1. Introduction
Bladder tumors represented around 2% of neoplasms diagnosed in dogs, being urothelial carcinoma or transitional cell carcinoma the most frequently identified [
1,
2,
3]. Due to the invasive nature, metastatic potential and its frequent development in the bladder trigone, the prognosis of these neoplasms is unfavorable [
4]. Different classification systems have been formulated for canine urothelial neoplasms, however, are not routinely used due to the discouragement of veterinarians from performing biopsies [
5].
Since the relationship between neoplasm morphology and its clinical behavior is a key point in oncology, different imaging modalities have been widely used in veterinary practice to identify and monitoring neoplasms evolution [
2,
6]. Two-dimensional (B-mode) ultrasonography is a highly accurate technique for detecting bladder lesions and when performed systematically may be superior to double-contrast cystography for identify bladder masses [
3]. It is a reliable tool for assessing the size of canine urinary bladder tumors [
7], in addition to presenting ultrasound characteristics that can be used as indicators of urothelial carcinoma prognosis [
8]. Despite being a non-invasive, accessible, and low-cost modality, the differentiation between benign and malignant lesions is still challenging [
9].
Elastography has been implemented as an auxiliary method in the study of different neoplasms in canine species and with promising results in predicting lesions malignancy [
10,
11,
12]. In humans, elastography is a useful tool in the evaluation of patients diagnosed with bladder masses, with malignant lesions being significantly stiffer than benign ones [
13], as well in the differentiation of low-grade and high-grade urothelial carcinoma [
14].
To our knowledge, there are no studies using ARFI (Acoustic Radiation Force Impulse) elastography to evaluate urinary bladder disorders in dogs. Considering that the determination of tissue elastic properties allows a better evaluation of lesions, we hypothesized that ARFI elastography would provide reproducible quantitative data that would be able to aid in the urothelial carcinoma identification, offering a noninvasive and accessible approach for veterinary clinical practice. Therefore, this study aimed to characterize the elastographic properties of urothelial carcinomas in dogs using ARFI elastography and verified its diagnostic potential comparing healthy tissues and neoplastic urinary blader tissues stiffness.
2. Materials and Methods
2.1. Animals
This study was approved by the Institutional Animal Care and Use Committee (protocol number 4280/22). Consisting in a prospective, observational study conducted between September 2022 and March 2024. In this study two types of animals were enrolled, dogs with urothelial carcinoma (UTC group) and healthy dogs (CON group).
In UTC group were included dogs treated by the oncology service of the "Governador Laudo Natel" University Veterinary Hospital and diagnosed with urothelial carcinoma by histopathological examination, by BRAF gene identification (PCR) in urine or by suggestive urinary sediment cytological analysis [
1,
15,
16], who have not received any type of chemotherapy previously, after tutor provided signed consent for participation in this study. There were no restrictions regarding sex, age, or breed. For the CON group were included healthy dogs from the Nutrition and Nutritional Diseases Research Laboratory.
All animals were clinical evaluated, previous history of urinary disease, as well as the presence of abdominal pain at palpation, stranguries and urinary urgency were noted. And finally, dogs were transferred to the radiology service to carry out the objective evaluations of this study.
2.2. Imaging Evaluation
Ultrasound examinations was performed using the ACUSON S2000® equipment (Siemens, Munich, Germany), with a 9.0 MHz multifrequency linear transducer, always by the same experienced evaluator. The abdominal area of the animals was previously depilated, ultrasound gel was applied, and they were positioned in dorsal or lateral recumbency according to the needs of the examination and causing minimal stress.
Initially, B-mode ultrasound was performed to assess the urinary bladder. The transducer was positioned in the caudal abdomen and after locating the organ, images were taken in longitudinal and transverse planes of its entire length. The bladder was assessed for its distension, thickness, regularity, integrity of the wall, general appearance, echogenicity and presence or absence of masses or particles (urine) within or around it. Specifically in dogs of the UTC group, the evaluation of the bladder masses included dimensions (height and length), echotexture pattern (homogeneous or heterogeneous), location (apex, body, trigone, or diffuse), contours (regular or irregular), tumor shape (pedunculated or nonpedunculated), bladder wall involvement (involvement of the muscular layer of the bladder wall or loss of normal stratification) and echogenicity (hypoechoic, hyperechoic, or mixed) [
8].
After B-mode examination, ARFI elastography was performed using Virtual Touch IQ software (Siemens, Munich, Germany). First, images of the bladder wall of all dogs, and of the neoformations in dogs of the UTC group were obtained. The quality of the images was tested using a software indicator in which homogeneous and greenish images indicated high quality, while heterogeneous and yellowish images indicated low quality, when the latter occurred, the image was repeated until it reached a quality standard. Based on this quality image, a qualitative and quantitative evaluation was carried out. In the qualitative one it was classified, the deformability (deformable or non-deformable) and the relative stiffness of the tissues (color elastogram, blue color indicated fewer rigid structures, green intermediate stiffness, and the reddish regions more rigid structures). On the quantitative evaluation, at least five square regions of interest (ROI of 1 x 1 mm) were randomly positioning in each evaluated tissues trying to include the maximum area, immediately the software provides the values of the shear wave velocity (SWV) of each of these ROIs [
17,
18]. In the CON group the same evaluation sequence was applied making the SWV measurements in the dorsal region of the bladder wall.
2.3. Statistical Analysis
Was performed using GraphPad Prism software (version 8.0.0 for Windows, GraphPad Software, Boston, Massachusetts USA). Initially, tissue shear wave velocities (SWV) of different regions of interest (ROIs) of the bladder wall and masses were compared to each other using the Kruskal-Wallis’s test. If this analysis not showed significance, the median SWV of the ROIs were calculated and used for subsequent analyses. Bladder SWVs and wall thickness were compared between UTC group and CON group using the Mann-Whitney test, if this test resulting significative, the SWV and/or wall thickness were subjected to a discriminative power analysis to identify the presence of neoplasia through Receiver operating characteristic (ROC) curve analysis and calculated the cut-off value, sensitivity, specificity, likelihood ratio and area under the curve (AUC), for this diagnosis, using the logistic regression model by mean of Wilson/Brown method. Qualitative variables were analyzed subjectively (relative stiffness) and compared by Fisher's exact test (presence or absence). Statistical significance was considered when P-value < 0.05, and data were presented as median ± interquartile range (IQR).
3. Results
Nine healthy dogs and seven dogs with urothelial carcinoma were confirmed through, urine identification of the BRAF gene (4/7 58%), histopathological examination (1/7 14%) and by mixed methods (cytological analysis of urinary sediment, BRAF and histopathological 2/7; 28%) were included in this study.
The UTC group was composed of five females (5/7; 71%) and two males (2/7; 29%), with a mean age of 12±3 years, and belonging to various races, Basset Hound (2/7), Border Collie (1/7), German Shepherd (1/7), Yorkshire Terrier (1/7) and mongrel (2/7). The CON group was composed of six females (6/9; 67%) and three males (3/9; 33%), with a mean age of 5±2 years, all of the beagle breeds.
All dogs of the UTC group showed clinical signs of dysuria and abdominal pain, as well as urinary incontinence or dribbling urination, while in the CON group, no dogs showed clinical signs. Furthermore, urine color varied between orange and red in 6/7 patients with neoplasia, and it was yellow in all animals in the healthy dogs.
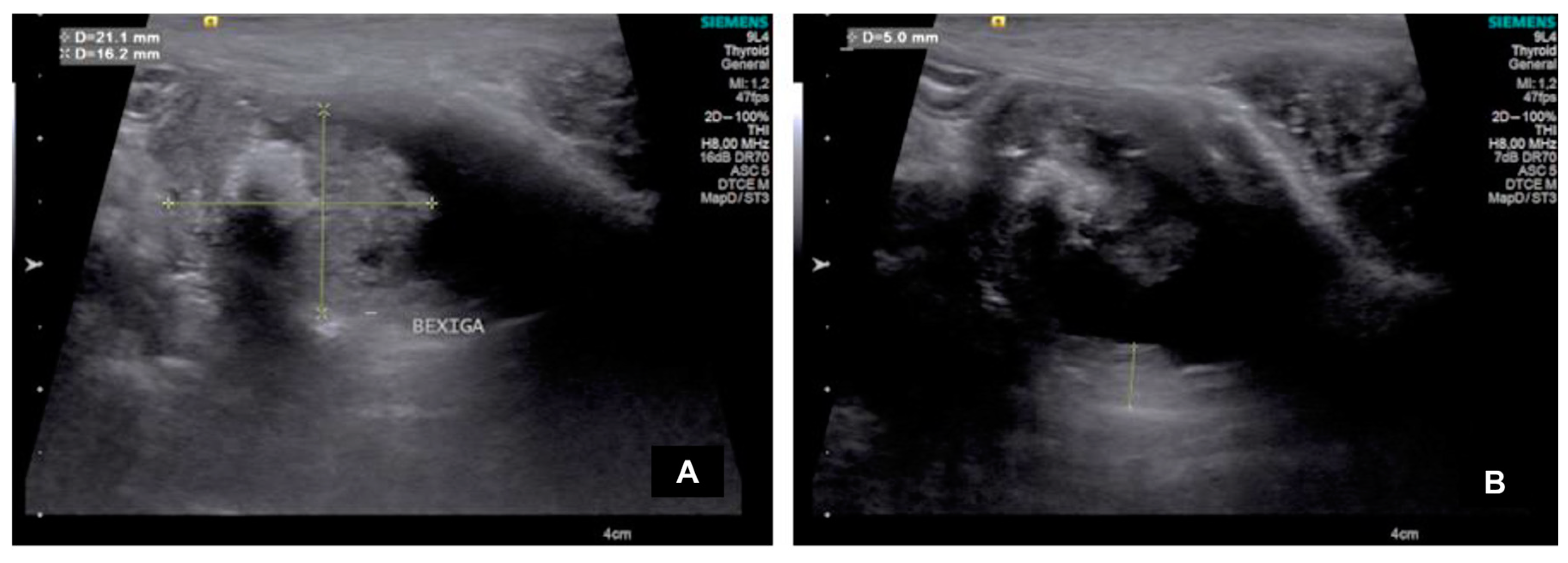
In the CON group, the bladder was full of urine, with a normal appearance and regular wall, while in the UTC group, 3/7 dogs had an irregular bladder wall, and all animals had an abnormal appearance. In both groups, the bladder content was predominantly anechoic and homogeneous (
Figure 1).
All neoformation had a pedunculated form with muscular wall invasion, its echogenicity was hyperechogenic in 5/7 dogs and mixed in 2/7 with heterogeneous characteristics and irregular borders. The mass size, echotexture and location varied among animals (
Table 1); however, its average size was 1.72 ± 1.04 cm in height and 3.08 ± 1.01 cm in length.
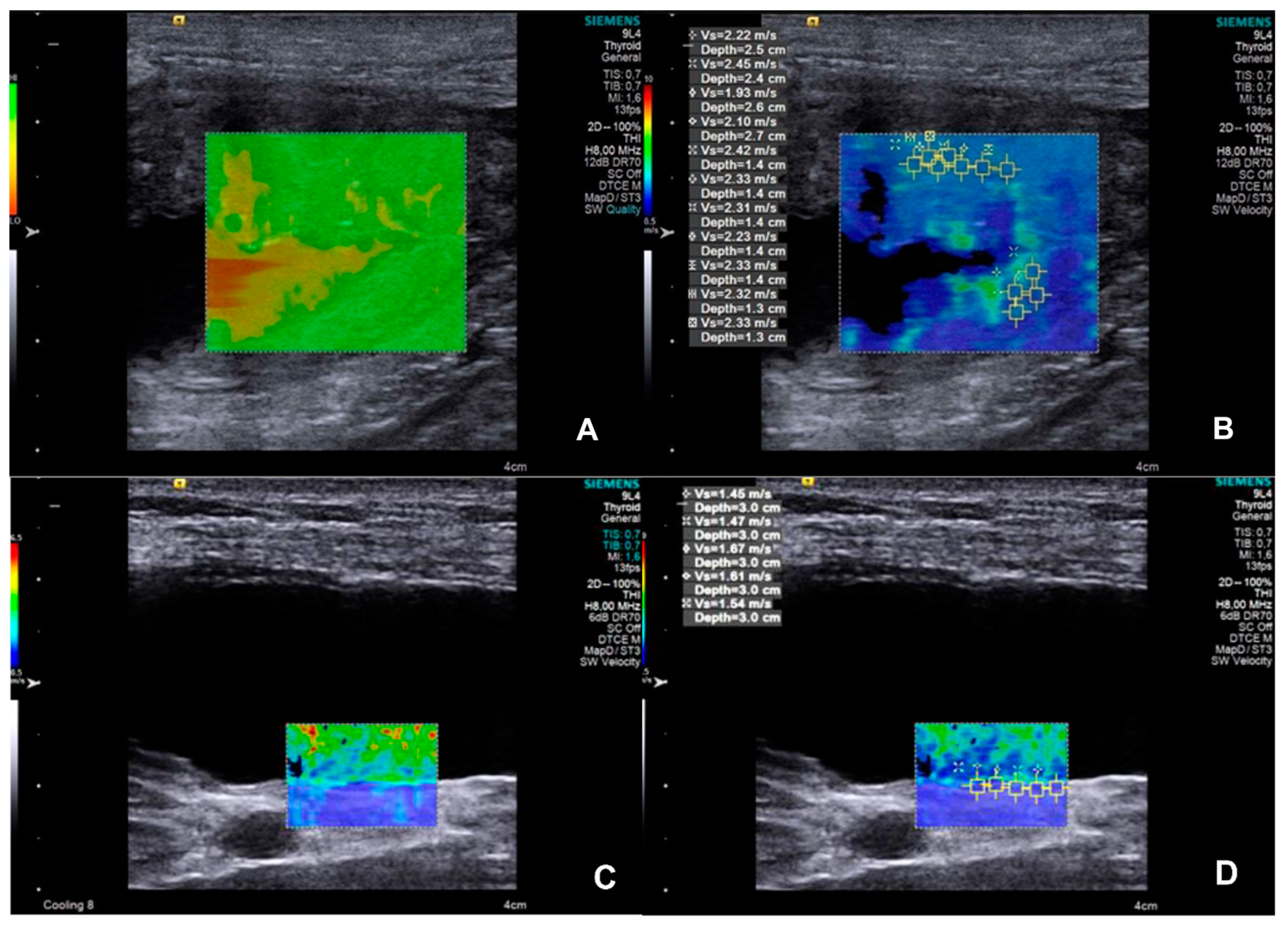
The SWVs measurements of different ROIs (
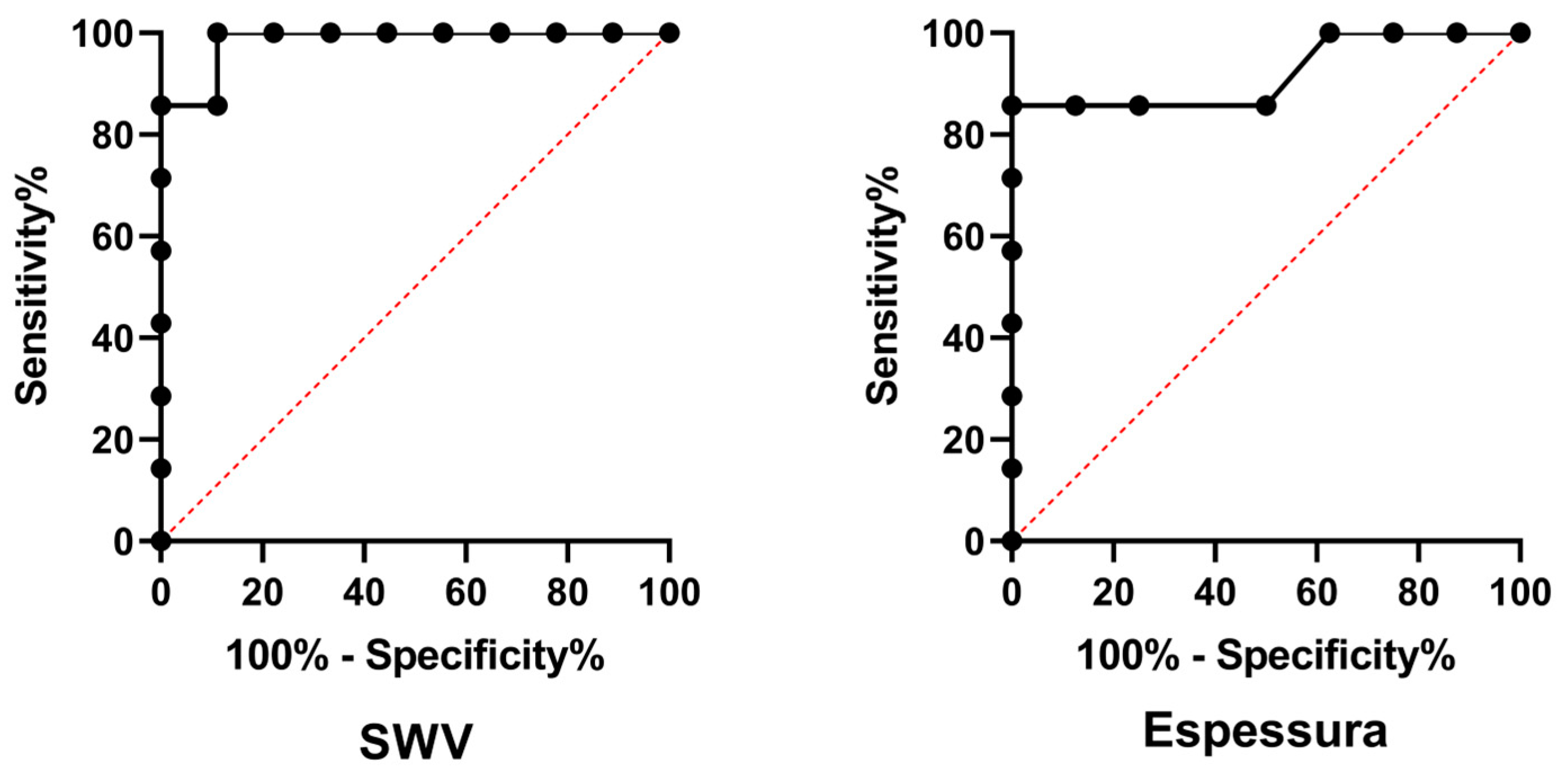
Figure 2) resulted in similar between each (p=0.2925), consequently, the median SWV of the bladder wall was used for the posterior analysis. SWV and thickness of the bladder wall resulted in higher (p=0.0045 and 0.0003; respectively) in the UTC patients. Denoting that both, bladder wall SWV and thickness showed discriminative power for identifying canine urothelial carcinoma (p=0.0012 and 0.0065; respectively), diagnostic features and median values were presented in
Table 2 and illustrated in
Figure 3.
4. Discussion
This study provides a significant contribution to the characterization of the elastographic properties of healthy bladder and urothelial carcinomas in dogs using the ARFI elastography technique. The analysis of the SWV, median of 2.53 m/s in the group of dogs with urothelial carcinoma, compared with 1.41m/s in the healthy dogs, and of bladder wall thickness 0.28 ± 0.05 cm in the UTC group, in contrast with 0.14 ± 0.26 cm in the CON group, revealed that both variables have a promising potential to detection bladder neoplasms and that ARFI elastography may be an important screening tool for urothelial carcinoma. Establishing reference values based on tissue characterization of healthy dogs is a fundamental step, and the cutoff value of 1.585 m/s for SWV stands out as an important diagnostic tool for urothelial carcinoma and serves as a starting point for future differentiation between benign and malignant lesions.
These results corroborate previous research in neoplasms from other organs, where greater tissue stiffness has been associated with histopathological changes, such as increased cell density, disordered proliferation, and stromal disorganization [
10,
19]. Studies performed in humans with liver and kidney diseases have shown that ARFI elastography has high sensitivity for detecting increased tissue stiffness, which is correlated with malignancy [
20,
21]. The thickening is consistent with tumor infiltration seen in invasive carcinomas, as reported in previous studies that used ultrasonography and computed tomography for the evaluation of bladder neoplasms [
1]. Thus, this tumor infiltration, reflected by the thickening of the bladder wall, is indicative of malignant behavior, highlighting the importance of ultrasound evaluation as a complementary tool for both the diagnosis and staging of urothelial carcinoma.
The analysis of the ROC curves in this study revealed a sensitivity of 100% for SWV and 85.7% for bladder wall thickness, indicating that the technique has the potential for excellent diagnostic capacity. The high sensitivity observed for SWV is consistent with the results of studies that used ARFI elastography for the diagnosis of breast and prostate cancers in humans, where the technique was demonstrated to be a valuable tool for the early detection of malignant lesions [
22,
23,
24].
In addition to the quantitative findings, the clinical signs reinforce that urothelial carcinomas are diagnosed late and that the clinical signs resemble those observed in cystitis, making early diagnosis difficult. The animals in the diseased group presented a variety of clinical signs, such as pain on palpation, urine with altered color and difficulty in keeping the bladder full, which are frequently associated with bladder neoplasms [
2]. Incontinence is commonly observed in patients with urothelial carcinoma [
25], one hypothesis is that it occurs precisely due to the increase in rigidity with a consequent decrease in compliance. These clinical signs, combined with the observation of heterogeneous bladder masses with irregular borders, corroborate previous studies indicating that these characteristics are associated with a more reserved prognosis [
8]. Furthermore, the reduced dependence on operator subjectivity compared to conventional ultrasound makes ARFI elastography a quantitative and reproducible tool, which is essential for the standardization of diagnoses [
11,
18].
Additionally, the fact that ARFI elastography can detect subtle tissue changes even before the lesions become macroscopically visible represents a significant advance in the diagnosis of several pathologies [
26]. Several studies have evaluated the application of ARFI elastography in the differentiation of malignant and benign tumors in different tissues of dogs, including splenic tumors, cutaneous neoplasms, and the identification of metastatic lymph nodes in bitches with mammary neoplasia, demonstrating that the technique can be effective in identifying lesions with malignant characteristics, contributing to a more accurate diagnosis and clinical decision-making [
12,
18,
27,
28]. The results of the present study, associated with the historical applicability of ARFI elastography in the differentiation between healthy and diseased tissues, demonstrate the application of the technique in the evaluation of canine bladder neoplasms, where high tissue stiffness is associated with the presence of urothelial carcinoma.
Future studies evaluating bladder wall stiffness in cases of neoplasia and comparing it with bladder wall stiffness in cases of chronic cystitis, where the wall is chronically thickened, as well as in cases of benign formations, such as polyps, are important to define whether there is a difference between neoplasia and benign lesions, between neoplasia and normal tissue, between benign lesions and normal tissue and to establish the cutoff values for each of them. After all, a study carried out in children with cystitis, where they compared the values of the stiffness of healthy and diseased tissue by two-dimensional shear wave elastography, also identified a correlation between the SWV and wall thickness [
29].
ARFI elastography and B-mode ultrasound examination can provide information that, although not strictly diagnostic, is essential for the early diagnosis process, providing information that can be used as a guide for decision-making by both the veterinary oncologist and the guardian/owner. Therefore, it is important to explore in future research the combination of ARFI elastography with other diagnostic techniques, such as the detection of genetic mutations, such as the BRAF mutation. The association between molecular methods and imaging diagnostics could further improve diagnostic accuracy and allow a more personalized approach in the treatment of patients with urothelial carcinoma [
30]. The literature suggests that the integration of techniques can significantly increase the sensitivity and specificity of diagnoses, especially in aggressive and difficult-to-detect neoplasms [
12].
Although the results of this study are promising, it is important to recognize its limitations. The limited number of cases evaluated, especially with few histopathological results, is one of the main limitations, which may affect the generalization of the results. In addition, it was not possible to evaluate local invasion (urethra and prostate) and metastatic behavior of the tumor in the population studied, as well as classification and staging of the tumors or serial evaluations that would allow the monitoring and evolution of these patients. Future studies are encouraged and should include larger and more diverse samples, both in terms of number of animals and racial diversity, to validate the findings and ensure that the ARFI technique is effective in a wide range of patients [
15].
5. Conclusions
The study demonstrated that ARFI elastography is a tool in the diagnosis of urothelial carcinoma in dogs, providing quantitative information that can complement traditional approaches. It suggests that new studies in the area should be carried out to reinforce the findings and expand the clinical use of ARFI elastography in veterinary practice. New studies based on this one should be carried out to expand its application for differentiating neoplastic and non-neoplastic diseases.
Author Contributions
Conceptualization, A.P.L.O., A.B.N. and M.A.R.F.; methodology, A.P.L.O., B.B.L., D.J.R. and G.C.L.E.; software, A.P.L.O., B.B.L., D.J.R. and R.A.R.U.; validation, A.P.L.O., B.B.L., D.J.R., G.C.L.E., I.C.K.C., R.A.R.U., A.B.N. and M.A.R.F; formal analysis, G.C.L.E., I.C.K.C., R.A.R.U., A.B.N. and M.A.R.F; investigation, A.P.L.O., A.B.N. and M.A.R.F.; resources, A.P.L.O., A.B.N. and M.A.R.F.; data curation, A.P.L.O., B.B.L. and D.J.R.; writing—original draft preparation, A.P.L.O., A.B.N. and M.A.R.F; writing—review and editing, A.P.L.O., G.C.L.E., I.C.K.C. and R.A.R.U.; visualization, A.B.N. and M.A.R.F.; supervision, A.B.N. and M.A.R.F.; project administration, M.A.R.F.; funding acquisition, A.B.N. and M.A.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (number: 30512/2020-0), and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (number: 2022/07366-0).
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of the Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista “Júlio de Mesquita Filho” (FCAV/Unesp – Jaboticabal) (protocol number 4280/22).
Informed Consent Statement
The animals’ owners were consulted, informed, and clarified in advance about all the study details and expressed their agreement with the evaluations proposed in the free consent form, approving the participation of their animal(s) in the research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We appreciate the support of FEALQ—Fundação de Estudos Agrários Luiz de Queiroz.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knapp, D.W.; McMillan, S.K. Tumors of the urinary systems. In: Withrow & MacEwen's Small Animal Clinical Oncology, 6th ed. Elsevier-Saunders, 2013, pp 572-582.
- Fulkerson, C.M.; Knapp, D.W. Management of transitional cell carcinoma of the urinary bladder in dogs: A review. Vet J. 2015, 205, 217–225. [CrossRef]
- Mutsaers, A.J.; Widmer, W.R.; Knapp, D.W. Canine Transitional Cell Carcinoma. J Vet Intern Med. 2003, 17, 136–144. [CrossRef]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary Bladder Cancer in Dogs, a Naturally Occurring Model for Cancer Biology and Drug Development. ILAR Journal. 2014, 55, 100–118. [CrossRef]
- Brambilla, E.; Govoni, V.M.; Cavalca, A.M.B.; Laufer-Amorim, R.; Fonseca-Alves, C.E.; Grieco, V. Grading Systems for Canine Urothelial Carcinoma of the Bladder: A Comparative Overview. Animals. 2022, 12(11), 1455. [CrossRef]
- Macrì, F.; Di Pietro, S.; Mangano, C.; Pugliese, M.; Mazzullo, G.; Iannelli, N.M.; Angileri, V.; Morabito, S.; De Majo, M. Quantitative evaluation of canine urinary bladder transitional cell carcinoma using contrast-enhanced ultrasonography. BMC Vet Res. 2018, 14(84), 1-8. [CrossRef]
- Honkisz, S.I.; Naughton, J.F.; Weng, H.Y.; Fourez, L.M.; Knapp, D.W. Evaluation of two-dimensional ultrasonography and computed tomography in the mapping and measuring of canine urinary bladder tumors. Vet J. 2018, 232,23-26. [CrossRef]
- Hanazono, K.; Fukumoto, S.; Endo, Y.; Ueno, H.; Kadosawa, T.; Uchide, T. Ultrasonographic findings related to prognosis in canine transitional cell carcinoma. Vet Radiol Ultrasound, 2014, 55(1), 79-84. [CrossRef]
- Léveillé, R.; Biller, D.S.; Partington, B.P.; Miyabayashi, T. Sonographic investigation of transitional cell carcinoma of the urinary bladder in small animals. Vet Radiol Ultrasound. 1992, 33(2), 103-107. [CrossRef]
- Park, H.; Park, J.Y.; Ahn, S.H.; Chon, C.Y.; Han, K.H.; Kim, S.U. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol, 2013, 19, 219–226. [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS One. 2017, 12(5), e0178143. [CrossRef]
- Da Cruz, I.C.K.; Carneiro, R.K.; De Nardi, A.B.; Uscategui, R.A.R.; Bortoluzzi, E.M.; Feliciano, M.A.R. Malignancy prediction of cutaneous and subcutaneous neoplasms in canines using B-mode ultrasonography, Doppler, and ARFI elastography. BMC Vet Res. 2022, 18, 1-13. [CrossRef]
- Dede, O.; Teke, M.; Daggulli, M.; Penbegül, N. Use of Acoustic Radiation Force Impulse Elastography to Discrimination Benign and Malignant Masses for Bladder. Int J Radiol Imaging Technol. 2018, 4, 039.
- Huang, X.Z.; Zhou, A.Y.; Liu, M.W.; Zhang, Y.; Xu, P. Shear Wave Elasticity Differentiation Between Low-and High-Grade Bladder Urothelial Carcinoma and Correlation With Collagen Fiber Content. J Ultrasound Med. 2021, 40(1), 113-122. [CrossRef]
- Childress, M.O.; Adams, L.G.; Ramos-Vara, J.A.; Freeman, L.J.; He, S.; Constable, P.D.; Knapp, D.W. Results of biopsy via transurethral cystoscopy and cystotomy for diagnosis of transitional cell carcinoma of the urinary bladder and urethra in dogs: 92 cases (2003-2008). J Am Vet Med Assoc. 2011, 239(3), 350-356. [CrossRef]
- Yamasaki, H.; Uematsu, Y.; Okano, K.; Ichikawa, M.; Tei, M.; Hirabayashi, M.; Hirao, H. Establishment and characterization of urothelial carcinoma cell lines with and without BRAF mutation (V595E) in dogs. In Vitro Cell Dev Biol Anim. 2022, 58(10), 898-911. [CrossRef]
- Abreu, T.G.M.; Feliciano, M.A.R.; Renzo, R. et al. Acoustic radiation force impulse elastography of the eyes of brachycephalic dogs. Arq Bras Med Vet Zootec. 2018, 70:949-958. [CrossRef]
- Maronezi, M.C.; Carneiro, R.K.; Da Cruz, I.C.K.; De Oliveira, A.P.L.; De Nardi, A.B.; Pavan, L.; Feliciano, M.A.R. Accuracy of B-mode ultrasound and ARFI elastography in predicting malignancy of canine splenic lesions. Sci Rep, 2022, 12(1), 4252. [CrossRef]
- Nightingale, K.; McAleavey, S.; Trahey, G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003, 29(12), 1715-1723. [CrossRef]
- Gallotti, A.; D’Onofrio, M.; Romanini, L.; Cantisani, V.; Mucelli, R.P. Acoustic Radiation Force Impulse (ARFI) ultrasound imaging of solid focal liver lesions. Eur J Radiol. 2012, 81(3), 451-455. [CrossRef]
- Yoǧurtçuoǧlu, B.; Damar, Ç. Renal elastography measurements in children with acute glomerulonephritis. Ultrasonography. 2021, 40(4), 575. [CrossRef]
- Kumm, T.R.; Szabunio, M.M. Elastography for the characterization of breast lesions: initial clinical experience. Cancer control. 2010, 17(3), 156-161. [CrossRef]
- Meng, W.; Zhang, G.; Wu, C.; Wu, G.; Song, Y.; Lu, Z. Preliminary results of acoustic radiation force impulse (ARFI) ultrasound imaging of breast lesions. Ultrasound Med Biol. 2011, 37(9), 1436-1443. [CrossRef]
- Cui, X.W.; Li, K.N.; Yi, A.J.; Wang, B.; Wei, Q.; Wu, G.G.; Dietrich, C.F. Ultrasound elastography. Endoscopic ultrasound. 2022, 11(4), 252-274.
- Reed, L.T.; Knapp, D.W.; Miller, M.A. Cutaneous metastasis of transitional cell carcinoma in 12 dogs. Vet Pathol. 2013, 50(4), 676-681. [CrossRef]
- Furuichi, Y.; Moriyasu, F.; Taira, J.; Sugimoto, K.; Sano, T.; Ichimura, S.; Miyata, Y.; Imai, Y. Noninvasive diagnostic method for idiopathic portal hypertension based on measurements of liver and spleen stiffness by ARFI elastography. J Gastroenterol. 2013, 48, 1061-1068. [CrossRef]
- Feliciano, M.A.R.; Maronezi, M.C.; Crivellenti, L.Z.; Crivellenti, S.B.; Simões, A.P.R.; Brito, M.B.S.; Garcia, P.H.S.; Vicente, W.R.R. . Acoustic radiation force impulse (ARFI) elastography of the spleen in healthy adult cats–a preliminary study. J Small Anim Pract. 2015, 56(3), 180-183. [CrossRef]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci Rep, 2018, 8(1), 17708. [CrossRef]
- Durmaz, M.S.; Yorulmaz, A.; Durmaz, F.G.; Arslan, S. Utility of 2-Dimensional Shear Wave Elastography for Assessment of the Bladder Wall in Children With Acute Cystitis. J Ultrassom Med. 2021, 40, 1105–1111. [CrossRef]
- Mochizuki, H.; Shapiro, S.G.; Breen, M. Detection of BRAF mutation in urine DNA as a molecular diagnostic for canine urothelial and prostatic carcinoma. PloS one, 2015, 10(12), e0144170. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).