1. Introduction
Under the thermal dependence of physiological processes, ectotherms must regulate their body temperature close to their preferred temperature range (Tpref /Tsel) for the optimal performance of various physiological functions, such as metabolic rate, locomotor performance, digestive efficiency, growth rate, egg production, spermatogenesis and sperm maturation [
1,
2,
3,
4,
5]. To this thermal dependence, reports on the susceptibility of germ cell damage to extreme temperature changes in lizards date back as far as the 1940 showed that high temperatures cause sterility [
6] and subsequently, asserting that there is a greater sensitivity of germ cells concerning somatic cells under conditions of temperature stress [
7].
A study in
Sceloporus siniferus [
8] revealed the possibility of a negative effect of temperature on epididymal sperm maturation due to CD retention since the collection of males between 2015 and 2016 shows a percentage of sperm with significantly different CD. It is observed that the sperm percentage with CD in the epididymis in 2015 was approximately 10%; in 2016, the percentage remained around 40%. Moreover, it was observed that the specimens collected in 2015 show a cloacal temperature of 28 ⁰C, while those collected in 2016 presented a temperature of 34 ⁰C
Studies in
Sceloporus aeneus detail the positive and negative repercussions of subjecting specimens to preferred temperatures, highlighting locomotor performance and sperm quality. In the first case, animals that are kept in a preferred temperature environment favor motility interval, sprint speed, and foraging, however, some sperm parameters are decreased, such as concentration, viability, and motility, as well as the increase of spermatozoa with CD [
3]. These aspects had been considered by Méndez-De La Cruz et al. (2014) [
2], who proposed that low temperatures, also given by the inactivity schedules of the organisms, may be the ones in charge of favoring such reproductive parameters.
Recently, in
Sceloporus megalepidurus, these aspects were shown, where temperatures below the preferred temperature favor viability and epididymal sperm maturation, contrary to individuals subjected to higher temperatures, where the cases of sperm DNA fragmentation and the presence of CD increased [
5].
Even more, in
Lepidophyma pajapanense, it was found that copulation occurs in the coldest months and the ovulation at the beginning of the warmest season; perhaps that asynchrony in reproduction is due to the favorable temperature for males’ mismatch with the ones that are favorable for the fecundation. Moreover, a significant percentage of infertile eggs was found, perhaps due to low sperm quality or to the long sperm retention in females, or both [
9]. Therefore, the interaction between sperm quality and temperature is an important issue to resolve.
The study of lizard thermal ecology is relevant to understanding their distribution, life history, ecology, and reproductive aspects. It also reveals how species can achieve physiologically active body temperatures, evaluating the risk of extinction in the face of climate change and its general consequences.
Also known as Gaiger's lizard [
10],
Lepidophyma gaigeae is an endemic species of Mexico, whose habitat includes pine and oak forests, grasslands and xerophytic scrub preferably in outcrops and between the crevices of limestone rocks [
11], at altitude of 1500 to 2500 m, in states such as Hidalgo and Querétaro [
12]. It is a small-sized viviparous lizard, between 47 and 54 mm (snout-vent length), one of the smallest species of the genus [
13]. It presents a diurnal activity period [
14], although some nocturnal individuals have been found [
13]. In areas of thermal ecology, Arenas-Moreno et al. (2018) [
13] state that
L. gaigeae has a preferred temperature range of around 24 °C, a range similar to that reported in other species of its genera [
15,
16].
The present study aims to determine if the preferred temperature could be the cause of the alteration in some sperm parameters in the testis and/or epididymis, as well as the migration of CD and the affectation of DNA integrity in spermatozoa of the lizard L. gaigeae.
2. Materials and Methods
As a species under special protection according to Mexican Official Standard NOM-059-ECOL-1994 and so as not to alter availability of adult males in the area and consider their distribution in the future, only 12 male individuals were captured during December 2020 in the surroundings of Landa de Matamoros, Querétaro, in the area known as "El Lobo" (21°17'32.5 "N 99°07'13.1 "W), with a collection schedule from 9 am to approximately 3 pm, which coincides with their diurnal usual activity schedule.
For the selection, male lizards with snout-vent length (SVL) greater than 47 mm and a visible bulging in the ventral region due to the presence of hemipenes inside the cloaca, characteristics described by Goldberg & Camarillo (2003) [
17].
The specimens were captured by direct capture methods, either manually or with the use of a fishing rod, depending on the location of the specimens and the availability of space for free collection of the specimens.
The captured animals were housed in 20 x 15cm blanket bags, with a maximum of 4 specimens each, to be later contained in plastic racks to facilitate their transfer and to be transported to the vicinity of the Laboratory of Morphophysiology and Biochemistry of Spermatozoa, Universidad Autónoma Metropolitana-Iztapalapa, to proceed with the acclimatization procedures. The transport vehicle maintained a temperature between 21 °C and 24 °C according to the preferred temperature range of the species (24 °C) [
13], avoiding stretches that generate sudden movements during their arrival at the facilities.
2.1. Inclusion of Specimens
The lizards were separated into 3 groups (4 individuals per group): control group, analyzed without treatment; preferred temperature (Tpref) (24
OC), and low temperature (LT) (21
OC), following the considered observations of [
2,
3,
5,
17]), where a range between the lower limits of the Tpref and without reaching the upper limit of the critical minimum temperature of the species is considered. Treatments were maintained during the activity time of the lizards (9 am to approximately 3 pm), placed in incubators "Hova Bator 1602N" of thermal radiation, with the required temperature, including the temperature during scotophase [
13]. They were checked daily and maintained with water and live food, ad libitum during the 14 days of treatment.
2.2. Collection of Biological Material
The control specimens were sacrificed by intraperitoneal injection with 0.10 mL of sodium pentobarbital (Sedalpharma, from Pet's Pharma de México, S.A. de C. V) 36 hours after the arrival of the specimens at the University facilities for the Control group and after 14 days of treatment for the remaining groups, with adherence to the guide for the care and use of laboratory animals (Institute of Laboratory Animal Resources, National Research Council, 1996), as well as the Mexican Official Standard NOM-033-SAG/ZOO-2014, Methods for killing domestic and wild animals. Both testes and epididymides were extracted, measured (diameters and lengths) with a digital caliper (precision=±0.5mm) and weighed with a METTLER TOLEDO AB204-S balance (precision =±0.1mg), of which, those on the left side were used for sperm parameters, although those on the right side were fixed in 4% paraformaldehyde (PAF) (Thermo Scientific) and subsequently sagittally sectioned for further study.
Each epididymis was sectioned into three regions with similar proportions: caput, corpus, and cauda.
To obtain sperm parameters, the organs were placed in a four-well Petri dish with 250 μL of physiological Ringer's solution (NaCl, KCl, KH2PO4, CaCl2 2H2O from Baker Laboratories) to obtain as many spermatozoa as possible, then the biological material was transferred to a 1.5mL Eppendorf tube [
18]. Subsequently, the material was centrifuged at 250 G for 5 minutes, the supernatant was removed, and the cell button was disaggregated with 250 μL of Ringer's solution twice. Thus, the resultant will be "washed spermatozoa.
The spermatozoa obtained were evaluated according to the World Health Organization specifications for the evaluation of human semen (WHO, 2010) with necessary adjustments for lizard cells obtained from the different organs. The variables and methods to obtaining them were as follows:
Total number of spermatozoa per organ (n spz X 106/ organ): obtained by counting the spermatozoa with the Neubauer chamber, making a dilution depending on the density of spermatozoa identified at a field of 40x, filling both chambers with 10 to 15 µL of the dilution.
To obtain the sperm concentration, the formula was used: N/n x (1/20) x dilution factor, where N is the total number of spermatozoa counted in both chambers and n is the number of squares counted.
To calculate the viability percentage and CD, a 5 µL aliquot of the sperm suspension was placed on a slide and mixed with an aliquot of the same volume of Spermavit supravital stain and then smeared and observed under brightfield microscopy for counting.
Percentage of DNA integrity: A 10 µL aliquot of the sperm suspension was placed on a slide to make a smear and allowed to dry at room temperature, then included in Carnoy's solution (ethanol from Meyer Laboratories, chloroform and acetic acid from Baker Laboratories) for approximately 24 hrs in a cool, dark place, Afterwards, the fixative was removed and the slides were included in acridine orange solution for 5 minutes, then, the excess dye was removed and left to dry isolated from light, finally, the slides were analyzed with the aid of fluorescence microscopy at 40x.
The handling of chemical-biological waste generated in the experiment was instructed by the Mexican Official Standard NOM-033-STPS-2015, safety conditions for working in confined spaces.
2.3. Statistical Analysis
It was used to identify the existence of significant differences among groups, performing parametric analysis using an analysis of variance (ANOVA) for the evaluation of the three treatments, as well as to evaluate variability and random errors so that possible environmental and temporal effects are distributed equally among the treatments, followed by a Tukey post-hoc test and a Kruskal-Wallis non-parametric analysis for significantly different means. The data were previously analyzed to meet the assumptions of the parametric test used (normality and homoscedasticity), considering the data from the different organisms analyzed as independent.
4. Discussion
The formation of spermatozoa takes place in the seminiferous epithelium of the testis, through a process known as spermatogenesis, a process by which, from diploid round cells (spermatogonia) located adjacent to the basement membrane of the seminiferous tubules, they differentiate until forming spermatozoa, advancing towards the tubule lumen [
19,
20]. However, even when the morphological differentiation of the spermatozoa culminates in the testis when the spermatozoa are released from it, they have not yet acquired the capacity to fertilize the oocyte, for this reason they need to pass through the epididymis, where they acquire this capacity, a process known as epididymal sperm maturation [
21,
22], where the following stand out: loss of cytoplasmic droplet (CD) and chromatin hypercompaction about the maintenance of DNA integrity [
8,
23,
24].
Climate change may cause the extinction of species, as well as a shift in their distribution in the coming decades [
2,
25]. Since 1975, 12% of local lizard populations have gone extinct, as well as 4% around the world. By 2080, extinction is projected to approach 39% globally and species extinction by 20%. If the maximum temperature derived from global warming continues unchanged, it is estimated that, in Mexico, in the year 2050, 56% of the viviparous species will become extinct and 66% by 2080. The causes of extinction were attributed to low thermal quality that impairs the lizards' ability to perform basic biological activities and increases the restriction hours [
25]. Nevertheless, the temperatures may decrease the thermal quality of the sperm, and the males become infertile. The results of this study show that the sperm is labile (in quality and morphology) when lizards are overexposed to the preferred temperatures and is even worse at higher temperatures.
For example, as occurred with the difference in testicular size, previous studies with
Sceloporus megalepidurus [
5] showed that individuals who remained in treatment at the preferred temperature for 14 days presented smaller testis and epididymal, being one of the first characteristics described in reptiles affected by temperature changes. It is known that the volume of reproductive organs can be an indirect indicator of sperm concentration [
26]. In the present study, we found that this difference in volume could be attributed to the decrease of spermatozoa in the group exposed to preferred temperature [
3,
21,
27]. In the
Lepidophyma pajanapense, a high percentage of infertile eggs was found [
26]. That low percentage could be attributed to the long sperm retention or the low quality of the sperm cells, even when copula occurs during the coldest months of the year, perhaps due to the inability to manage the warm temperatures during the ovulation months.
It is known that chronic hyperthermia stress can cause alterations in spermatogenesis [
28], as has also been shown in mammalian species such as rats [
29], and sheep [
30]. In addition, these thermal conditions tend to influence reproductive conditions from the hypothalamus-pituitary-gonad axis through the increase of glucocorticoids and the repression of gonadotropin-releasing hormone (GnRH), fundamental for the promotion of spermatogenesis and testosterone production [
31], also causing a reduction in the number of Leydig cells as well as spermatogonia in the seminiferous tubule [
32].
The integrity of the plasmatic membrane is one of the most important characteristics to consider for the evaluation of viable spermatozoa [
33], therefore the use of the eosin-nigrosin dye allowed demonstrating the decrease of this characteristic in individuals maintained at preferred temperatures, bringing it below 50%, a value that resembles that shown in other reptile species [
3,
5]. On the other hand, knowing that sperm viability in each of the epididymal regions may be different according to the functionality of its cellular structure and the achievement of non-functional sperm absorption [
34], the coincidence in the percentage, even in the four regions evaluated allows us to question whether the problem may even come from the concentration of androgens. Given that the epididymis is a hormone-dependent organ, mainly dihydrotestosterone [
35] different analyses have coincided in that the lack of these contributes in the decrease of epididymal weight, as well as in the regulation of secretions that reach the luminal region, such as proteins and transcription factors important for sperm viability and flagellar motility mainly [
13,
36,
37,
38,
39,
40,
41,
42]. In addition, reduction of epididymal epithelium volume may also occur as a consequence of the effect of temperature, with increased apoptosis of constituent cells, such as principal cells [
43].
Although once again the effect that CD causes in lizard spermatozoa has not been analyzed in depth, it is clear with the present study, as well as those reported [
3,
5,
8], that thermal modifications tend to influence its presence along the different regions of the epididymis. This coincides with numerous reports in mammals that document the increase in CD under conditions of testicular hyperthermia, being indicative of immature sperm, given by changes in ambient osmolarity or decreased antioxidant system and increased reactive oxygen species (ROS) and plasma membrane lipid peroxidation [
22,
44,
45,
46].
However, some studies have raised the possibility of a protective function in the face of unfavorable conditions for spermatozoa. [
47] showing that CD spermatozoa may have a higher tolerance to heat stress. In addition to this, it is known that CD has the presence of aquaporins, which can collaborate with osmotic adaptation, migration or sperm storage [
48], besides being enzymatically active [
49] and being an important source of energy for the different maturational aspects of the cells, which is why they can remain in the cell throughout the epididymis [
50].
The relationship that CD has had around the exposure of organisms to high temperatures is given in many cases by its relationship with lipid droplets (LD), which, although in some cases, supplements the function of the latter to provide energy [
50], can also cause lipoperoxidation of the plasma membrane by increasing ROS given mainly by the decompensation of antioxidant enzymes. In hyperthermia treatments, GL has been found along Leydig cells, important for testosterone production and sperm maintenance [
46], but mostly involved in the breakdown of disulfide bridges involved in DNA compaction [
51]. The relationship of increased ROS with sperm viability and morphology also derives from the cell death by apoptosis, where studies show the activation of caspase 3 in seminal samples with high DNA fragmentation index [
52].
Lizards around the world are facing problems to survive; one of the most important is global warming. This study determines that warming temperatures affect not only the hours of activity but also the fertility of males. It was found that rising temperatures arrested female fertility during the El Niño Southern Oscillation event in
Sceloporus mucronatus [
53] (Rodríguez Romero and Méndez de la Cruz, 2004). This study determines that sperm quality could be severely affected by environmental temperatures and may be the cause of that infertility. Therefore, the declining of populations may also be due to the infertility of males exposed to warmer temperatures. Furthermore, it may promote dissociation in reproductive cycles, as in
L. pajapanense, where males copulate during the colder months while females ovulate in the warmer ones [
9]. This study shows that the conditions for fully fertile males are an issue that demands more studies as there is a connection between physiology and environmental temperatures.
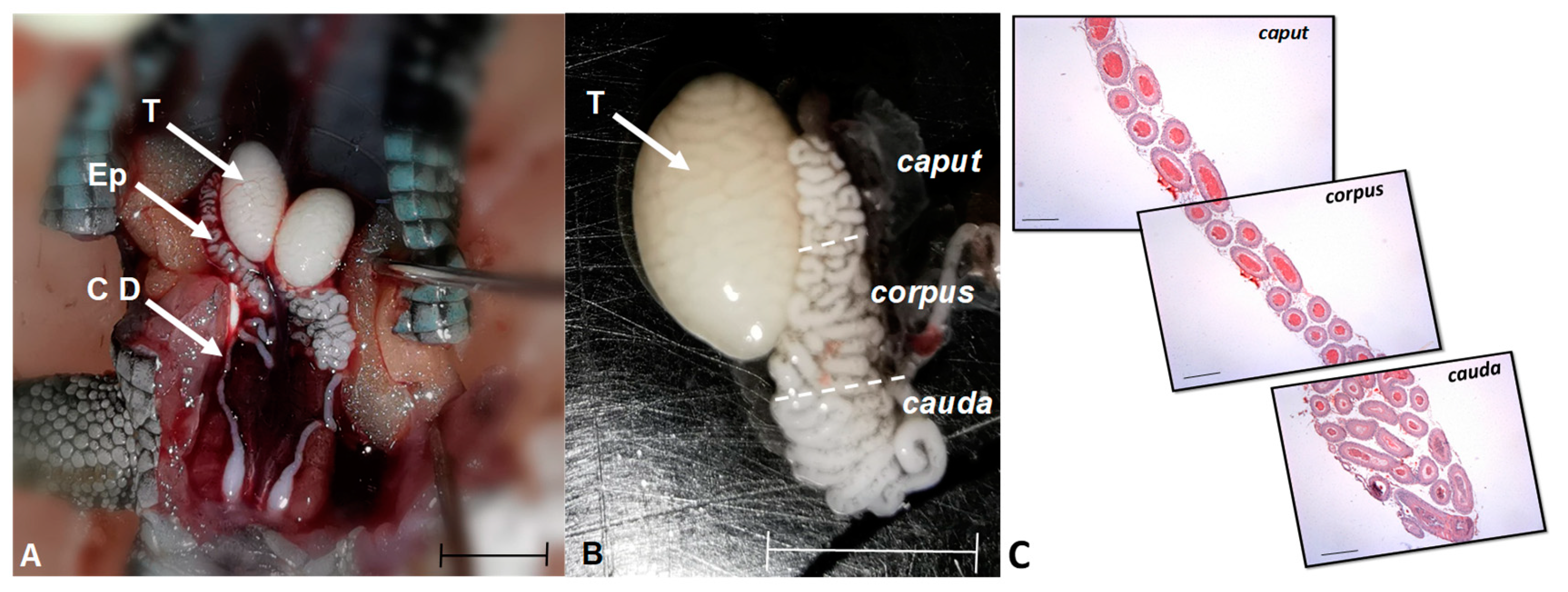
Figure 1.
Position and regionalization of male gonads of L. gaigeae in reproductive stage. In A: Location of the gonadal position. Arrows show the testis (T) and epididymis (Ep) of the specimen. In B: Testis and epididymis with regionalization to identify the area of the caput, corpus and cauda. Bar: 0.5 cm. In C: Photomicrographs in sequence showing the different histomorphology of the three epididymal regions in longitudinal section, obtained with H&E at 5x. Bar at 50 µm.
Figure 1.
Position and regionalization of male gonads of L. gaigeae in reproductive stage. In A: Location of the gonadal position. Arrows show the testis (T) and epididymis (Ep) of the specimen. In B: Testis and epididymis with regionalization to identify the area of the caput, corpus and cauda. Bar: 0.5 cm. In C: Photomicrographs in sequence showing the different histomorphology of the three epididymal regions in longitudinal section, obtained with H&E at 5x. Bar at 50 µm.
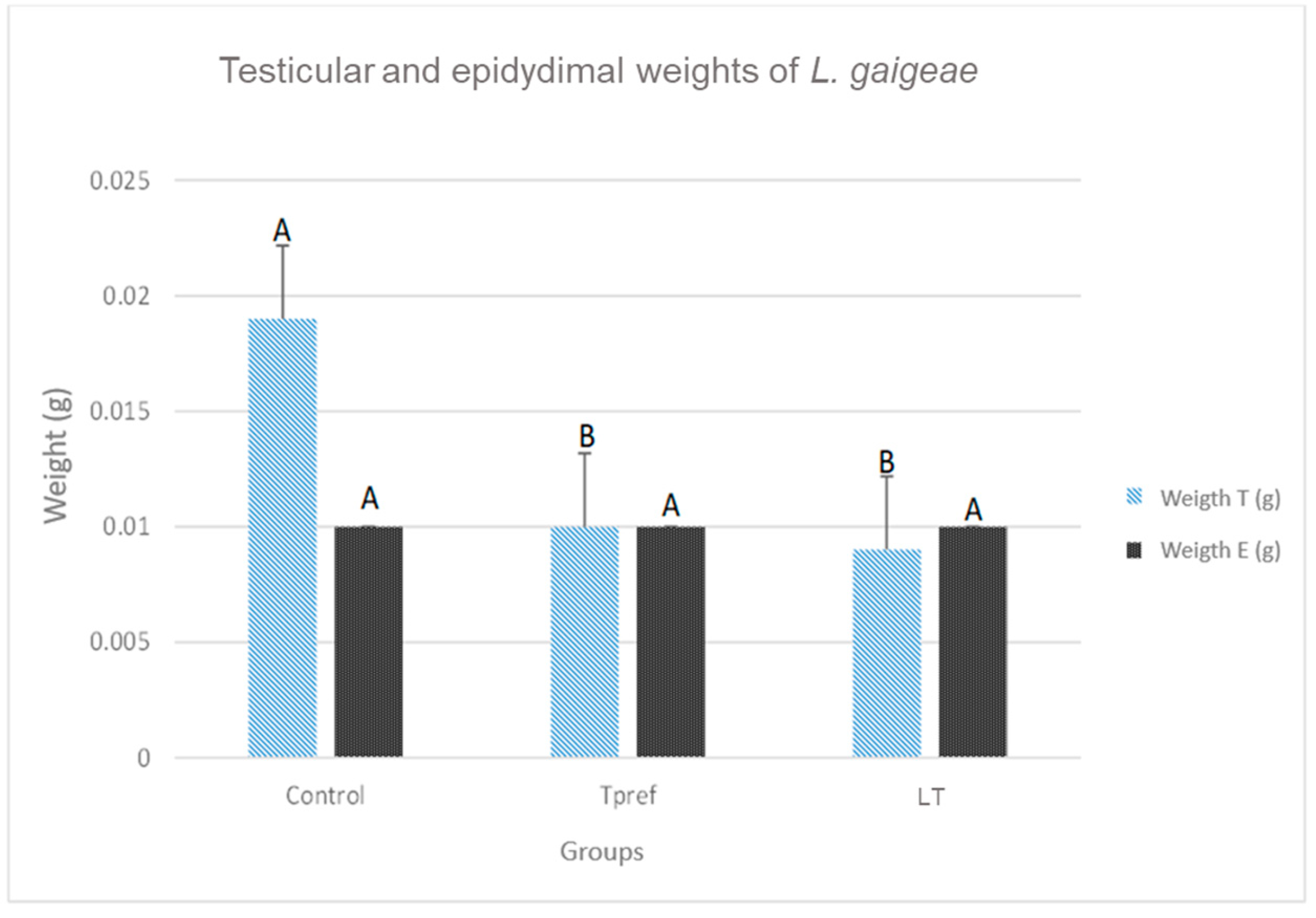
Figure 2.
Weight (g) of testis and epididymis of L. gaigeae specimens with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
Figure 2.
Weight (g) of testis and epididymis of L. gaigeae specimens with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
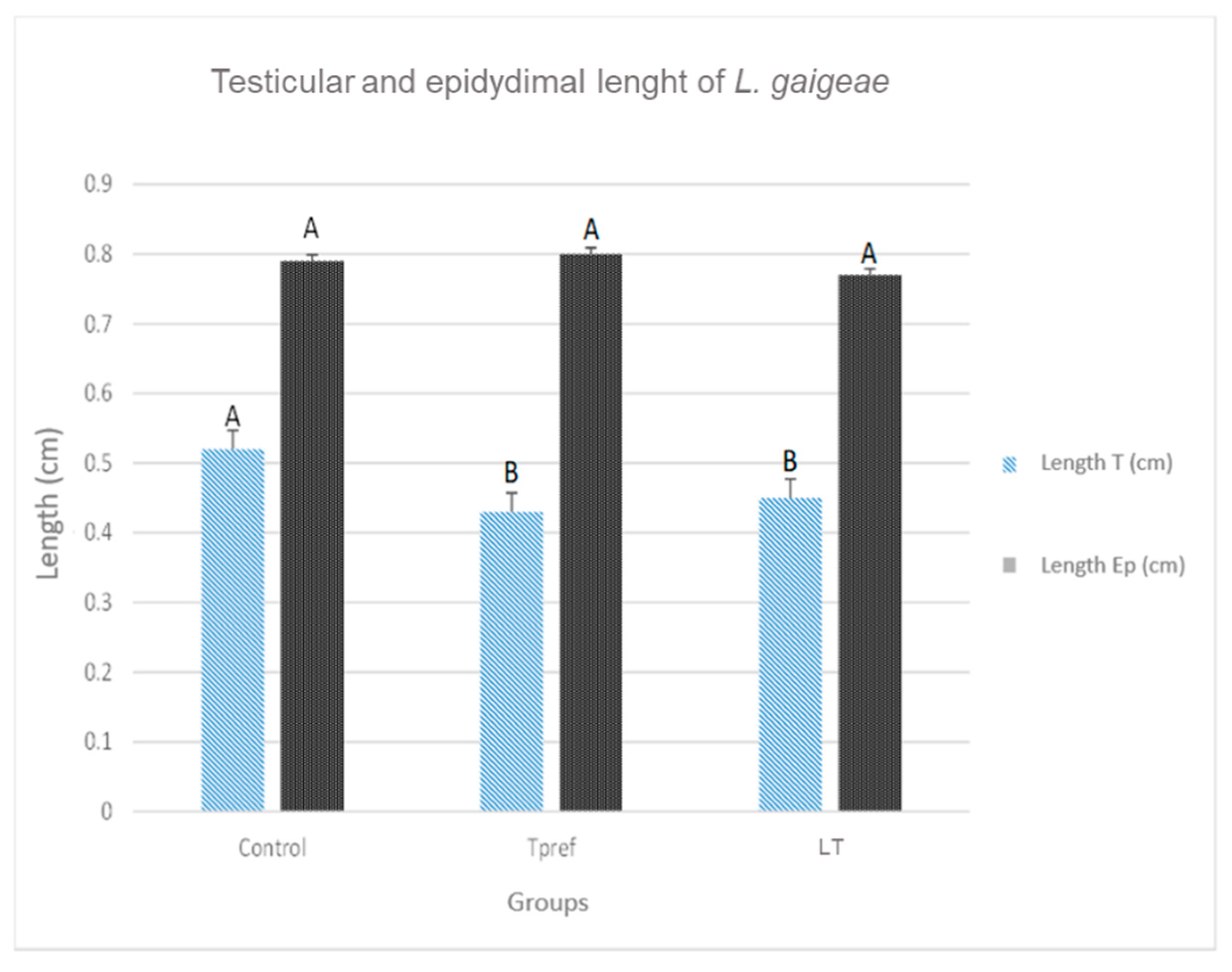
Figure 3.
Testicular and epididymal lengths of L. gaigeae specimens with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
Figure 3.
Testicular and epididymal lengths of L. gaigeae specimens with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
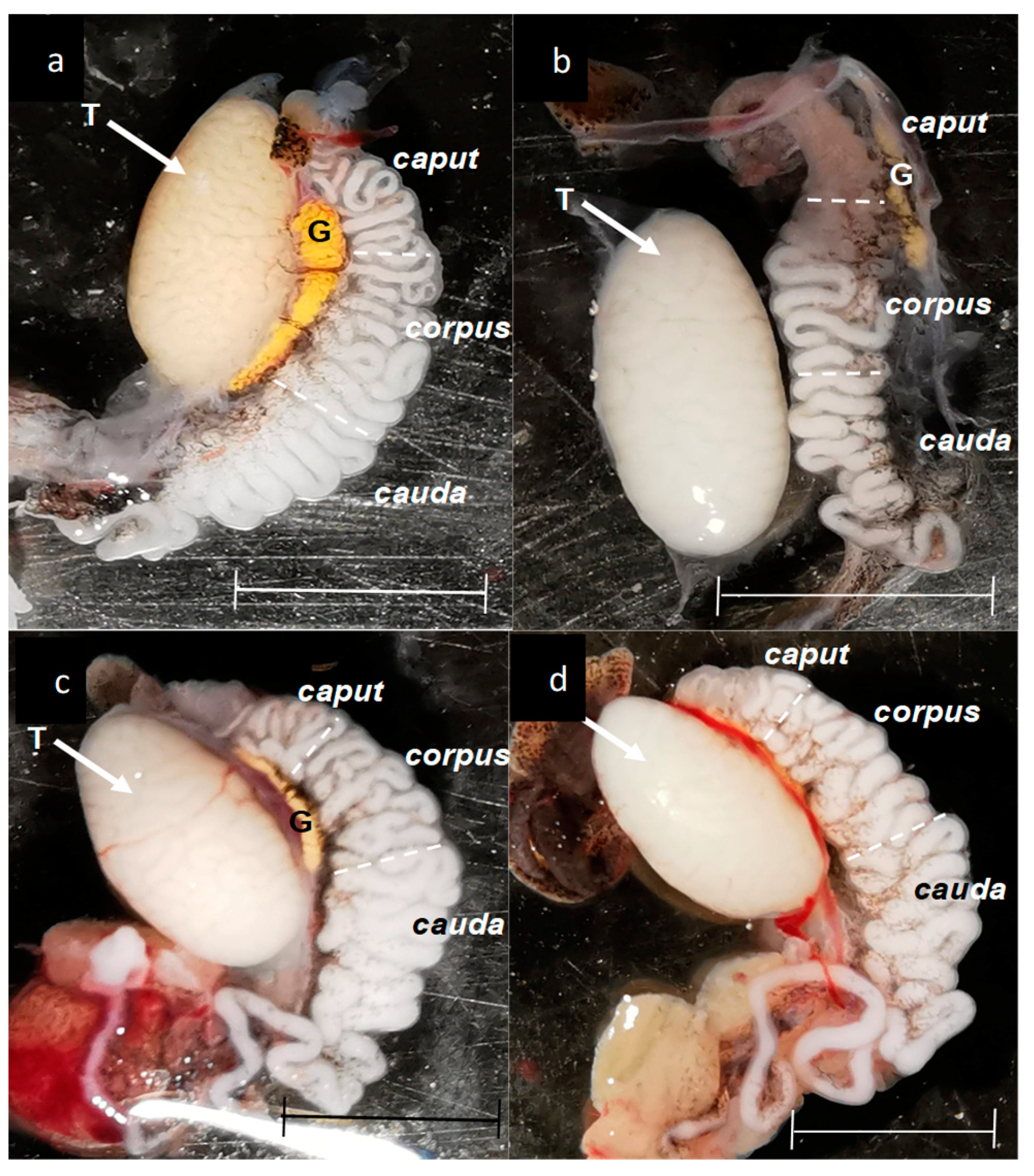
Figure 4.
Physical variations of testis and epididymis found in some individuals of L. gaigeae subjected to different temperatures. Images a and c correspond to organs collected in the preferred temperature treatment (Tpref); Images b and d correspond to organs collected in the low temperature treatment (LT). G: Fat body. T: Testis. Bars at 0.5 mm.
Figure 4.
Physical variations of testis and epididymis found in some individuals of L. gaigeae subjected to different temperatures. Images a and c correspond to organs collected in the preferred temperature treatment (Tpref); Images b and d correspond to organs collected in the low temperature treatment (LT). G: Fat body. T: Testis. Bars at 0.5 mm.
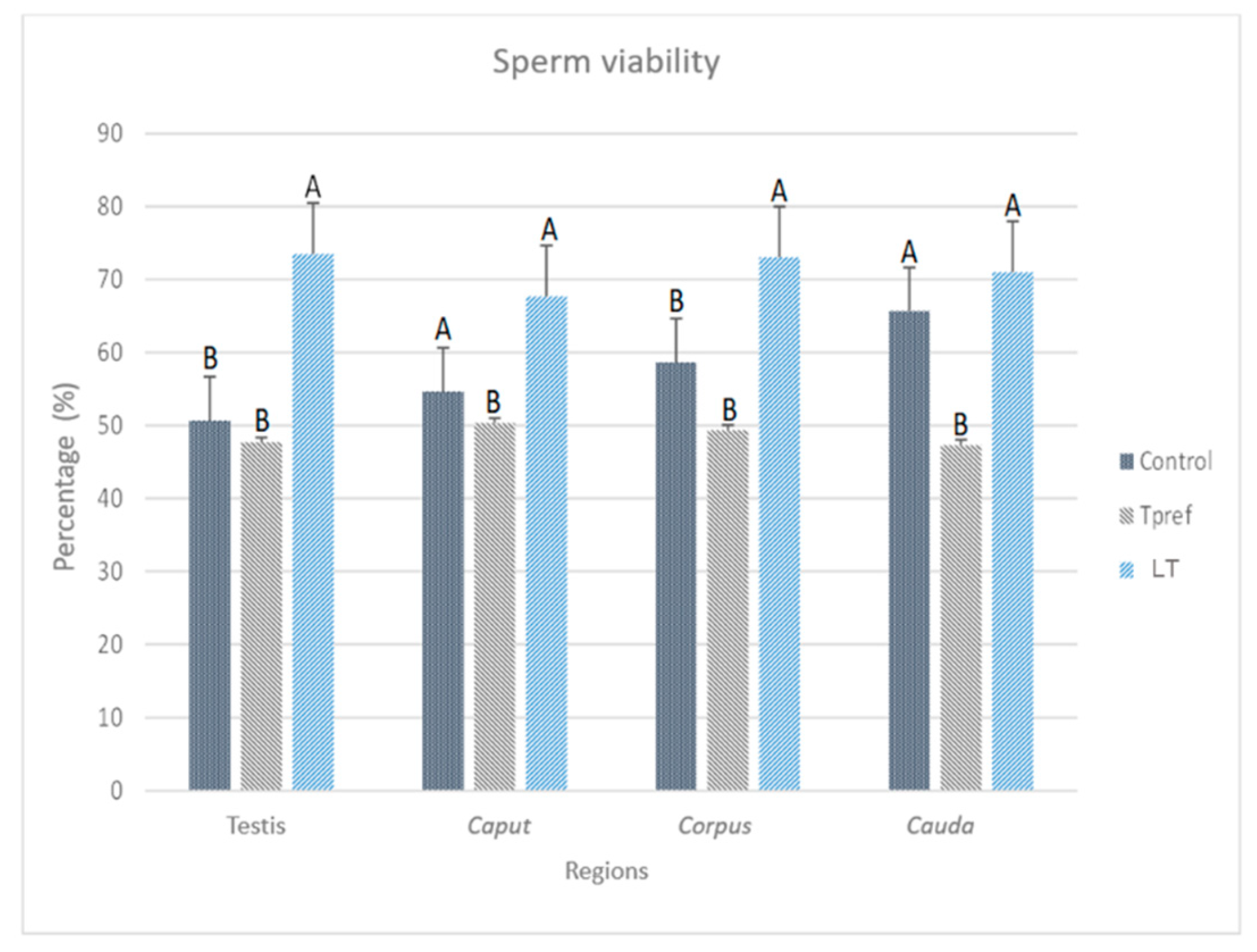
Figure 5.
Percentage of sperm viability of L. gaigeae with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. It is evident the maintenance of the viability percentage in the groups with low temperature treatment (21°C) and the increase of sperm viability in the control group compared to the remaining group, whose values decrease notably and maintain a viability of 50%. Different letters indicate significant differences between the group comparisons, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
Figure 5.
Percentage of sperm viability of L. gaigeae with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. It is evident the maintenance of the viability percentage in the groups with low temperature treatment (21°C) and the increase of sperm viability in the control group compared to the remaining group, whose values decrease notably and maintain a viability of 50%. Different letters indicate significant differences between the group comparisons, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
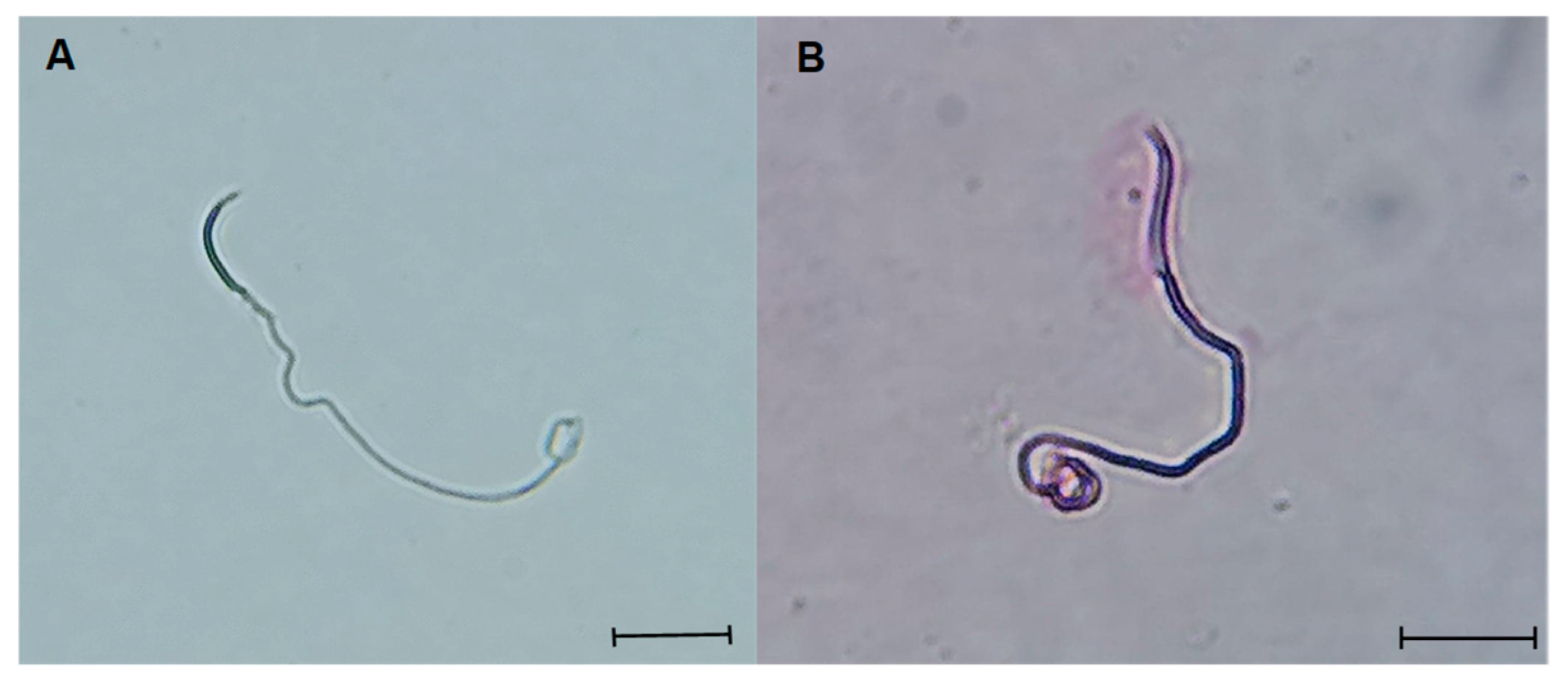
Figure 6.
Photomicrographs of L. gaigeae spermatozoa with and without treatment at different temperatures, stained with eosin-nigrosin for viability assessment with the plasma membrane dye penetration method, obtained by brightfield microscopy showing A: viable spermatozoa. B: non-viable spermatozoa. X400. Bars corresponding to 10 µm.
Figure 6.
Photomicrographs of L. gaigeae spermatozoa with and without treatment at different temperatures, stained with eosin-nigrosin for viability assessment with the plasma membrane dye penetration method, obtained by brightfield microscopy showing A: viable spermatozoa. B: non-viable spermatozoa. X400. Bars corresponding to 10 µm.
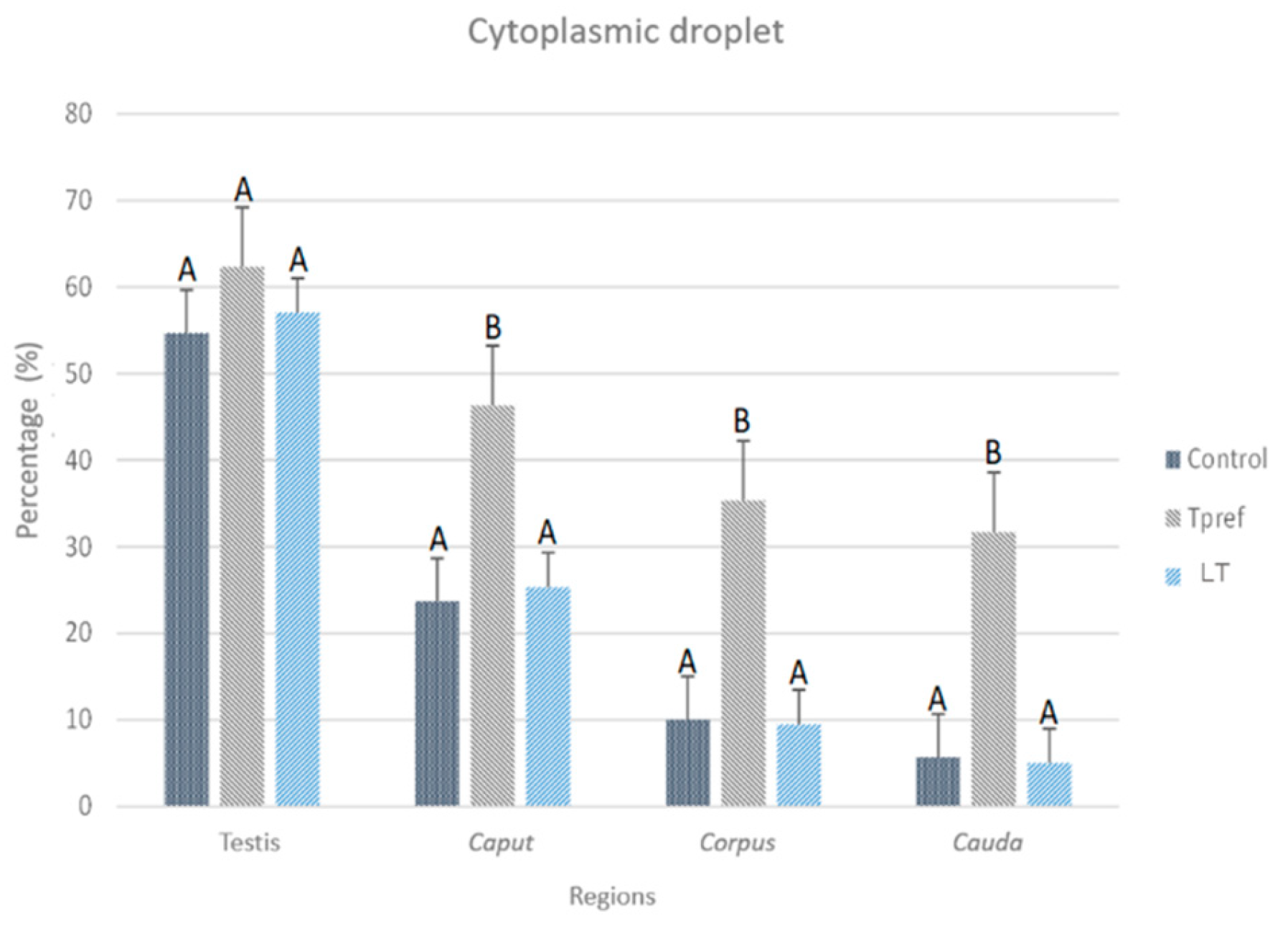
Figure 7.
Percentage of spermatozoa with cytoplasmic droplet (CD) of L. gaigeae with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Evidence of CD removal at the testicular level is shown in the control group and with low temperature treatment (21°C). All groups showed a decrease in CD as they moved to the caput zone, with a difference in the percentage of spermatozoa found with this characteristic. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
Figure 7.
Percentage of spermatozoa with cytoplasmic droplet (CD) of L. gaigeae with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. Evidence of CD removal at the testicular level is shown in the control group and with low temperature treatment (21°C). All groups showed a decrease in CD as they moved to the caput zone, with a difference in the percentage of spermatozoa found with this characteristic. Different letters indicate significant differences between the comparison of groups, according to the analysis of variance (ANOVA) and the Tukey and Kruskal-Wallis post hoc test given with a p-value < 0.05.
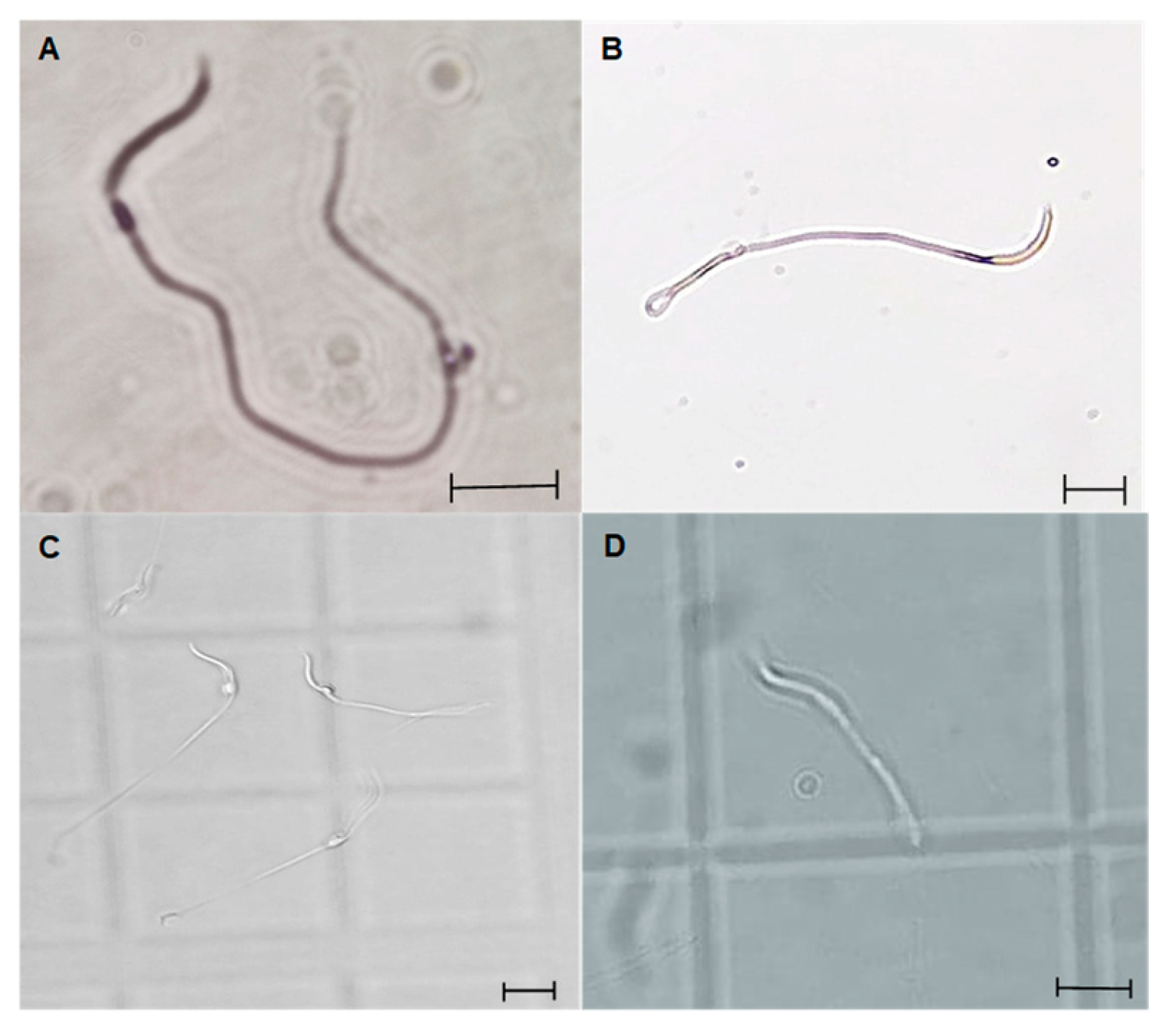
Figure 8.
Photomicrographs of L. gaigeae spermatozoa stained with eosin-nigrosin (A and B) and unstained (C and D) for evaluation of the presence of cytoplasmic droplet (CD) by brightfield microscopy. A and C: Spermatozoa with CD. B and D: Spermatozoa without CD. X400. Bars indicate the dimensions of the photomicrographs, corresponding to 10 µm.
Figure 8.
Photomicrographs of L. gaigeae spermatozoa stained with eosin-nigrosin (A and B) and unstained (C and D) for evaluation of the presence of cytoplasmic droplet (CD) by brightfield microscopy. A and C: Spermatozoa with CD. B and D: Spermatozoa without CD. X400. Bars indicate the dimensions of the photomicrographs, corresponding to 10 µm.
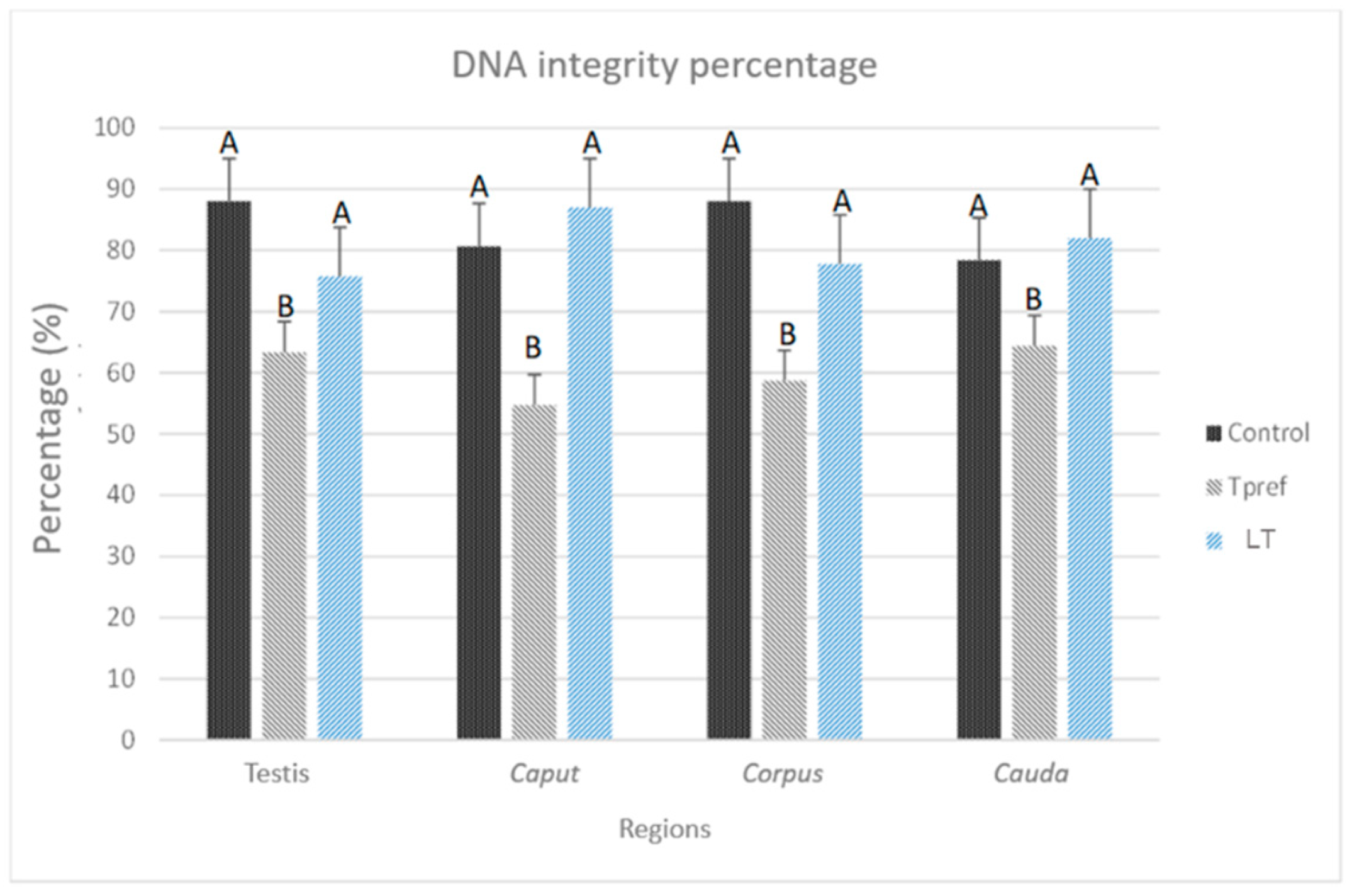
Figure 9.
Percentage of DNA integrity of L. gaigeae spermatozoa with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. The percentage of spermatozoa with intact DNA is maintained, except for the group with treatment at the preferred temperature (Tpref) in all the areas evaluated, which seems to be increasing, shortening the difference between the remaining groups.
Figure 9.
Percentage of DNA integrity of L. gaigeae spermatozoa with and without treatment at different temperatures. The bars indicate the distribution of the mean value of the data obtained from each treatment and region. The percentage of spermatozoa with intact DNA is maintained, except for the group with treatment at the preferred temperature (Tpref) in all the areas evaluated, which seems to be increasing, shortening the difference between the remaining groups.
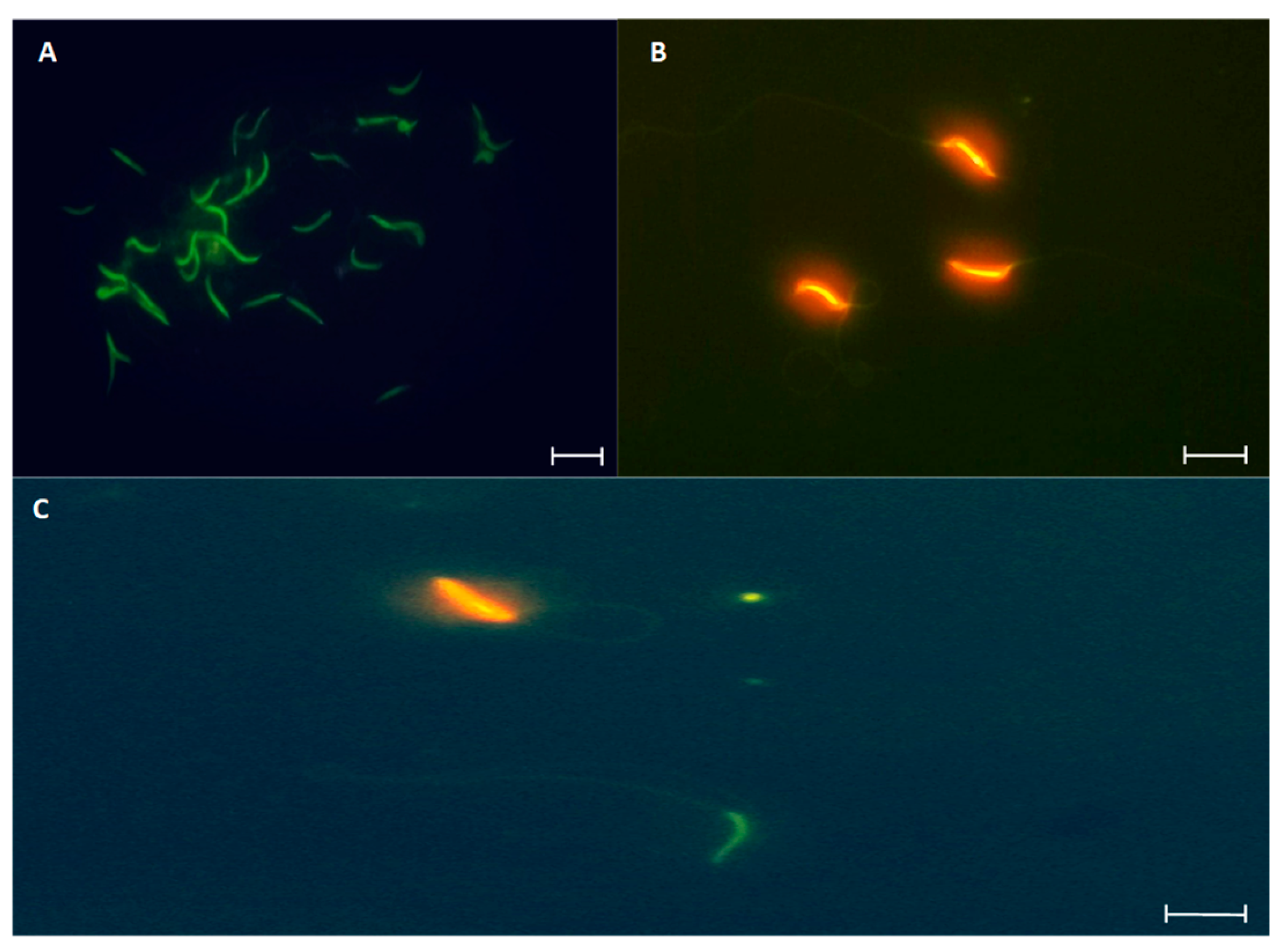
Figure 10.
Photomicrographs of L. gaigeae spermatozoa stained with acridine orange for DNA integrity evaluation by fluorescence microscopy. A: Spermatozoa with intact DNA. B: Spermatozoa with fragmented DNA. C: Indicates the bipolarity of the staining found in the cell samples. X400. Bars at 10 µm.
Figure 10.
Photomicrographs of L. gaigeae spermatozoa stained with acridine orange for DNA integrity evaluation by fluorescence microscopy. A: Spermatozoa with intact DNA. B: Spermatozoa with fragmented DNA. C: Indicates the bipolarity of the staining found in the cell samples. X400. Bars at 10 µm.
Table 1.
Length and weight records of 12 male L. gaigeae collected. The average length is shown to verify the status of an adult male.
Table 1.
Length and weight records of 12 male L. gaigeae collected. The average length is shown to verify the status of an adult male.
| Organisms |
SVL (mm) |
Body weight (g) |
| L. gaigeae |
51.60 ± 2.17 |
4.37 ± 0.33 |
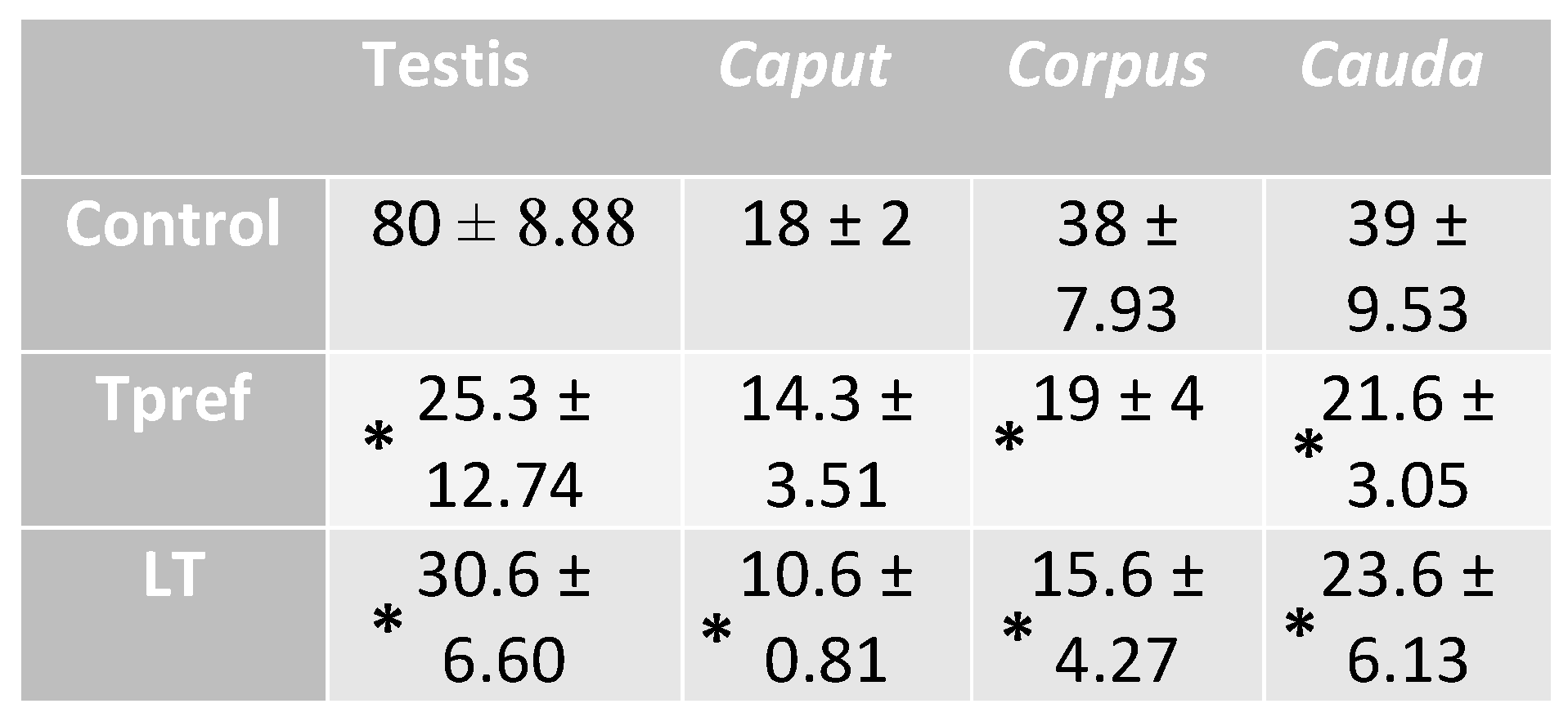
Table 3.
Sperm concentration of L. gaigae with and without treatment at different temperatures. Data represent n106 spz/mL. Asterisks indicate significant differences between group comparison, according to analysis of variance (ANOVA) and Tukey's and Kruskal-Wallis post hoc test given at p-value < 0.05.
Table 3.
Sperm concentration of L. gaigae with and without treatment at different temperatures. Data represent n106 spz/mL. Asterisks indicate significant differences between group comparison, according to analysis of variance (ANOVA) and Tukey's and Kruskal-Wallis post hoc test given at p-value < 0.05.