1. Introduction
The domestication of cats represents a unique human-animal relationship in history; evidence dates back 9,500 years to a human and cat shared burial in Cyprus [
1]. Unlike other animals, cats were not primarily domesticated for practical purposes such as food. Instead, their natural ability to control pests, like mice and snakes, contributed to their integration into human settlements [
2]. This mutually beneficial relationship gradually evolved into a more complex social bond, where the cats' inherent hunting prowess was complemented by their appealing physical features and independent, yet affectionate nature [
1,
3]. As a result, cats have become companion animals that maintain much of their ancestral behavior while forming deep emotional bonds with humans, a partnership that continues to evolve [
3].
Today, cats are popular pets globally. Although they retain a significant degree of independence, as evidenced by the large population of feral cats that thrive without regular human care [
4], the human-cat bond has grown stronger [
5].
However, this close relationship also brings health considerations, particularly regarding parasitic infections. Cats can harbor both endoparasites and ectoparasites, with varying prevalence rates among countries and cat populations [
6,
7]. Prevalence of ectoparasites and endoparasites in owned cats in nine European countries was 29.6% and 35.1% respectively;
Otodectes cynotis (17.4%) and fleas (15.5%) were the most frequently identified ectoparasite while
Toxocara cati (19.7%) was the most prevalent nematode. Notably, 14% of the cats were co-infested with endoparasites and ectoparasites, demonstrating the need for effective treatment against both [
6]. Similar findings come from other continents, with reported pooled prevalences for
T. cati: 27.9% in Asia, 21.4% in Africa, and 18.5% in North America [
8]. Other studies reached similar conclusions and highlighted the need for year-round treatment in cats [
9,
10] and higher frequency of parasitic infections in stray or feral cats [
11].
The management of parasites has evolved significantly; endo- and ectoparasiticides and endectocides are used prophylactically or therapeutically. Current treatment options include, among others, macrocyclic lactones, pyrethroids, organophosphates, and the newer class of isoxazolines [
12]. According to European Scientific Counsel Companion Animal Parasites (ESCCAP) guidelines, cats living outdoors should receive endoparasitic treatments at least four times annually, while cats living indoors require treatment one to two times yearly. As the infestation risk increases based on the lifestyle of the cats and the local parasite prevalence, they should receive year-round protection against ticks, fleas, and heartworm disease [
13,
14,
15]. The increasing cat population in urban areas worldwide [
16] necessitates continued innovation in parasite control strategies. Developing effective and more convenient treatment options with broad coverage of endo- and ectoparasites in one application remains essential for ensuring feline health and the safety of the human-animal bond [
13].
Veterinary professionals play a crucial role in communicating cat and human health risks due to parasites, and they are also responsible for recommending appropriate treatments [
17,
18]. Antiparasitics are available worldwide with a veterinary prescription. However, regulations on their prescription vary across countries [
19]. In some regions, there is no prescription requirement, placing the responsibility of choosing antiparasitic treatments on the pet owners. However, consulting a veterinarian is recommended to ensure the appropriateness of the treatment. Moreover, despite the availability of antiparasitics, adherence to recommended protocols is challenging, and the actual compliance rates fall far short. In a Portuguese study, over 90% of cats received antiparasitics, but only 38% of owners dewormed quarterly for endoparasites and 28% for ectoparasites. Just 2% followed monthly deworming, while 26% used ectoparasiticides monthly [
20].
The success of parasite control relies heavily on two key factors: owner willingness and ability to comply with the recommended treatment and veterinarians' communication skills. To ensure better compliance, it is crucial to understand what influences owners' decisions when selecting antiparasitic products for their cats and to consider these factors during client communication. While previous studies have identified barriers to compliance like dosage frequency, the complexity of using multiple products, the difficulty of application, and insufficient knowledge about available products, parasitic diseases, and their public health significance [
18,
21,
22,
23], there remains a need for a more systematic understanding of how cat owners prioritize and weigh different product attributes in their decision-making process.
To address this knowledge gap, our study employs a Discrete Choice Experiment (DCE), a methodological approach that has demonstrated robust results in both human and veterinary medicine [
24]. DCE offers several advantages over traditional preference assessment methods: it reduces measurement bias by simulating real-world decision-making scenarios, forces explicit trade-offs between attributes, and enables quantification of relative importance for different factors. While DCEs have been successfully applied to various veterinary contexts, pet owner communication preferences regarding willingness to pay for recommended services, antimicrobials, and antiparasitic treatments for dogs [
23,
24,
25,
26,
27], its application to preferences for feline antiparasitic treatments represents a novel opportunity to understand this specific aspect of feline healthcare.
To this end, we employed a multifaceted approach comprising a physical properties study (Phase 1) to evaluate the features and usability aspects of seven commercially available topical antiparasitic formulations for cats, an ease-of-use study (Phase 2) to explore the cat owners’ application experiences with three topical antiparasitics, and a DCE study (Phase 3) to identify cat owners' preferences for feline antiparasitics and the key drivers influencing their preferences. By understanding these aspects, we aim to provide insights that can inform veterinarians to improve their communication strategies, ultimately increasing compliance rates and enhancing feline health and public health outcomes.
2. Materials and Methods
2.1. Study Overview
This study comprises three Phases: (a) a physical properties study (Phase 1), (b) an ease-of-use study (Phase 2) and (c) a DCE study (Phase 3). An overview of the three study Phases is presented in
Figure 1.
Phase 1, a physical properties study, comparatively evaluated the features and usability aspects of seven commercially available topical antiparasitic formulations for cats. Within Phase 2, an ease-of-use study was conducted, which involved pet owners evaluating the application experience of three deidentified topical antiparasitic formulations. Phase 3 comprised a qualitative study phase to inform DCE, during which, product attributes most valued by cat owners were identified through cat owner interviews (development stage) and validated through consultations with veterinary experts (validation stage). The product attributes identified during the qualitative study phase informed the quantitative DCE survey, which included a pilot stage to gather preliminary insights into the study design and a main study focusing on pet owner preferences across product profiles mirroring four topical antiparasitic formulations in various countries.
2.2. Phase 1: Physical Properties Study
In this comparative study, product attributes, including the odor, stickiness, drying time properties, and viscosity, as well as certain usability aspects like the dosage, the volume of the solution, the administration process, and the container usability of seven spot-on antiparasitic formulations for cats were evaluated by the independent agency Shoku-kan-ken Inc. (561-21 Arakuchi-machi, Maebashi-shi, Gumma, Japan). The products assessed were designated as Treatments A, B, C, D, E, F and G corresponding to selamectin-sarolaner, moxidectin-fluralaner, moxidectin-imidacloprid, eprinomectin-esafoxolaner-praziquantel, fipronil-(s)-methoprene, moxidectin-imidacloprid, and moxidectin-imidacloprid respectively (
Table 1).
2.2.1. Enrolled Cats
A total of 21 clinically healthy male and female cats, aged 4 to 10 years, with no differences in type, fur, or skin condition, were enrolled in the study. The cats were randomly allocated into seven groups, each consisting of three cats. Body weight was measured immediately before product administration, ranging from 2.5 to 5.0 kg. Cats were housed individually in stainless steel cages. They were fed a predetermined daily amount of food calculated based on body weight and administered once each morning. Additionally, the cats had ad libitum access to water via an automatic water dispenser.
2.2.2. Study Design
For all the cats assigned to the seven groups, a single dose of the products under evaluation was successfully administered by applying the solution to the skin along the midline of the neck, between the base of the skull and the shoulder blades.
2.2.3. Assessment of Product Features
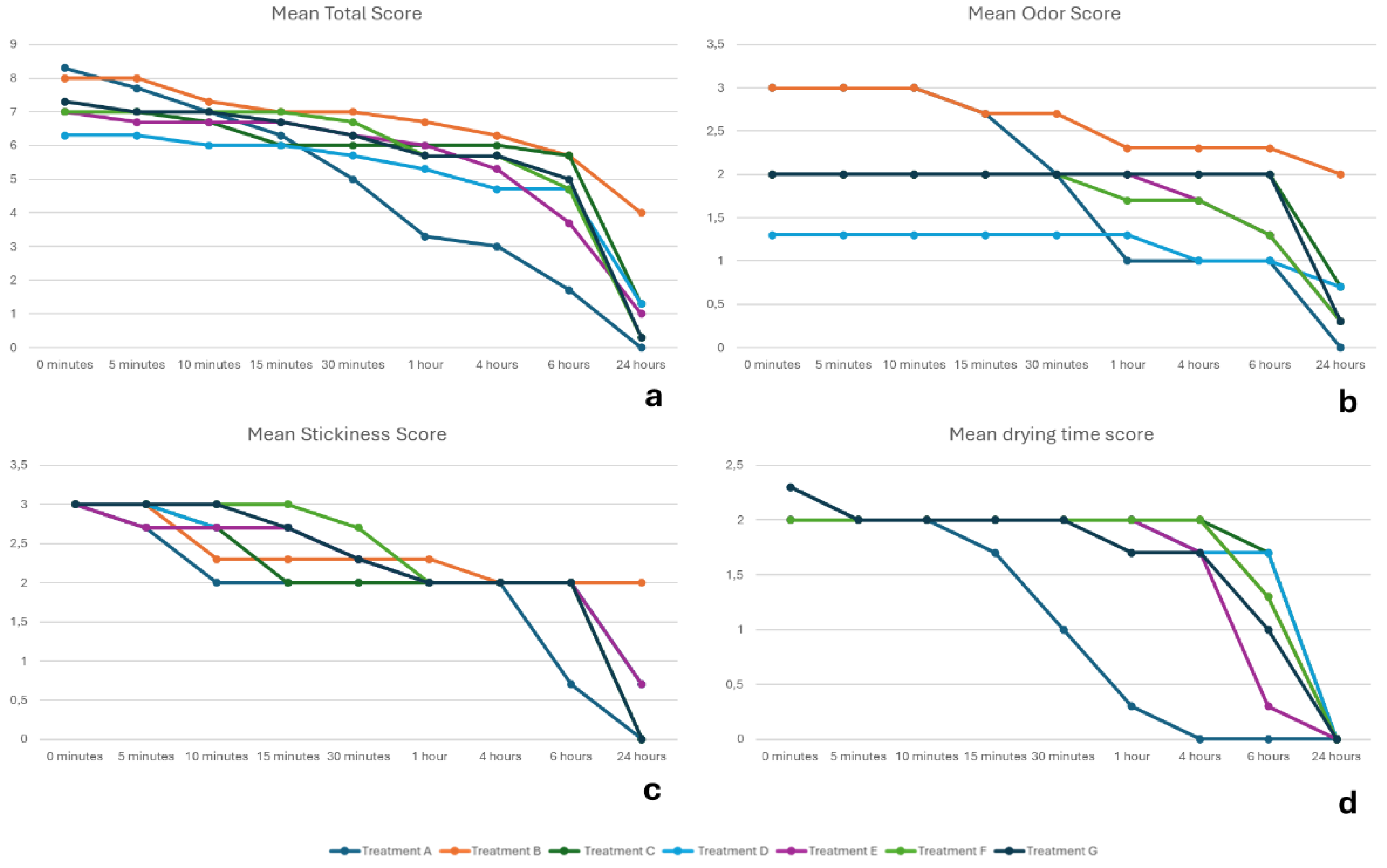
The researchers observed each cat's general condition in terms of abnormalities immediately after the administration of each spot-on formulation. The product features odor, stickiness, and drying time were scored using specific criteria (
Table 2) at eight time points: 5, 10, 15, and 30 minutes, 1, 4, 6, and 24 hours.
The assessment of the products' usability focused on the attributes of dosage, volume of solution, administration process, and container usability. The scoring system used was based on specific criteria summarized in
Table 3.
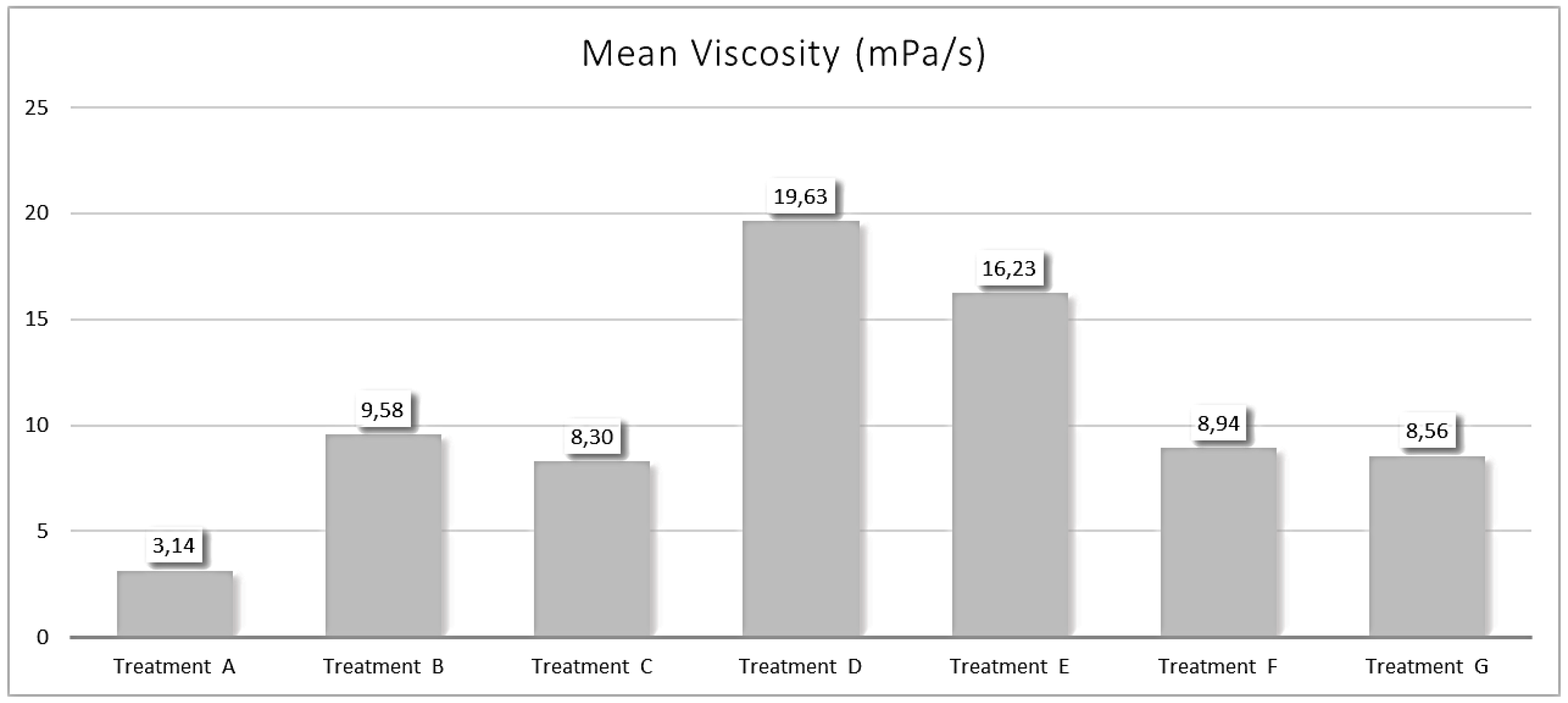
The viscosity measurement was performed three times for each product using a tuning-fork vibration viscometer (SV-10, A&D Company, Limited, Tokyo, Japan).
2.3. Phase 2: Ease-Of-Use Study
In the ease-of-use study, cat owners assessed their application experiences with deidentified products representing formulations combining selamectin-sarolaner, moxidectin-fluralaner, and eprinomectin-esafoxolaner-praziquantel.
2.3.1. Participants and Inclusion Criteria
Potential participants were enrolled in the study by a recruiter guided by a screener, which was the basis of the study’s inclusion/exclusion criteria. Participants were introduced to the study’s nature and assured about confidentiality. They verified eligibility by sharing a picture of the current antiparasitic treatment used on their cat. Various demographic and qualifying questions were asked, including gender, age, cat ownership, veterinary visits, and product usage. Participants were also asked about their role in health decisions and their comfort handling products. A crucial aspect of screening was ensuring that participants would be comfortable discussing product attributes in detail.
2.3.2. Evaluation of Cat Owners’ Application Experience
This study aimed to explore cat owners’ experiences and preferences regarding the use of the application device of the three blinded topical antiparasitic formulations under evaluation. To this end we conducted ten dyadic interviews with 20 cat owners (ten from Philadelphia and ten from Indianapolis, USA). In these in-depth interviews, two participants interacted as a dyad to facilitate an evaluation of the application device ease of use.
The three products evaluated were labelled as Treatment A, B, or D, representing the topical formulations of selamectin-sarolaner, moxidectin-fluralaner, and eprinomectin-esafoxolaner-praziquantel, respectively. The moderator guided the discussions during the interviews using a comprehensive interview guide. Each dyadic interview session lasted approximately 60 minutes.
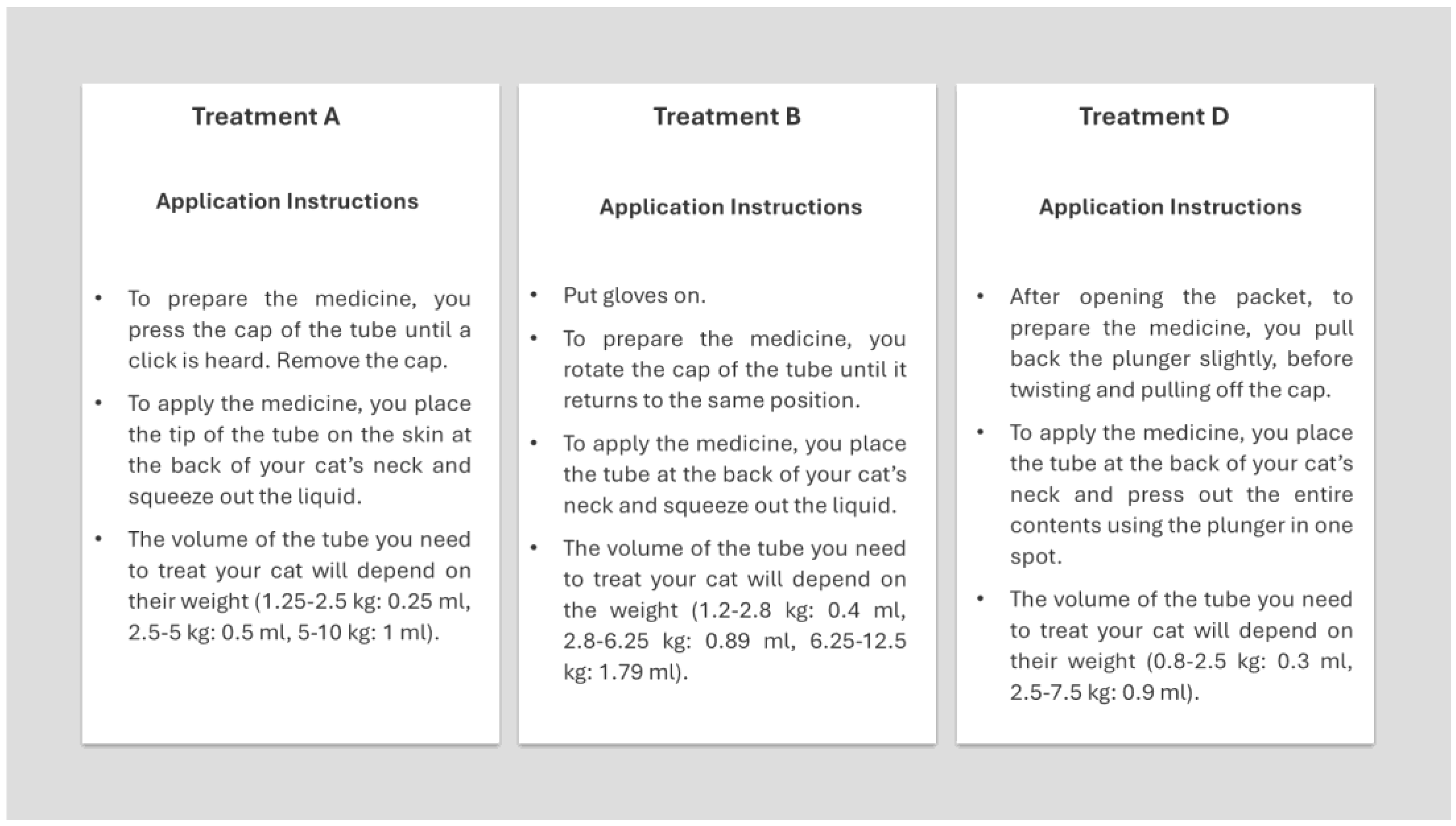
During the interviews, participants applied each product to a plush cat toy (hereafter referred to as an "animal prototype") to simulate the look and feel of a cat. Product identities remained concealed to ensure unbiased assessments. Blinded to the products, participants described their experiences applying products to animal prototypes. Each participant was provided gloves for each application and instructions on how to open each applicator and apply the product to the animal prototype (
Figure 2).
2.4. Phase 3a: Qualitative Study Phase to Inform DCE - Development Stage
The development stage of the qualitative study phase aimed to identify the product attributes and their levels that cat owners value most highly. For this purpose, 12 cat owners from Australia/New Zealand (n=3), Canada (n=3), Greece/Spain (n=3), and the UK (n=3) who aligned with predefined screening criteria were recruited and interviewed to explore their perception of each product attribute that is related to ease of administration and usability and their opinions on how these should be presented to ensure understanding.
The interview included a mixture of structured open-ended and closed questions aiming (a) to understand what and how preventative treatments for feline parasites were selected by cat owners, (b) identify the characteristics that would define the “ideal” preventative treatment for cats and (c) to uncover the key administration and usability drivers that influence cat owners' choices in selecting these treatments.
We also provided the treatment profiles and visual stimuli to the participants to (a) explore the relevance and impact of the content included in the treatment profiles, ensuring they effectively capture and visualize the key factors influencing treatment decisions; (b) test the treatment profiles for general comprehension, verifying that the wording is appropriate, and easily understandable for cat owners; (c) assess the overall utility and effectiveness of the visual stimuli in supporting the treatment profile descriptions; (d) investigate whether any pertinent information, explanations, or context was missing from the treatment profiles or the visual stimuli. Finally, we summarized any key points made earlier in the discussion and identified any final comments the respondent would like to capture. Participating cat owners were blinded to the products.
Participants were also asked to rank-order all the attributes based on their perceived importance (rate 1-lowest level of importance to 7-highest level of importance) when choosing a topical preventative therapy for feline parasitic infection/infestation.
2.5. Phase 3b: Qualitative Study Phase to Inform DCE - Validation Stage
The second stage of the qualitative study phase aimed to validate the previously identified attributes and record new ones that cat owners had not identified. For this purpose, four veterinary experts, including veterinarians, veterinary nurses/technicians, and pet retail specialists from Australia/New Zealand (n=1), Canada (n=1), Greece/Spain (n=1) and the UK (n=1) who aligned with predefined screening criteria were recruited and interviewed to discuss and validate the relevance and accuracy of the developed treatment profiles and attribute descriptions.
The interview guide included open-ended and closed questions to explore expert perceptions of each treatment attribute related to ease of use and opinions on how these should be presented to ensure understanding. Questions were designed to identify any modifications that should be made to increase their relevance and clarity. The questions aimed to (a) identify who primarily influences the decisions and recommendations made by participants, as well as the motivations behind therapy recommendations; (b) capture the therapy options currently offered—or previously offered—for preventing parasitic infections in cats, including a breakdown of the most prescribed treatments; (c) explore the satisfaction levels of both prescribers and cat owners with current preventatives, focusing on ease of administration and usability in terms of formulations and applications; (d) examine how satisfaction influences cat owner behaviors related to ease of use and compliance, and how preventative therapy impacts the attitudes of cat owners and veterinarians towards feline behaviors and welfare; (e) test product profiles and attribute descriptions for general comprehension, ensuring that all product profiles were blinded to cat owners. We also explored the impact of each attribute on treatment decision-making and investigated any missing relevant information or explanations. Finally, we summarized key points from earlier discussions and identified any additional comments respondents wished to capture.
The veterinary experts were also asked to rank-order all the attributes based on their perceived importance (rate 1-lowest level of importance to 7-highest level of importance) when choosing a topical preventative therapy for feline parasitic infection/infestation.
Participating veterinary experts were blinded to the products. When they mentioned individual product brand names, it remained unconfirmed by the interviewer.
2.6. Phase 3c: Quantitative DCE Survey - Pilot Stage
A pilot study with 40 cat owners (10 from each market: Australia/New Zealand, Canada, Greece/Spain, and the UK) was conducted to validate the main study design. After reviewing the pilot survey results, minor amendments were made.
2.7. Phase 3d: Quantitative DCE Survey – Main Stage
The main stage of the quantitative DCE survey aimed to understand the factors that are most important to cat owners when choosing topical antiparasitics to treat their cats for parasitic infections and infestations. To this end, we conducted an international survey involving cat owners from different countries and using the product attributes previously identified by the cat owners during Phases 3a and 3b. The main stage was designed, launched, and shared with a sample of cat owners from Australia/New Zealand, Canada, Greece/Spain, and the UK, with a target sample size of N=260 per country. The inclusion criteria were cat owners owning at least one cat and no more than four cats, with previous experience with topical preventative treatment for parasitic infections and infestations, aged ≥18 years, residents in one of the countries of interest, primary caretakers/decision-makers for their cat(s), not employees in animal health or market research.
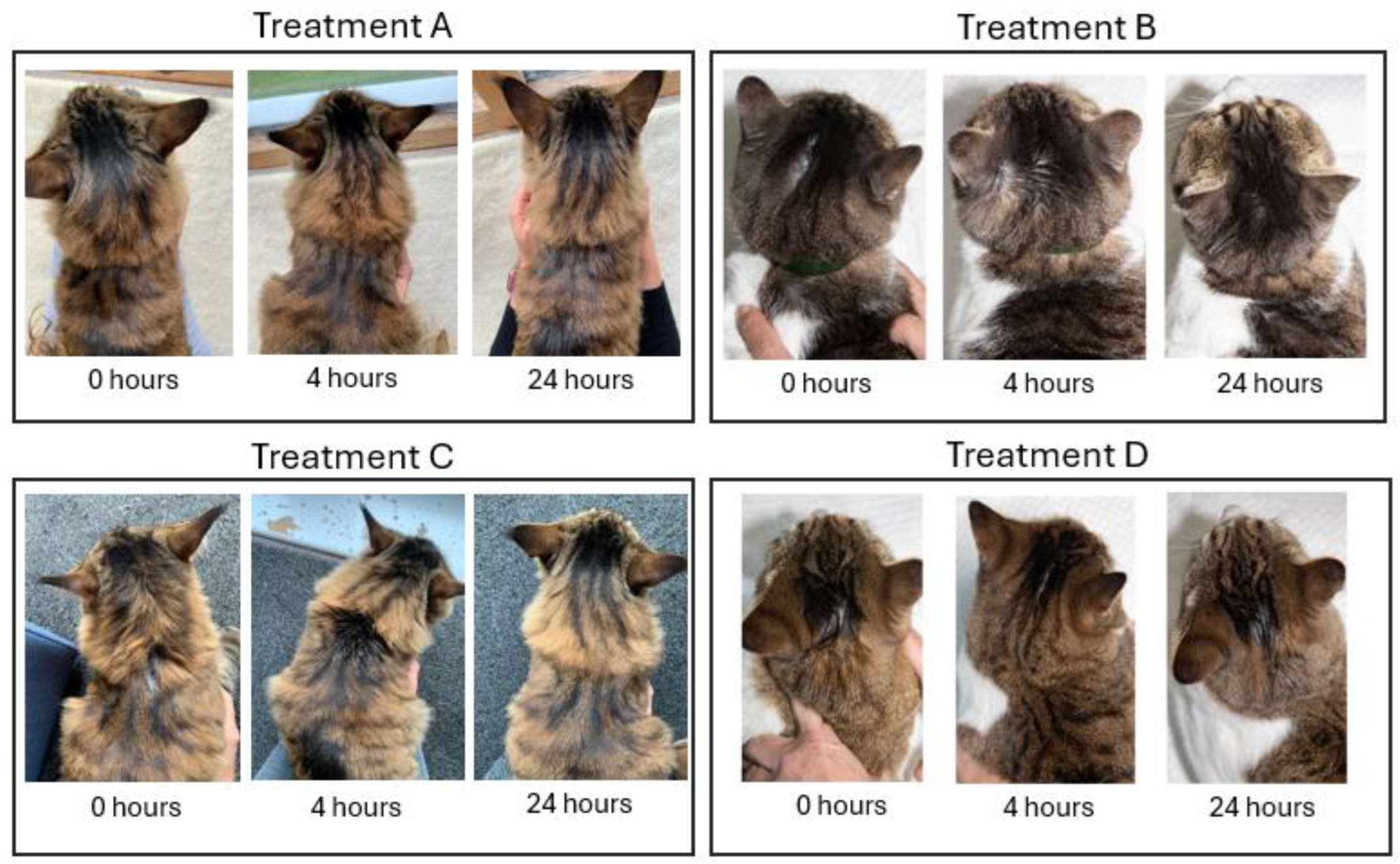
The survey was web-based and designed to last no longer than 45 minutes to establish cat owners’ participation eligibility and collect their informed consent. Participants were briefed about the purpose of the study and familiarized with the set of attributes and their varying levels. They were provided with treatment profiles, each designed according to product labels that were accurate for each region at the time of the study. Each treatment profile was referred to anonymously (i.e., Treatment A, Treatment B) and mirrored a topical antiparasitic formulation: selamectin-sarolaner (Treatment A), moxidectin-fluralaner (Treatment B), moxidectin- imidacloprid (Treatment C), and eprinomectin-afoxolaner-praziquantel (Treatment D). Photographs of live cats following the application of the products corresponding to the treatment profiles under evaluation at 0, 4, and 24 hours were also provided (
Figure 3).
The survey comprised primarily closed-ranking or preference-based questions, including screening questions (e.g., age, location, cat ownership), demographic questions (e.g., employment, income, pet insurance), and experience with topical therapies and parasitic infections (e.g., previous infection/infestation, importance of the spectrum of parasites covered by topical antiparasitics). The participants were also asked to rate the importance of different product attributes when choosing a topical preventative therapy for their cat using a seven-point scale, where one represented "not very important" and seven signified "very important”.
They were then presented with DCE choice sets in which they had to choose between the following treatment profiles: Treatment A vs. Treatment B, Treatment A vs. Treatment C, and Treatment A vs. Treatment D. To minimize bias, the order of choice sets and their visual presentation on the screen (left-hand vs. right-hand positions) were randomized. Within the DCE, participants’ reactions to treatment profiles were recorded by presenting specific product profiles alongside visual aids.
2.8. Data Analysis
Descriptive statistics were employed to analyze the data collected from the physical properties study. For each product, mean endpoint total scores, mean score values for odor, stickiness, drying time, and the mean viscosity values were calculated.
Qualitative data originating from the ease-of-use study, in the form of direct quotes from participants, was summarized using thematic analysis to identify patterns and themes.
Demographic data were analyzed using descriptive statistics, including means, medians, standard deviations, and confidence intervals. Preference data of respondent subgroups for the different treatment profiles were analyzed using the chi-squared test.
The relative importance of product attributes and their effects on treatment preferences were compared using regression analysis. Statistical significance for differences between subgroups was set at p < 0.05. All statistical analyses were conducted using R software (version 4.3.3).
2.9. Ethics
Ethical approval for the physical properties study was sought from the Kalamazoo Zoetis Ethical Review Board (ERB) post-study. The protocol, procedures, and results were reviewed, and this report acknowledges that the Kalamazoo ERB evaluated and approved the study design retroactively.
4. Discussion
Our study provides comprehensive insights into cat owners' preferences for antiparasitic treatments through a multi-faceted approach: the evaluation of physical properties and usability aspects of seven topical antiparasitics (Phase 1), the evaluation of the application experience of deidentified products representing three antiparasitics by cat owners (Phase 2), and the international quantitative DCE study for cat owner preferences when choosing antiparasitics for their cats (Phase 3). This integrated methodology revealed that treatment selection is influenced by an intricate interplay of physical properties, application experience, usability aspects, and owner-pet dynamics, with implications for both veterinary practice and product development.
The physical properties study showed that topical antiparasitics for cats vary in odor, stickiness, and drying time. To this end, the selamectin-sarolaner formulation (Treatment A) showed the lowest odor score one hour after application, with a complete absence of odor at 24 hours. This aspect is especially important for cats living indoors, where lingering odors can be unpleasant for the owners. Regarding stickiness, this formulation had the lowest score at 10 minutes post-application and exhibited no stickiness by 24 hours. Non-stickiness is important for topical antiparasitics to avoid adherence to the cat's fur or the owner's clothing, which could reduce the drug's efficacy. Additionally, the selamectin-sarolaner formulation exhibited high speed of drying, achieving complete dryness within four hours, a score reached by the other formulations at 24 hours. Quick drying is essential for topical products because it allows the treated animals, especially those living indoors, to return to normal activities sooner and provide peace of mind to the cat owner. Finally, the selamectin-sarolaner formulation showed the lowest viscosity compared to the other formulations.
These differences can be attributed to the specific excipients used, particularly the choice of solvents [
28]. Supporting our findings, the study by Chansiripornchai and Jantanawaranon (2020) emphasized the importance of stickiness and drying time as key usability parameters in cat spot-on parasiticides. Their research demonstrated that formulations like selamectin and fluralaner presented reduced stickiness and faster drying times, achieving complete dryness within 24 hours post-application [
29]. Understanding these formulation characteristics can help veterinarians make informed recommendations based on the user experience and their specific needs, ultimately improving adherence to prescribed therapies.
Considering the usability aspects, that is, the product characteristics that enable patients and caregivers to effectively use pharmaceutical products in their everyday environments [
30], we assessed the dosage, the solution volume, the administration process, and the container usability. This evaluation showed that the administration was straightforward and the solution volumes were appropriate for all the products, except for the case of Treatment G (moxidectin-imidacloprid formulation for > 4 kg to 8 kg). Moreover, the dosing regimen effectively minimized drug spillage, and the container design facilitated precise administration, allowing for gradual dosage adjustments. This finding underscores the overall user-friendliness of the formulations, which is crucial for ensuring adherence to treatment protocols. To this end, veterinarians should remember that the application experience associated with topical formulations is particularly important, as pet owners may administer these treatments themselves.
Although similar studies in veterinary medicine are lacking, studies on patients' preferences in human medicine have repeatedly shown that the physical properties of drugs, like color, size, taste, smell, and shape, are important determinants of patient acceptance and preferences [
30]. These findings are relevant in veterinary practice, as pet owners, who are involved in their pets’ health care, may have similar emotional responses to the physical attributes of antiparasitic treatments. For instance, whether choosing a product or not can be influenced by its odor, drying time, and stickiness.
This link between usability aspects and the cat owner preference for one formulation over another was further validated by the ease-of-use study, which included the hands-on application of blinded products representing three topical antiparasitics by the cat owners on animal prototypes. This study complemented the physical properties study and delved deeper into the application process and user experience. The participants reported that the product representing the selamectin-sarolaner formulation had a seamless application, characterized by rapid dispensing and a sense of control for the cat owner. They also reported a hassle-free experience, contrasting it with Treatment B, which, while user-friendly, raised concerns about application efficiency and the need for gloves. Likewise, Treatment D presented rapid dispensing but unexpected challenges during application. These findings highlight the importance of minimizing stress for the cat owner and their cats during product application.
The perceived importance of product attributes among cat owners is critical for further understanding their preferences. To this end, during the quantitative DCE study, participants rated the importance of the product attributes. Eleven product attributes were perceived as important based on the ratings assigned, indicating their role in treatment selection. Notably, seven attributes were related to overall ease-of-use, underscoring the importance of user-friendly designs. Attributes such as the ease of use of the applicator device, the spectrum of parasites covered, the familiarity with current treatment, and the ability to confirm successful administration were highly rated. These insights emphasize the need for veterinarians to consider product usability when recommending treatments. Attributes related to negative precautions following administration and physical properties also garnered attention. However, the highest-rated attributes were exclusively linked to overall ease-of-use, highlighting a clear preference among cat owners for products that simplify the treatment process.
The findings from the quantitative DCE study further showed a preference for the product profile mirroring the selamectin-sarolaner formulation among a global sample of 1,040 cat owners. When data from all the countries were considered together, it was significantly favored over other formulations in all comparisons. Seemingly, when preferences were analyzed based on the countries of origin of the cat owners, it remained significantly favored over all other treatment profiles, except in Australia/New Zealand, where the difference was not statistically significant (p>0.05).
Demographic characteristics such as gender, age, and insurance status influenced the cat owner's preferences. Male cat owners preferred the product profile mirroring the selamectin-sarolaner formulation over all the other formulations. At the same time, female owners significantly favored the product profile mirroring selamectin-sarolaner formulation over Treatments C (mirroring moxidectin- imidacloprid formulation), and D (mirroring eprinomectin-afoxolaner-praziquantel formulation). Age also played a role, with a significant preference for the product profile mirroring the selamectin-sarolaner formulation over Treatment B (mirroring moxidectin-fluralaner formulation) among those aged 31-50 years and across all ages over Treatments C (p < 0.01) and D (p < 0.01). Insurance status positively influenced preferences for the product profile mirroring the selamectin-sarolaner formulation over C and D (p < 0.01) but not B (p > 0.05). These findings indicate that demographics play a significant role in cat owners' treatment preferences, which could help veterinarians make informed recommendations in daily practice.
The analysis of cat owner treatment preferences, when considering the perceived importance of the product attributes, showed that those who rated the product attributes as "very important" showed a significant preference for the product profile mirroring the selamectin-sarolaner formulation over Treatment B for the following attributes: ability to confirm successful administration, drying time, and the length of time before the cat can be touched following application. Even among those who rated the attributes "not very important", the product profile mirroring the selamectin-sarolaner formulation was preferred over Treatment B for all attributes assessed, except for drying time, where no significant difference was found. Overall, cat owners favored the product profile mirroring the selamectin-sarolaner formulation, with significant differences noted in multiple scenarios compared to Treatments B, C, and D.
Further analysis of the data obtained through the quantitative DCE study identified several key predictors influencing the preference for the product profile mirroring the selamectin-sarolaner formulation over the comparative treatment profiles. In brief, cat owners who prioritized the ability to confirm successful administration and age restrictions were more likely to prefer the product profile mirroring selamectin-sarolaner formulation. Conversely, increased concerns about treatment transfer to family members or furniture following application decreased this preference. In comparing the product profile mirroring selamectin-sarolaner formulation with Treatment C, factors such as the time before the cat could be touched post-application and the preparation required for the applicator device negatively impacted the likelihood of preferring the product profile mirroring the selamectin-sarolaner formulation. For the comparison of the product profile mirroring the selamectin-sarolaner formulation vs. Treatment D, ease-of-use during application and the time before the cat could sit on furniture following application positively predicted preference for the product profile mirroring selamectin-sarolaner formulation. In contrast, the oiliness of the formulation negatively affected it. These findings collectively suggest that cat owners prioritize safety, ease of use, and the overall experience of administering treatments.
However, the overall explanatory power of the model for the comparison between the product profile mirroring the selamectin-sarolaner formulation and Treatment B was limited, and the models comparing the product profile mirroring selamectin-sarolaner formulation to Treatment C and D exhibited a lack of robustness. This suggests that identifying explanatory predictors for variance in pet owners' treatment preferences is complex and requires the consideration of several other factors to build robust prediction models and reach solid conclusions.
For instance, treatment cost could affect treatment preferences, and its impact should be investigated in future research. Besides, treatment cost is often presumed to be an important barrier to pet owners’ compliance, which could be indirectly related to treatment preferences. However, in a study conducted in Hong Kong, the cost of preventive products was one of the least frequent reasons for not using tick prevention, with other factors such as lifestyle and lack of knowledge being more prominent barriers [
31]. In the same context, a study showed that 69% of cat owners were willing to spend any amount necessary for their cats' health [
23]. Another study showed that cat owners may be less willing to pay for the recommended care than dog owners unless they clearly understand the needs and benefits [
23]. Thus, the preferences of pet owners may constitute a dynamic situation influenced by several other factors, including cultural differences, awareness of health risks, and available products and understanding of the treatment benefits and needs. These scenarios should be investigated further in future studies.
Another limitation of this study was that the physical properties assessment, while comprehensive, was conducted under controlled laboratory conditions, and experienced researchers applied the treatments. These conditions may not fully reflect real-world application scenarios. Environmental factors such as temperature, humidity, and the variability in the cat owners' application technique could influence the performance of these formulations in practice. In addition, while the quantitative DCE study provides valuable insights, it has inherent limitations. While our sample size of 1,040 participants provided substantial statistical power, the self-selected nature of participation could have potentially led to overrepresenting more engaged and conscientious pet owners. As previously mentioned, the product labels were accurate for each country when the study was conducted, which may account for differences such as the recommended separation times before the cat could be touched after the product application. Despite the above-mentioned limitations, this study provides valuable insights into pet owners' preferences for spot-on antiparasitics in cats, supported by three distinct assessments: a physical properties study, an ease-of-use study, and a quantitative DCE study. Combining different methodologies enhances the validity of the key attributes that matter most to pet owners.