Bee J., 1999, 1 (II), 36‐42.
- Priti; Sihag, R. C. Diversity, visitation frequency, foraging behaviour and pollinating efficiency of different insect pollinators visiting fennel (Foeniculum vulgare L.) blossoms. Asian Bee J, 2000, 2, 57‐64.
- Sihag, R. C. Phenology of migration and decline in colony numbers, and crop hosts of giant honeybee (Apis dorsata F.) in semiarid environment of Northwest India. J. Insects, 2014, 2014, 1‐9, Article ID 639467. [CrossRef]
- Wadhwa, N.; Sihag, R. C. Melittophilous mode of pollination predominates in European plum (Prunus domestica L.) in the semi-arid environment of Northwest India. Asian J. Agric. Res, 2015, 9 (5), 189‐207. [CrossRef]
- Saini, Reena; Sihag, R. C. Abundance, foraging behavior and pollination efficiency of insects visiting the flowers of Aonla (Emblica officinalis). EUREKA: Life Sciences, 2023, 2023 (1), 40‐56. [CrossRef]
- Priti; Sihag, R. C. Diversity, visitation frequency, foraging behaviour and pollinating efficiency of insect pollinators visiting carrot (Daucus carota L.var. HC-I) blossoms. Indian Bee J, 1998, 60 (I), 1‐8.
- Gahlawat, S. K.; Narwania, S. K.; Sihag, R. C. ; Ombir. Studies on the diversity, abundance, activity duration and foraging behaviour of insect pollinators of cucumber (Cucumis sativus L.) at Hisar. J. Apiculture, 2002, 17 (2), 69‐76.
- Gahlawat, S. K.; Narwania, S. K. ; Ombir; Sihag, R. C. Pollination studies on Pracitrullus fistulosus at Hisar, India. Ecoprint, 2002, 9 (1), 1‐6.
- Gahlawat, S. K.; Narwania, S. K. ; Ombir; Sihag, R. C. Diversity, abundance, foraging rates and pollinating efficiency of insects visiting wanga (Cucumis melo ssp. melo) blossoms at Hisar (India). J. Apiculture, 2003, 18 (1), 29‐36.
- Ahaiao, R. . Methods to control migration by Apis dorsata colonies in Pakistan. Bee Wld, 1989, 70 (4), 160‐162. [CrossRef]
- Mahindre, D.B. Developments in the management of Apis dorsata colonies. Bee Wld., 2000, 81 (4), 155 –163. [CrossRef]
- Koeniger, N.; Koeniger, G. Observations and experiments on migration and dance communication of Apis dorsata in Sri Lanka, J. Apic. Res., 1980, 19, 21–34. [CrossRef]
- Venkatesh, G.; Reddy, C.C. Rates of swarming and absconding in the giant honey bee, Apis dorsata F, Proc. Indian Acad. Sci. (Anim. Sci.), 1989, 98 (6), 425–430. [CrossRef]
- Woyke, J.; Wilde, J.; Wilde, M. Swarming and migration of Apis dorsata and Apis laboriosa honey bees in India, Nepal and Bhutan. J. Apic. Sci, 2012, 56 (1), 81–91. [CrossRef]
- Shrestha, J. B.; Mandal, C. K.; Shrestha, S. M.; Ahmad, F. The trend of the giant honeybee, Apis dorsata Fabricius colony migration in Chitwan, Nepal. The Wildlife, 2002 , 7 (10), 16– 20.
- Pokhrel, S. Climato-cyclic immigrations with declining populations of wild honeybee, Apis dorsata F. in Chitwan valley, Nepal. J. Agric. Environ, 2010, 11 (6), 51–58. [CrossRef]
- Dyer, F. C.; Seeley, T. D. Colony migration in the tropical honey bee Apis dorsata F. (Hymenoptera: Apidae). Insect. Soc, 1994, 41 (2), 129–140. [CrossRef]
- Sihag, R.C. Nesting behavior and nest site preferences of the giant honey bee (Apis dorsata F.) in the semi-arid environment of northwest India. J. Apic. Res, 2017, 56 (4), 452‐466. [CrossRef]
- Joshi, S.R. Non-destructive method of honey hunting. Bee Wld, 2005, 86, 63-64. [CrossRef]
- Oldroyd, B.P.; Nanork, P. Conservation of Asian honey bees. Apidologie, 2009, 40, 296–312.
- Oldroyd, B.P.; Wongsiri, S. Asian honey bees. Biology, conservation, and human interactions. Cambridge and London: Harvard University Press. 2006.
- Strickland, S. S. 1982. Honey hunting by the Gurungs of Nepal. Bee Wld, 1982, 63 (4), 153‐161. [CrossRef]
- .
- Tan, N. Q.; Chinh, P. H.; Thai, P. H.; Mulder, V. Rafter beekeeping with Apis dorsata: some factors affecting the occupation of rafters by bees. J. Apic. Res. 1997, 36 (1), 49‐54. [CrossRef]
- Tan, N. Q.; Chinh, P. H.; Ha, D. T. Socio-economic factors in traditional rafter beekeeping with Apis dorsata in Vietnam. Bee Wld, 2002. 83(4), 165‐170. [CrossRef]
- Waring, C. and Jump, D.R. Rafter beekeeping in Cambodia with Apis dorsata. Bee Wld, 2004, 85(1): 14‐ 18. [CrossRef]
- Thakar, C.V. A preliminary note on hiving Apis dorsata colonies. Bee Wld., 1973, 54(1), 24‐27. [CrossRef]
- Sihag, R. C. Characterization of the pollinators of cultivated cruciferous and leguminous crops of sub-tropical, Hisar, India. Bee Wld, 1988, 69 (4), 153‐158. [CrossRef]
- Sihag, R.C.; Shivrana, S. Foraging behaviour and strategies of the flower visitors. In: Pollination Biology: Basic and Applied Principles (Ed.: R. C. Sihag). Rajendra Scientific Publishers, Hisar, 1997; 53-73.
- Snedecor, G.W.; Cochran, W. G. Statistical Methods (6th Edn.). Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, 1967, 593p.
- https://www.graphpad.com/quickcalcs/ttest2/.
- https://www.statskingdom.com/180Anova1way.html.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).