1. Introduction
The study of social behaviour in animals is a cornerstone of behavioural ecology, offering crucial insights into the biological and ecological dynamics of different species. Social animals, ranging from insects to primates, exhibit complex behavioural repertoires that serve to facilitate communication, cooperation, and group cohesion [
1,
2]. In primates, for instance, research on social dynamics has elucidated behaviours related to hierarchy, dominance, submission, agonism, affiliation, and reconciliation [
3]. Analogous dominance–submission patterns have also been described in species as diverse as gorillas [
4], elephants [
5], dolphins [
6], and killer whales [
7].
Despite the relevance of such research, cetacean social behaviour remains particularly challenging to study due to the aquatic environment in which most of their activity occurs, largely beneath the water’s surface. This constraint has historically limited direct observation and led researchers to infer social interactions based on proximity at the surface. For example, Thomsen [
8] categorised the surface behaviour of resident killer whales (
Orcinus orca) near Vancouver Island into social and non-social contexts, depending on whether individuals were within or beyond one body length of each other—an approach grounded in earlier work by Ford [
9]. This proximity-based framework has since been employed in various studies, including those by Gibson [
10] on bottlenose dolphins (
Tursiops sp.) in Shark Bay, Parsons [
11] on killer whales, and Marley [
12] on
Tursiops aduncus in Western Australia. While useful, such methods often simplify the complexity of cetacean interactions, underscoring the need for more granular approaches supported by emerging technologies.
Efforts to refine our understanding of cetacean sociality have increasingly focused on describing specific patterns of synchronised behaviour, particularly among animals under human care. Clegg et al. [
13], for instance, analysed synchronised swimming and tactile interactions in bottlenose dolphins (
Tursiops truncatus) in managed environments. Serres et al. [
14] extended these observations to multiple captive cetacean species. However, these studies remain largely constrained to behaviours visible at the surface, offering limited access to the full social repertoire displayed underwater.
To overcome these observational limitations, underwater studies have proved essential. Christiansen et al. [
15] examined the surface-level social behaviour of Indo-Pacific bottlenose dolphins (
Tursiops aduncus) off Zanzibar, noting that the presence of tourist vessels reduced resting and social time while increasing travel and foraging. In contrast, Dudzinski et al. [
16] and Delfour [
17] employed underwater methodologies to provide more detailed behavioural descriptions. More recently, Manitzas Hill [
18] combined both surface and subsurface observations to document activity budgets in killer whales under human care, thereby offering a more comprehensive understanding of behavioural variation in managed populations.
A pivotal contribution to the ethology of killer whales was the ethogram compiled by Martínez and Klinghammer in 1978 [
19], based on both captive and free-ranging individuals. Their work catalogued over 50 behaviours, including both surface and underwater actions. Among the behaviours described was a peculiar interaction termed “nibbling,” in which one whale gently mouthed the tongue of another.
Decades later, Sánchez-Hernández et al. [
7] revisited this behaviour under human care, describing it for the first time in detail and interpreting it as an affiliative interaction. The study found that nibbling occurred predominantly among females and juveniles, and situated it within a broader analysis of reconciliation behaviours, suggesting its importance in promoting group cohesion.
However, in the absence of comparable reports from wild populations, the ethological validity of such behaviours has sometimes been questioned. Some could have hypothesised that such behaviours could be a stereotypy, aberrant behaviour, or ephemeral fad, akin to the placement of dead salmon atop the head—a behaviour previously reported in killer whales.
This study presents the first documented case of tongue-nibbling between two wild killer whales in Norway. This finding confirms that the behaviour, previously observed only under human care, also occurs in the wild, thereby supporting its interpretation as part of the natural social repertoire of the species.
2. Materials and Methods
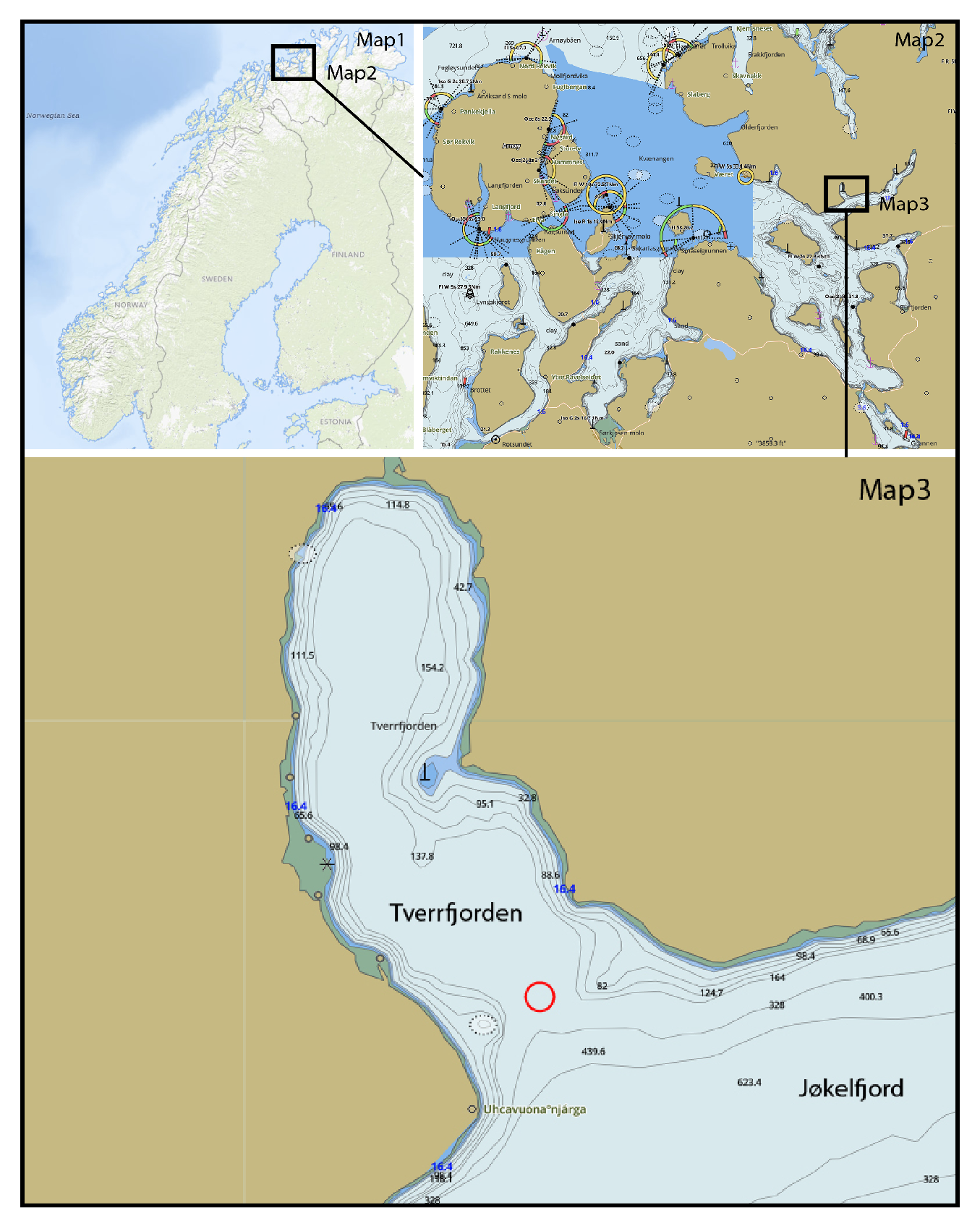
The present observations were conducted in the Kvænangen fjords, northern Norway, approximately 109 km northeast of Tromsø. Specifically, the focal interaction occurred at the entrance of Tverrfjorden, a smaller bay situated on the northern side of Jøkelfjord. The fjord extends approximately 15 km inland along the eastern coast of Spildra Island. This location is characterised by sheltered waters that offer protection from harsh winter conditions, making it ideal for marine wildlife observation. The calm environment allows for detailed behavioural monitoring from both Zodiac platforms and in-water perspectives during snorkelling operations.
The data were collected during the annual Winter Whales of Norway expeditions, operated by Waterproof Expeditions. These expeditions have been conducted for over 15 years and occur between 22 October and 22 January each year, with a brief pause between 11 December and 4 January due to limited daylight. Since 2022, the company Wild-Encounters has joined as expedition leaders and experts aboard one of the participating vessels. In the 2023 and 2024 seasons, an additional six-day, seven-night expedition was added to accommodate the earlier arrival of whales in the region.
All observations described herein were carried out aboard the Vestland Explorer, a 46-metre-long, 11-metre-wide passenger and cargo vessel that has supported the Winter Whales of Norway programme since 2022. The ship is equipped with two inflatable boats (Zodiacs), which are deployed for close-range cetacean observations. Each expedition accommodates a maximum of 12 guests and lasts six nights and seven days, departing from and returning to Tromsø. Across each season, eight such expeditions are conducted aboard this vessel.
Prior to departure, all guests receive a comprehensive safety and conduct briefing led by the expedition team. This includes protocols for Zodiac boarding and disembarkation, wildlife interaction guidelines, and behaviour expectations during whale-watching activities. Particular emphasis is placed on minimising disturbance to cetaceans and never entering the water without explicit permission from the expedition leaders.
On the first evening of each trip, guests are fitted with snorkelling gear. Each participant is provided with thermal overalls, a drysuit, a 10mm neoprene hood in orange for visibility, a mask, snorkel, fins, and 7mm gloves to ensure warmth and safety in Arctic waters.
Daily expedition routes are determined each morning following a weather and wildlife activity briefing between the captain and expedition leaders. At approximately 08:30, the vessel departs based on environmental conditions, whale sightings, and the location of fishing and whale-watching vessels in the area. Cetacean detection is conducted from the vessel’s bridge, where guests are encouraged to assist in wildlife spotting under the guidance of the expedition team. When a group of whales or orcas is sighted, the vessel may alter course for further observation, subject to safety and ethical considerations.
The decision to launch Zodiacs is based on multiple criteria, including wind speeds below 30 knots, swell heights under one metre, and limited vessel traffic in proximity to the observed animals. Compliance with local regulations requires maintaining a distance of at least 370 m from fishing vessels (740 m when in Zodiacs). If the animals are resting, socialising, or travelling at high speed, Zodiacs are not launched. When animals are feeding or travelling slowly, and conditions are favourable, the expedition leaders initiate snorkelling operations with the goal of maintaining non-invasive, respectful engagement.
Once the decision to deploy Zodiacs is made, guests are instructed to prepare their snorkelling gear. The preparation time varies between individuals, ranging from 15 to 45 minutes. Each Zodiac carries six guests and one expedition leader. Upon launching, animal behaviour is continually monitored. The approach is made in parallel with the group of cetaceans from the side, with expedition leaders carefully assessing group dynamics, breathing patterns, and reactions to the Zodiac. If no signs of disturbance or avoidance are detected, the Zodiac positions itself at approximately a 2 o’clock angle to the group.
Once in position, guests complete their final gear checks, including adjusting masks, activating cameras, and positioning fins. Upon instruction from the expedition leader, guests gently enter the water and swim forward to form a group in front of the Zodiac, which then reverses to maintain a safe distance. A dive flag is raised at the rear of the boat to indicate the presence of snorkellers to nearby vessels. Guests remain floating in a passive position to observe cetaceans without initiating contact or pursuit.
Throughout the expedition, guests record their experiences using personal devices, including GoPro, Insta360, and other action or professional-grade cameras. These recordings capture both onboard and in-water interactions. Upon returning to the main vessel, guests are invited to voluntarily share their footage with the expedition team. With prior informed consent, media are transferred to a designated laptop or database managed by the team. Guests are informed that shared materials may be used for scientific research and educational or communication purposes related to the expedition. All data are handled respectfully and in accordance with participant consent.
Each evening, one of the expedition leaders compiles a curated recap of the day’s observations using the collected footage. This is presented in the lounge before dinner, providing an opportunity for guests to review the day’s highlights and learn more about observed species and behaviours. These presentations are supplemented with interpretive commentary and informal lectures. After the expedition, the media summaries are shared with all guests, creating an archive of both educational and observational material for participants and the research team.
3. Results
On 31 October 2024, at approximately 13:30 local time, a behavioural interaction between two free-ranging killer whales (Orcinus orca) was recorded during a snorkelling expedition at the entrance of Tverrfjorden, a sheltered bay located on the northern side of Jøkelfjorden in the Kvænangen fjords, northern Norway. The weather conditions were calm and overcast, with light winds between 4 and 6 knots and mirror-like water surfaces within Tverrfjorden. These conditions provided a stark contrast to the adjacent fjord systems, where wind gusts exceeded 35 knots and wave heights reached approximately 50 cm in Jøkelfjorden. No other vessels or RIBs were present in the area at the time of the observation.
The research team operated two Zodiacs, each carrying six guests and an expedition leader. The main vessel, Vestland Explorer, remained anchored outside Jøkelfjorden and did not enter Tverrfjorden during the operations. Multiple groups of killer whales were present in the fjord throughout the day, with an estimated total of approximately 30 individuals. Observations began at around 11:00, with initial sightings focused on a group of approximately 12 individuals engaged in sub-surface feeding behaviour on dispersed bait balls. Additional groups were observed resting near the coastline.
At the time of the focal event, the group of 12 guests was floating in a single line formation at the entrance of Tverrfjorden. Within this context, two killer whales were observed engaging in a prolonged mouth-to-mouth interaction (
Figure 2) that lasted for a total of 1 minute and 49 seconds. The individuals approached one another and maintained contact between the anterior portions of their heads. The interaction comprised three discrete episodes: the first lasting 10 seconds, the second 26 seconds, and the third 18 seconds. Following the final episode, the individuals separated and swam away (See complete video in the complementary materials).
The observed behaviour is consistent with the affiliative tongue-nibbling interaction previously described in a 2013 study at Loro Parque involving two killer whales under human care (
Figure 3). In that case, one individual protruded its tongue while the other made gentle nibbling movements. The behaviour occurred in three sequences, interrupted by the withdrawal and re-extension of the tongue, lasting a total of approximately 15 seconds (See complete video in the complementary materials).
To assess the rarity and broader context of this behaviour, the authors consulted several professional divers and underwater videographers with extensive experience documenting killer whales in different geographic locations. None of the individuals consulted reported having recorded or directly observed tongue-nibbling interactions. One diver did recall an incident in which several killer whales approached a RIB while the group was preparing to enter the water. At that time, some observers on board remarked that the animals appeared to be “kissing” beneath the boat—a description identical to that provided independently by the guests who recorded the event in Tverrfjorden. Additionally, the authors sought confirmation from the senior marine mammal trainers at Loro Parque, who affirmed their familiarity with the behaviour. They reported that tongue-nibbling was observed repeatedly among four individuals housed at the facility, although the behaviour had not been observed in subsequent years.
Figure 1.
Map showing the location of the Kvænangen fjords, northern Norway, where the observed interaction between two wild killer whales (Orcinus orca) was recorded.
Figure 1.
Map showing the location of the Kvænangen fjords, northern Norway, where the observed interaction between two wild killer whales (Orcinus orca) was recorded.
Figure 2.
Still frame from video footage recorded in the Kvænangen fjords, Norway, in 2024, showing the tongue-nibbling interaction between two free-ranging killer whales. See
Supplementary Materials for the complete video sequence.
Figure 2.
Still frame from video footage recorded in the Kvænangen fjords, Norway, in 2024, showing the tongue-nibbling interaction between two free-ranging killer whales. See
Supplementary Materials for the complete video sequence.
Figure 3.
Still frame from video footage recorded at Loro Parque in 2013, illustrating the tongue-nibbling behaviour between two killer whales under human care. See
Supplementary Materials for the full video sequence.
Figure 3.
Still frame from video footage recorded at Loro Parque in 2013, illustrating the tongue-nibbling behaviour between two killer whales under human care. See
Supplementary Materials for the full video sequence.
4. Discussion
Although the video footage recorded in the Kvænangen fjords lacks the resolution required to discern fine-scale details of the tongue and mouth movements of the wild killer whales involved, the sustained frontal facial contact between the individuals, the prolonged duration of the episodes, and the overall behavioural pattern strongly support the interpretation that the observed interaction corresponds to the tongue-nibbling behaviour originally described by Martínez and Klinghammer in 1978 [
19] and later documented in detail in a zoological context by Sánchez-Hernández et al. (2019) [
7]. These new observations provide empirical support for the hypothesis that tongue-nibbling forms part of the natural behavioural repertoire of
Orcinus orca, and is not a behavioural artefact induced by captivity-related conditions such as stereotypy, aberrant expression, or transient cultural novelty.
The reappearance of this behaviour more than three decades after its initial description under human care—first noted shortly after killer whale husbandry was established, and subsequently recorded in detail in 2013—suggests remarkable behavioural continuity across generations in zoological environments. This temporal consistency implies that tongue-nibbling may be a socially conserved behaviour. Furthermore, the fact that it has taken 47 years since its original ethological description to obtain comparable footage from a wild population highlights the considerable difficulty in documenting rare or cryptic behaviours in natural contexts, particularly in highly mobile marine species whose social interactions occur predominantly beneath the water’s surface.
Notably, tongue-nibbling in killer whales exhibits significant parallels with recently documented mouth-to-mouth interactions in belugas (
Delphinapterus leucas) under human care, as reported by Ham et al. (2023) [
20]. In both species, these interactions primarily involve younger individuals and appear to serve a clearly affiliative function. As with the behaviour described by Martínez and Klinghammer [
19] and later detailed by Sánchez-Hernández et al. [
7], the beluga interactions are characterised by gentle, coordinated oral contact without any evident signs of aggression or dominance. This congruence supports the affiliative interpretation advanced by Sánchez-Hernández and colleagues, and suggests that oral behaviours may represent socially meaningful interactions in odontocetes.
The cross-species resemblance reinforces the hypothesis that oral contact behaviours in toothed whales may contribute to the development of both social and motor skills during early ontogeny. In this context, tongue-nibbling and similar behaviours may serve as low-conflict mechanisms for strengthening social bonds among conspecifics not yet involved in adult roles such as mating or dominance competition. The recurrence of such behaviours in distantly related taxa, including killer whales and belugas, suggests that affiliative oral interactions may represent a phylogenetically conserved socio-developmental strategy in odontocetes.
The individual in which Martínez and Klinghammer [
19] first recorded tongue-nibbling—Hugo—was a juvenile male of approximately 13 years, originating from the Southern Resident killer whale population off Vancouver Island in the Pacific Ocean [
21]. By contrast, the individuals observed by Sánchez-Hernández et al. [
7] at Loro Parque in 2013 were all born under human care and descended from a mix of lineages: some of Icelandic (North Atlantic) and Canadian (North Pacific) origin, while one female, Morgan, was a rescued individual from the Norwegian (North Atlantic) population [
22]. This diversity of geographic and genetic origins suggests that tongue-nibbling is not population-specific, but rather a widely distributed behaviour within the species.
Historically, studies of cetacean social behaviour have relied heavily on surface-based observations from vessels or coastal vantage points [
8,
9], which severely constrain the ability to describe the subtleties and complexity of social interactions. These methodological limitations have often led to the grouping of heterogeneous behaviours under broad, catch-all categories such as “socialising” [
10,
11,
12], thereby obscuring distinctions between specific types of interaction. Only in recent years has progress been made in refining ethological classifications at the surface, with increased attention to behavioural patterns such as synchronised swimming and physical contact in multiple cetacean species [
13,
14,
15]. Simultaneously, the use of underwater observation techniques has yielded more detailed behavioural data, facilitating the identification of specific interactions such as petting, rubbing, contact swimming, nibbling, and nudging in dolphins [
16,
17], and more recently in killer whales [
18].
That tongue-nibbling in killer whales—an interaction primarily expressed underwater—remained undescribed in the wild for nearly five decades underscores the indispensable role of subaquatic methodologies in cetacean behavioural research. Omitting the underwater dimension risks underestimating the richness of cetacean sociality and overlooking behaviours that may be central to group cohesion and individual bonding. Continued investment in underwater technologies and observation protocols is therefore vital to developing a more comprehensive and ecologically valid understanding of cetacean social systems.
Although killer whales under human care are unable to exhibit certain behaviours seen in wild populations, such as complex coordinated hunting strategies [
23], the sustained occurrence of tongue-nibbling—a clearly affiliative behaviour—across multiple decades in zoological facilities calls into question generalised criticisms regarding the relevance of managed populations for the study of natural social behaviour in cetaceans [
24]. These findings support the argument that killer whales, and potentially other cetaceans in human care, may serve as valuable models for investigating naturally occurring social dynamics under controlled, observable conditions.
Moreover, the long-term maintenance of affiliative interactions such as tongue-nibbling directly challenges reductionist perspectives that emphasise aggression or stress-related behaviours in captivity [
25]. A scientifically robust analysis of cetacean sociality in zoological settings must encompass the full behavioural spectrum—including affiliative, sexual, and reconciliatory interactions—as well as the dynamic processes that contribute to social stability [
7,
18].
Accordingly, evaluating the social complexity of killer whales in managed environments demands methodologically rigorous approaches, grounded in comprehensive ethograms and incorporating both surface and subaquatic observations. Such an integrative framework enhances the interpretive resolution of behavioural studies, facilitates meaningful cross-context comparisons, and contributes to a more nuanced understanding of cetacean socioecology.
The successful documentation of a rare and cryptic behaviour such as tongue-nibbling in wild killer whales was made possible in part through contributions from citizen science and the increasing global prevalence of recreational in-water cetacean encounters. While it is valuable that such interactions occasionally yield data of scientific relevance, it is well established that tourism-based activities—such as whale watching and swim-with-cetaceans programmes—may pose significant risks to wild populations. Numerous studies have documented potential adverse effects, including altered behavioural patterns, increased physiological stress, and disruptions to group cohesion [
26,
27,
28,
29,
30].
It is therefore essential that all wildlife interaction activities comply strictly with established local and international regulations. Moreover, continuous welfare monitoring and robust impact assessments should be mandatory components of such programmes. Balancing the research potential of citizen-generated data with the ethical imperative to minimise anthropogenic disturbance is critical to ensuring both the integrity of behavioural science and the conservation of cetacean populations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
JA conceptualized the study, wrote the manuscript, analyzed the data and produced the figures. J.V. and D.B. run the citizen science program and participated in the review and editing of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research in Loro Parque has been approved by the Research Ethics and Animal Welfare Committee of the University of La Laguna. Reg. Num. (CEIBA2024-3515).
Data Availability Statement
Acknowledgments
The authors would like to express their sincere gratitude to the individuals who captured the footage of the observed behaviour: Allison Kelly Estevez, and Michael Estevez. We also extend our appreciation to the 95 guests who voluntarily shared their recordings during the Winter Whales of Norway Expeditions, operated by Waterproof Expeditions and led by Wild-Encounters under the direction of Johnny van Vliet and Debbie Bouma, between 22 October and 9 December 2024. Their generous contributions provided invaluable material for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alexander, R.D. The Evolution of Social Behavior. Annual Review of Ecology and Systematics 1974, 5. [CrossRef]
- Eberhard, M.J.W. The Evolution of Social Behavior by Kin Selection. The Quarterly Review of Biology 1975, 50, 1–33. [CrossRef]
- de Waal, F.B.M.; Veenema, H.C., CHAPTER 2 The First Kiss: Foundations of Conflict Resolution Research in Animals. In Natural Conflict Resolution; 2023. [CrossRef]
- Robbins, M.M.; Robbins, A.M.; Gerald-Steklis, N.; Steklis, H.D. Long-Term Dominance Relationships in Female Mountain Gorillas: Strength, Stability and Determinants of Rank. Behaviour 2005, 142, 779–809. [CrossRef]
- Wittemyer, G.; Getz, W.M. Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Animal Behaviour 2007, 73, 671–681. [CrossRef]
- Tamaki, N.; Morisaka, T.; Taki, M. Does body contact contribute towards repairing relationships? Behavioural Processes 2006, 73, 209–215. [CrossRef]
- Sánchez-Hernández, P.; Krasheninnikova, A.; Almunia, J.; Molina-Borja, M. Social interaction analysis in captive orcas ( Orcinus orca ). Zoo Biology 2019, pp. 1–11. [CrossRef]
- Thomsen, F.; Franck, D.; Ford, J.K.B. On the communicative significance of whistles in wild killer whales (Orcinus orca). Naturwissenschaften 2002, 89, 404–407. [CrossRef]
- Ford, J.K.B. Acoustic behaviour of resident killer whales ( Orcinus orca ) off Vancouver Island, British Columbia. Canadian Journal of Zoology 1989, 67, 727–745. [CrossRef]
- Gibson, Q.A.; Mann, J. The size, composition and function of wild bottlenose dolphin (Tursiops sp.) mother-calf groups in Shark Bay, Australia. Animal Behaviour 2008, 76, 389–405. [CrossRef]
- Parsons, K.M.; Balcomb, K.C.; Ford, J.K.B.; Durban, J.W. The social dynamics of southern resident killer whales and conservation implications for this endangered population. Animal Behaviour 2009, 77, 963–971. [CrossRef]
- Marley, S.A.; Kent, C.P.S.; Erbe, C.; Parnum, I.M. Effects of vessel traffic and underwater noise on the movement, behaviour and vocalisations of bottlenose dolphins in an urbanised estuary. Scientific Reports 2017, 7, 13437. [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Delfour, F. Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behavioural Brain Research 2017, 322, 115–122. [CrossRef]
- Serres, A.; Hao, Y.; Wang, D. Swimming features in captive odontocetes: Indicative of animals’ emotional state? Behavioural Processes 2020, 170. [CrossRef]
- Christiansen, F.; Lusseau, D.; Stensland, E.; Berggren, P. Effects of tourist boats on the behaviour of Indo-Pacific bottlenose dolphins off the south coast of Zanzibar. Endangered Species Research 2010, 11, 91–99. [CrossRef]
- Dudzinski, K.M.; Gregg, J.D.; Ribic, C.A.; Kuczaj, S.A. A comparison of pectoral fin contact between two different wild dolphin populations. Behavioural Processes 2009, 80, 182–190. [CrossRef]
- Delfour, F.; Vaicekauskaite, R.; García-Párraga, D.; Pilenga, C.; Serres, A.; Brasseur, I.; Pascaud, A.; Perlado-Campos, E.; Sánchez-Contreras, G.J.; Baumgartner, K.; et al. Behavioural diversity study in bottlenose dolphin (Tursiops truncatus) groups and its implications for welfare assessments. Animals 2021, 11. [CrossRef]
- Hill, H.M.M.; Themelin, M.; Dudzinski, K.M.; Felice, M.; Robeck, T. Individual variation in activity budgets of a stable population of killer whales in managed care across a year. Behavioural Processes 2025, 224. [CrossRef]
- Martinez, D.R.; Klinghammer, E. A partial ethogram of the killer whale (Orcinus orca). Carnivore 1978, 1, 13–27.
- Ham, J.R.; Lilley, M.K.; Wincheski, R.J.; Miranda, J.; Ángel G. Velarde Dediós.; Kolodziej, K.; Pellis, S.M.; Hill, H.M.M. Playful mouth-to-mouth interactions of belugas (Delphinapterus leucas) in managed care. Zoo Biology 2023, 42, 730–743. [CrossRef]
- Wearing, S.; Buchmann, A.; Jobberns, C. Free Willy: The whale-watching legacy. Worldwide Hospitality and Tourism Themes 2011, 3, 127–140. [CrossRef]
- Kremers, D.; Lemasson, A.; Almunia, J.; Wanker, R. Vocal sharing and individual acoustic distinctiveness within a group of captive orcas (Orcinus orca). Journal of comparative psychology 2012, 126, 433–45. [CrossRef]
- Williams, R.; Lusseau, D. A killer whale social network is vulnerable to targeted removals. Biology Letters 2006, 2. [CrossRef]
- Corkeron, P., Marine Mammal Captivity, an Evolving Issue; 2022. [CrossRef]
- Marino, L.; Rose, N.A.; Visser, I.N.; Rally, H.; Ferdowsian, H.; Slootsky, V. The harmful effects of captivity and chronic stress on the well-being of orcas (Orcinus orca), 2020. [CrossRef]
- Erbe, C. Underwater noise of whale-watching boats and potential effects on killer whales (Orcinus orca), based on an acoustic impact model. Marine Mammal Science 2002, 18, 394–418. [CrossRef]
- Constantine, R.; Brunton, D.H.; Dennis, T. Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behaviour. Biological Conservation 2004, 117, 299–307. [CrossRef]
- Schaffar, A.; Madon, B.; Garrigue, C.; Constantine, R. Avoidance of whale watching boats by humpback whales in their main breeding ground in New Caledonia. International Whaling Commission 2009, SC/61/WW6, 9.
- Christiansen, F.; Rasmussen, M.H.; Lusseau, D. Inferring energy expenditure from respiration rates in minke whales to measure the effects of whale watching boat interactions. Journal of Experimental Marine Biology and Ecology 2014, 459, 96–104. [CrossRef]
- Oliveira, C.; Pérez-Jorge, S.; Prieto, R.; Cascão, I.; Wensveen, P.J.; Silva, M.A. Exposure to whale watching vessels affects dive ascents and resting behavior in sperm whales. Frontiers in Marine Science 2022, 9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).